Contents
1.Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 244
2.Electrospinning nanofiber technology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 244
2.1. Development history of electrospun nanofibers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 244
2.2. Different electrospinning techniques. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .245
3. Electrospun nanofibers as tissue engineering scaffolds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 245
3.1. Ideal scaffold for tissue engineering . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 245
3.2. Advantages of electrospun nanofibers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .247
4. Electrospun nanofibers for skin tissue regeneration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 247
5. Electrospun nanofibers for blood vessel tissue regeneration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 248
6. Electrospun nanofibers for nerve tissue regeneration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 250
6.1. Simple hollow luminal structure NGC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 250
6.2. Growth factor incorporated into NGC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 251
6.3. Conductive nanofiber NGC and electrical stimulation for nerve regeneration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 251
6.4. Filaments-containing NGC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 252
6.5. Sponge-containing NGC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 252
1. Introduction
Fibers are no stranger to human beings. Natural fibers from plants and animals, including cotton, hemp, wool, and silk, have been used for thousands of years. With the advent of modern polymeric materials, synthetic fibers have increasingly grown in popularity. They continue to be used in a wide variety of applications, including everyday consumer products, environmental applications, optoelectronics, biomedical research and technology, energy, filtration systems, and protective equipment [1]. In recent years, various fabrication methods have emerged to prepare nanofibers, such as phase separation [2], self-assembly [3] stretching, and template synthesis [4]. However, these methods are limited by their technical complexity, cost, yield, and ability to control the scale of the resulting fibers.
As an alternative, Formhals [5] invented a device capable of producing nanoscale polymer fibers using a high-voltage electric field and applied for a patent in 1934. It was the first international patent for the preparation of nanofibers using an electrical potential difference as a driving force, which coined the term electrospinning. Electrospinning provides a simple and straightforward approach to produce continuous, polymer fibers with diameters ranging from nanometers to microns [1], [6]. The typical electrospinning apparatus includes a high-voltage power supply, a syringe pump to control polymer flow rate, a spinneret (e.g., a medical needle with a blunt tip), and a conductive collector to catch the electrospun fibers. Simply, electrospinning can be considered an electrohydrodynamic process dependent on the potential difference to create a liquid jet, followed by mechanical stretching, elongation, and drying to generate fibers [7], [8]. As the fibers collect, an electrospun membrane is built with 3D topography, high porosity, and large surface area, while retaining mechanical integrity and fiber continuity [9]. As such, the unique structure of electrospun membranes have broad applications in many fields but are of particular interest as scaffolds for tissue engineering [10], [11]. This tissue engineering application is because most human tissues and organs are composed of nanofibers in the ECM, which makes it possible for electrospun nanofiber use in tissue and organ repair as a mimic [12], [13]. More importantly, a variety of natural and synthetic polymers can be electrospun that have biocompatibility and biodegradability and are reabsorbed by the human body. Together, these attributes of electrospun nanofibers have attracted the attention of biomedical researchers and led to investigation for use in skin, bone, cartilage, nerve, blood vessel, tendon, and other tissue regeneration applications.
The field of tissue engineering attempts to restore or regenerate the functionality of healthy tissues and organs using the three essential components of cells, biomolecules, and scaffolds [14]. The tissue engineering scaffold provides the 3D microenvironment needed for the growth of cells damaged by disease, injury, or congenital defects, and the selection of the material depends, to a great extent, on the tissue-specific application [15]. However, no matter the intended application, a primary challenge is scaling biomaterials into 3D constructs that recapitulate the biological, chemical, and mechanical properties of the tissue microenvironment [16]. With its relative simplicity and versatility, electrospinning can be a process used to fabricate these 3D porous, fiber membranes resembling the ECM and modified with biomolecules to facilitate cell adhesion, migration, differentiation, and proliferation. Thus, we review here the current advances in the fabrication of electrospun fiber materials and their use as scaffolds for tissue engineering.
2. Electrospinning nanofiber technology
2.1. Development history of electrospun nanofibers
Electrospun nanofibers have existed since the beginning of the 20th century (Table 1). Formhals originally published a series of patents on solution electrospinning using a polymer dissolved in solution to obtain ultrafine fibers in the nanometer to micrometer diameter range. In 1980, the United States Patent Office disclosed another method of electrospinning, polymer melt electrospinning, where the polymer is melted to produce the electrospinning solution [17]. This process results in fibers that are 1-2 orders of magnitude larger than those obtained by solution electrospinning, indicating that it may not be useful for all applications of electrospun fibers [18]. Nonetheless, the invention of both solution and melt electrospinning laid the foundation for the further development and advancement of new technologies.
Table 1 Development history of electrospun nanofibers.
| Particular year | Development of electrospinning technology |
|---|---|
| 1902 | Solution Electrospinning |
| 1980 | Melt Electrospinning |
| 1999 | Preparation of Electrospun Nanocomposites |
| 2000 | Electrospun Nanofibers for Tissue Engineering |
| 2003 | Coaxial Electrospun Nanofibers |
| 2005 | Emulsion Electrospinning Nanofibers |
| 2007 | Dynamic Water Flow Electrospun nanoyarns |
| 2012 | Electrospun Continuous Nanoyarn |
| 2014 | Preparation of three-dimensional porous aerogel by electrospinning combined with freeze-drying technology |
Since the turn of the 21 st century, traditional electrospinning technologies have developed rapidly worldwide in scientific research and industrial circles, partly due to the growing interest by the tissue-engineering field. In 2003, Sun et al. [19] reported on a new electrospinning technology, coaxial electrospun nanofibers with core-shell structure, in which two different solutions are simultaneously ejected through a coaxial nozzle, enabling encapsulation of materials. Building on this technology, Xu et al. [20] reported the core-shell structure of nanofibers containing an anticancer drug by emulsion electrospinning, which does not require a coaxial nozzle, but instead uses the emulsion of a non-spinnable component within a solution that can be electrospun. In an attempt to increase the three-dimensionality of electrospun membranes, Teo et al. [21] created an electrospinning system that incorporated a water vortex to collect the nanofibers, resulting in a highly porous yarn that could be used as a tissue engineering scaffold. Likewise, in 2012, Ali et al. [22] created a system for continuous, electrospun nanoyarns that can be knitted or weaved into tissue engineering scaffolds. Most recently, Si et al. [23] combined electrospinning with freeze-drying to generate a composite material with nanofibers embedded in a 3D porous aerogel. These examples illustrate the progression of electrospinning over the last two decades from simple mats to more complex, 3D structures.
Since 2000 due to the growing interest in electrospun membranes, the number of articles published related to electrospinning has increased exponentially (Fig. 1). In the ranking of the total number of articles published by country, China ranks first, accounting for almost 50 % of the total number of papers published worldwide, followed by the United States and South Korea (Fig. 2). Thus, rendering China as the most active country in the field of electrospinning.
Fig. 1.
Fig. 1.
Graphical representation of the number of articles on electrospinning published worldwide for each year since 2000.
Fig. 2.
Fig. 2.
Proportion of electrospinning papers published by countries in the world.
2.2. Different electrospinning techniques
The traditional method of electrospinning and its equipment is illustrated in Fig. 3(A). In this method of electrospinning, a high voltage is applied to the polymer solution to create a Taylor cone at the tip of the nozzle, typically a blunt needle. Using a syringe pump to control the polymer solution flow rate, a jet erupts from the Taylor cone when the potential difference between the charged solution and collector overcomes the surface tension of the solution. The erupted liquid jet creates fibers that continue to travel towards the collector, causing the solvent to evaporate and resulting in the collection of dried nanofibers. Depending on the shape of the receiver, membranous or tubular nanofibrous structures can be fabricated, and the fiber alignment can be regulated (aligned to random) depending on the rotation speed of the receiver.
Fig. 3.
Fig. 3.
Schematic of different electrospinning techniques. (A) The traditional electrospinning; (B) Coaxial electrospinning; (C) Emulsion electrospinning; (D) Dynamic water flow electrospinning.
Similar to traditional electrospinning, coaxial electrospinning incorporates a coaxial nozzle, which contains two nozzles of different sizes with one forming a sleeve around the other (Fig. 3(B)). The nozzle with the smaller inner diameter transports the core solution, and the nozzle with the larger inner diameter transports the shell solution. In this way, the shell solution and the core solution, contained in separate reservoirs, are ejected simultaneously through the coaxial nozzle, and the core-shell nanofiber is created through the same mechanism as traditional electrospinning using a potential difference. An advantage of coaxial electrospinning is that only the shell solution must be spinnable, so some non-spinnable drugs, growth factors, and other functional additives can be incorporated into the core solution, and thus the electrospun biomaterial.
Like coaxial electrospinning, emulsion electrospinning creates core-shell structure nanofibers but uses polymer emulsions for the preparation. This process is advantageous because the emulsion can be spun by a single spinning nozzle to obtain the nanofiber with a multi-core structure or core-shell structure without using a coaxial nozzle (Fig. 3(C)). During this process, the emulsion droplets can be drawn out in the nanofibers to create a core-shell structure or can remain as droplets to create a multi-core structure. Again, non-spinnable drugs, growth factors, and other active substances can be dispersed in the polymer solution and electrospun with emulsion electrospinning.
Another type of electrospinning is dynamic water flow electrospinning where nanofibers are first collected on a water surface, twisted by a water vortex, and subsequently collected on a rotating collector, as shown in Fig. 3(D). In this method, an upper and a lower water basin are utilized. The upper water basin contains a small hole at the base from which water can flow by gravity, thereby forming a vortex on the water surface. Then, as the nanofibers are electrospun on the water surface, they flow through the vortex and are wound into yarn. The water and yarn flow from the upper basin to the lower water basin from where water is recycled by a pump to the upper one to continue the process. As the yarn flows to the lower basin, it is collected on a rotating receiver, which generates a porous nanoyarn scaffold. Compared to traditionally electrospun membranes, these materials have rougher surfaces, larger pore sizes, and higher porosities, suggesting that they may be more conducive to the 3D growth of cells when used as tissue engineering scaffolds. [24], [25]
Another approach to creating nanoyarns utilizing a double-spray of electrospun nanofibers to prepare continuous nanoyarns is shown in Fig. 4(a) and (b). One spinning nozzle produces positively charged nanofibers through the application of a positive high voltage, and another spinning nozzle produces negatively charged nanofibers through the application of a negatively charged high voltage. This arrangement results in the winding together of oppositely charged fibers that are collected on a rotating funnel to form a coil (Fig. 4(c) and(d)), resulting in a nanoyarn (Fig. 4(e)) [22].
Fig. 4.
Fig. 4.
Diagram illustrating the double-spray electrospinning [22]. (a) The schematic of double-spray electrospinning. (b) A photograph of the receiving funnel. (c) A photograph of the process of nanoyarn formation and winding. (d) A photograph of yarn coil. (e) Scanning electron microscopy (SEM) images of a single nanoyarn.
3. Electrospun nanofibers as tissue engineering scaffolds
3.1. Ideal scaffold for tissue engineering
Tissue engineering scaffolds are designed to function as a temporary substitute for tissue defects that induce cell ingrowth and neotissue formation while degrading in vivo as the tissue matures. Electrospinning is a technology with tremendous potential for fabricating tissue engineering scaffolds because it fabricates biomimetic structures similar to the scale and morphology of the native ECM. In fact, the ECM of the human body is a nanofiber gel network composed of several structural proteins, such as collagens and elastin, interwoven with non-structural proteins, like glycosaminoglycans. The diameter of the ECM structural fibers is usually 50-300 nm, and the fibers provide anchoring points for cell attachment and maintain overall tissue/organ shape and form [26]. As shown in the SEM of an example tissue after freeze-drying (Fig. 5), the fibroblasts extend their processes in the direction of the collagen fibrils [27]. As the cell receptors bind to ligands on the ECM, outside-in signaling can induce changes in cell behavior, which creates a dynamic crosstalk between the cells and the ECM. Therefore, the tissue engineering scaffold should not only be biomimetic of the ECM structure but also of the signals contained within the ECM. Research has shown that the scale of the fibers is of utmost importance for inducing appropriate outside-in signaling with nanofibrous structures, significantly improving the function of tissue engineering scaffolds for bone, cartilage, cardiovascular, nerve, and bladder regeneration [28], [29].
Fig. 5.
Fig. 5.
SEM of fibroblasts in a native tissue after freeze-drying [27].
Several studies illustrate the regulatory effects of nanomaterials on cell behavior. For example, Pattison et al. [30] used nanoscale poly lactic-co-glycolic acid (PLGA) scaffolds and smooth muscle cells to create a tissue-engineered bladder in vitro and demonstrated that the cells adhered better, were more proliferative, and secreted more collagen and elastin on the nanoscale compared to microscale scaffolds. Similarly, carbon nanofibers have been shown to increase the proliferative capacity of osteoblasts and their secretion of alkaline phosphatase and calcium [31]. These changes in cell behavior are thought to be related to changes in cell morphology [32]. While adhering to a flat morphology on microporous and microfibrous scaffolds (Fig. 6(A, B)), cells exhibit stretching behavior, but when they adhere to the larger surface area of nanofibrous scaffolds the morphology was similar to that observed in native tissues (Fig. 6(C)). The high surface area of nanofiber scaffolds facilitates more significant protein adsorption and provides more adhesion sites for receptors on the cell membranes. As a result, nanofibrous scaffolds that have a similar structure to the ECM may induce more appropriate cell behavior through changes in cell morphology during attachment and migration, providing a microenvironment more conducive to guided tissue regeneration.
Fig. 6.
Fig. 6.
Schematic of how a scaffold structure affects cell adhesion and spreading [32]. (A), (B) Cells adhere in a flat morphology on the microporous and microfiber scaffolds, similar to that observed on flat surfaces. (C) Nanofibers have a greater surface area, and therefore adsorbed more protein, providing more adhesion sites for cell membrane receptors.
3.2. Advantages of electrospun nanofibers
Electrospun nanofibers are advantageous for fabricating tissue engineering scaffolds because the electrospinning process is versatile and tailorable for the specific tissue application. Most biocompatible synthetic and natural polymers can be electrospun into nanofibers independently or as blends of multiple polymers [33], [34]. The orientation of nanofibers can be controlled to guide cell attachment and orientation within the scaffolds, which is beneficial for tissues with an aligned ECM, such as tendons and ligaments [35]. Because of the recent advances in electrospinning technology, bioactive factors and functional drugs can also be encapsulated in the electrospun nanofibers through coaxial or emulsion electrospinning and released over time. Imparting bioactivity, in addition to the structural features of the scaffold, has enabled the creation of multiple tissue engineering scaffolds with tissue-specific functions.
4. Electrospun nanofibers for skin tissue regeneration
Skin is the largest organ of the body and provides an external barrier to protect against pathogen invasion [36]. As such, chronic, non-healing wounds that are often infected or large-scale traumatic injuries, such as those created by burns or automobile accidents, pose a significant threat to health due to the risk of systemic infection. Autologous skin grafts are commonly used to accelerate wound healing and recover the function of the damaged skin; however, they result in donor site morbidity and may not be available depending on the extent of tissue injury [37]. As an alternative, a tissue engineering scaffold could be used to guided healing and provide protection. Additional essential functions of the scaffold are to keep the wound and surrounding area moist, adsorb secretions, have good air permeability, and inhibit bacterial growth. Thus, the features of electrospun scaffolds are appealing for use in skin tissue regeneration applications.
A wide variety of natural and synthetic polymers have been electrospun and investigated for use in skin tissue engineering. Chitosan blended with collagen or silk fibroin has been shown to support cell proliferation and accelerate wound healing compared to control gauze [38]. Similarly, an antimicrobial peptide blended with silk fibroin was used to create a composite electrospun nanofiber matrix that exhibited significant antibacterial activity against gram-positive bacteria and gram-negative bacteria and accelerated wound healing in an in vivo model [39]. Li et al. [40] used electrospinning to generate nanofibers made from tilapia skin, which is primarily composed of type I collagen, and demonstrated that the nanofiber membranes had good biocompatibility and low immunogenicity, suggesting that tilapia skin may be a good source for collagen used in skin tissue engineering. Focusing on creating a hemostatic biomaterial, Ma et al. [41] incorporated ligustrazine into a silk fibroin electrospun scaffold and found that it promoted wound hemostasis, was anti-inflammatory, possessing blood compatibility, and induced healing. To improve the mechanical properties of an electrospun biomaterial, bioactive glass has been incorporated into electrospun fish collagen nanofibers [42]. The composite material had greater tensile strength, antibacterial activity against Staphylococcus aureus, and promoted the adhesion and proliferation of human skin fibroblasts compared to pure, fish collagen control nanofibers. Xie et al. [43] created an electrospun scaffold with five O-quaternary ammonium chitosan, which has broad-spectrum antibacterial activity, and demonstrated that it had good blood compatibility and biocompatibility, inhibited the formation of biofilm, was hemostatic, and promoted wound healing. Lastly, Yu et al. [44] recently developed a four-step process including electrospinning, mechanical cutting/mincing, freeze-drying, and heat treating to fabricate a scaffold with enhanced porosity and adsorption properties (Fig. 7). The scaffold made from polycaprolactone-poly-ethylene glycol-polycaprolactone exhibited a unique, hierarchical porous structure with larger pores and high porosity, which resulted in a 3.3-fold increase in water absorption compared to a two-dimensional (2D) membrane. In addition, the scaffold improved the adhesion, proliferation, and migration of mouse fibroblasts compared to the 2D mats and resulted in a minimal inflammatory response, early re-epithelialization, and formation of granulation tissue in vivo. Together, these examples illustrate the diversity of scaffolds that can be fabricated by electrospinning for skin tissue engineering.
Fig. 7.
Fig. 7.
Schematic of the fabrication of a scaffold for skin tissue engineering by a four-step process and the animal experiment to evaluate wound repair [44]. (1) 2D nanofibrous mats were fabricated via electrospinning. (2) A homogeneous short fiber solution was prepared by high-speed mechanical cutting. (3) The 3D scaffold was obtained after freeze-drying the solution. (4) The 3D scaffold was reinforced through heat treatment.
In the future development of scaffolds for skin tissue engineering, additional bioactive components should be investigated, such as adding temperature or pH-sensitive materials to the dressing. Additives like these could provide biofeedback through color changes for real-time monitoring of wound healing and infection. This feedback could provide additional insight into the wound care plan and improve patient outcomes. Ultimately, the simplicity and versatility of the electrospinning process facilitates the development of these multifunctional, bioactive scaffolds of skin tissue engineering.
5. Electrospun nanofibers for blood vessel tissue regeneration
More than 1.4 million patients in the United States receive a vascular graft every year, which costs around $25 billion US dollars [45]. This extensive market has a largely unmet need for small-diameter (i.e. inner diameter less than 5 mm) vascular grafts that can be used for tissue engineering. Currently available, non-resorbable small-diameter vascular grafts fail by thrombosis and neointimal hyperplasia at the anastomosis due to the reduced velocity of blood flow and lack of endothelization, ultimately resulting in stenosis and occlusion of the lumen [46]. Therefore, many tissue engineers are developing electrospun, resorbable small-diameter vascular grafts to guide blood vessel regeneration and circumvent the problems associated with current, synthetic grafts.
Several groups have explored the use of heparin and vascular endothelial growth factor (VEGF) to reduce thrombosis and improve endothelialization. Huang et al. [47] prepared an electrospun poly (L-lactic acid-co-caprolactone) (P(LLA-CL) tubular graft containing heparin by coaxial electrostatic spinning. When implanted in the femoral artery of a dog, the heparin functioned as an anticoagulant and improved patency. In order to promote the proliferation of endothelial progenitor cells, Chen et al. [48] loaded VEGF into the heparin-containing scaffold by emulsion electrospinning. The controlled release of heparin and VEGF from vascular grafts showed good anticoagulation and promoted endothelial progenitor cell proliferation. These examples illustrate the ability to fabricate electrospun vascular grafts with anticoagulant properties that encourage neointimal regeneration.
To further increase the biocompatibility of vascular grafts, natural polymers have been incorporated into the electrospun scaffolds. In one example, Yin et al. [49] blended collagen and chitosan with P(LLA-CL). Incorporating collagen and chitosan significantly increased endothelial cell proliferation and spreading compared to the P(LLA-CL) control, suggesting an improvement in biocompatibility. Similar work by Wu et al. [50] optimized the blend of collagen and chitosan with P(LLA-CL) and found that the weight ratio of 3:1 was optimal for mechanical integrity, biodegradability, and in vitro cellular compatibility (Fig. 8). When used in a canine femoral artery model, the graft demonstrated excellent structural integrity, higher patency rates, better endothelial and smooth muscle cell growth, and higher expression of angiogenesis-related genes and proteins compared to P(LLA-CL) control grafts without the natural polymers. These examples illustrate that the addition of natural polymers, such as collagen and chitosan, can improve biocompatibility and vascular remodeling for blood vessel tissue engineering.
Fig. 8.
Fig. 8.
Confocal microscopy images of human smooth muscle cells (hSMCs) and L929 murine fibroblasts after culturing for 5 days on the P(LLA-CL), 3: 1 and 1:1 composite scaffolds (A). Cell viability of hSMCs (B) and L929 cells (C) cultured for 1, 3, and 5 days [50].
In another approach, Kuang et al. [51] prepared the inner layer of an electrospun vascular graft by coaxial electrospinning with a core containing heparin and a shell containing salvianolic acid B (SAB) loaded in mesoporous silica nanoparticles (MSN) (Fig. 9). The release of heparin and SAB was sustained for almost 30 days, and synergistically promoted human umbilical vein endothelial cell (HUVEC) growth and blood compatibility. With an emphasis on regulating smooth muscle cell arrangement and infiltration, Wu et al. [52] used dynamic liquid electrospinning to prepare nanoyarns and conjugate electrospinning to prepare conjugated nanoyarns from 75 % P(LLA-CL) and 25 % collagen. The dynamic nanoyarns regulated smooth muscle cell infiltration while the conjugate nanoyarns guided the directional growth of the cells, suggesting that both scaffolds may be advantageous for guiding the regeneration of tunica media.
Fig. 9.
Fig. 9.
Process of fabricating the core (heparin)-shell (PC/SAB-MSN) fiber (left) [51]. Transmission electron microscope images of MSN (A) and core (heparin)-sheath (P(LLA-CL) and collagen with SAB-MSN) fiber (B), SEM of the nanofiber with MSN particle on the surface (C), SEM-EDS image of the fiber membrane (D).
Because controlling the regeneration of the neointima, important for anticoagulation, and the tunica media, important for mechanical integrity, requires independent cues, several groups have developed multi-layered electrospun vascular grafts with each layer serving a different function. Wu et al. [53] designed and fabricated a bi-layered vascular scaff ;old with a dense nanofiber inner layer to avoid transmural blood leakage and a loose nanoyarn outer layer to guide smooth muscle cell ingrowth. Likewise, heparin and CD133 antibodies, to encourage endothelial cell adherence, were incorporated into an inner layer of an electrospun scaffold by coaxial electrospinning, which was then covered by an outer layer of nanoyarns generated by dynamic liquid electrospinning. The resulting bi-layer graft had compliance comparable to that of the human saphenous vein and was improved relative to the commercially available expanded-polytetrafluoroethylene grafts. Moreover, the sustained release of heparin and CD133 over a period of approximately 40 days encouraged the regeneration of an endothelial cell monolayer and infiltration of smooth muscle cells when implanted in the abdominal aorta of a rat for 2 months (Fig. 10). Wu et al. [54] has also developed a three-layer electrospun vascular graft through a three-part electrospinning process using blends of P(LLA-CL), PLGA, collagen, and silk fibroin. The inner layer contains P(LLA-CL) and collagen in axially aligned nanofibers, the middle layer is composed of PLGA and silk fibroin nanoyarns aligned circumferentially, and the outer layer is made from randomly deposited P(LLA-CL) and collagen nanofibers to support the composite structure. The mechanical properties were found to be suitable for use as a vascular graft, and fluorescent staining of endothelial cells and smooth muscle cells illustrated that the inner and middle layers regulated cell morphology in the lumen and tunica media, respectively. Moreover, the in vivo results showed that the three-layered vascular graft supported cell infiltration, scaffold biodegradation, and abundant collagen production after subcutaneous implantation for 10 weeks. Together, these studies suggest that a multi-layered scaffold to mimic the anatomy and physiology of native blood vessels may improve the performance of small-diameter grafts for vascular tissue regeneration.
Fig. 10.
Fig. 10.
Fluorescence micrographs of (A-D) the bilayer vascular scaffold after implantation for 2 months and (E-H) the autologous vessel both after immunohistochemical staining of (A, E) DAPI for nuclei, (B, F) CD31 for endothelial cells, and (C, G) α-SMA for smooth muscle cells. (D, H) The corresponding merged fluorescence micrographs [53].
6. Electrospun nanofibers for nerve tissue regeneration
Peripheral nerve injury can lead to sensory, motor, or autonomic problems in patients. The capacity of the peripheral nerves to regenerate is profound compared to the central nervous system. If the defect is less than 5 mm, the nerves can repair themselves, illustrating that there are endogenous mechanisms to regeneration the nerve and restore function. Unfortunately, when the length of the nerve defect is greater than 5 mm, the self-repair process is limited, and a biomaterial is needed to secure both ends of the injured nerve to guide regeneration. In recent years, electrospinning has been investigated to create neural scaffolds, and the resulting materials have evolved from simple, hollow tubes into more complex structures, known as nerve guidance conduits (NGC) [55]. NGCs can contain internal filaments, sponge fillers, and physical and chemical cues to mimic the structure and bioactivity of nerve bundles (Fig. 11) [56]. The following sections will discuss several types of NGC developed via electrospinning.
Fig. 11.
Fig. 11.
A schematic showing how ideal NGCs are constructed by incorporating a diverse array of physical and biological cues into a neural scaffold with different configurations [56].
6.1. Simple hollow luminal structure NGC
Simple hollow NGC provides a lumen for the regenerating nerve to grow while limiting surrounding tissue ingrowth, and the material selection influences the potential for regeneration. Blends of silk fibroin and P(LLA-CL) have been shown to be biocompatible and enhance cell proliferation compared to P(LLA-CL) controls [57]. Based on these findings, Wang et al. [58] prepared silk fibroin and P(LLA-CL) blended nanofiber scaffolds with aligned fibers and created a NGC by wrapping the electrospun scaffold around a stainless steel rod. The NGC was implanted into a 10- mm sciatic nerve defect in rats to evaluate nerve regeneration. The results demonstrated that the NGC improved regeneration, resulting in a nerve with a more mature structure containing thicker nerve fibers at a higher density, and function when compared to the P(LLA-CL) control. These findings highlight the impact of the polymer selection on the potential for nerve regeneration and suggest that silk fibroin enhances nerve regeneration
6.2. Growth factor incorporated into NGC
Nerve growth factor (NGF) promotes the development, growth, differentiation, and maturation of central and peripheral neurons, plays a role in the maintenance of normal nerve function, and accelerates the repair of nervous system injuries. Thus, the incorporation of NGF into aligned, electrospun fibers by coaxial electrospinning to enhance nerve regeneration has been investigated [59]. In this study, NGF was released slowly from the electrospun scaffold and retained activity over 60 days, which is important for the long-term regenerative outcome. Additionally, the NGF containing scaffold had enhanced performance compared to the control after a 12-week in vivo study. These data indicate that the bioactivity of NGF and the structural guidance from the aligned scaffold work in tandem to more effectively promote nerve regeneration. Similarly, Wang et al. [60] investigated the use of NGF in the core of PLGA shell fibers, which were collected in an aligned orientation, wrapped on a stainless steel rod, and sealed with a nylon filament to form the NGC. After 12 weeks in a 13-mm sciatic nerve defect in rats, the scaffold stimulated nerve regeneration, and electrophysiology and muscle weight tests showed that the functional recovery of the regenerated nerve was significantly improved, relative to the non-growth factor containing control. These studies suggest that NGF plays a critical role in promoting the repair of injured peripheral nerves.
6.3. Conductive nanofiber NGC and electrical stimulation for nerve regeneration
Conductive materials, such as polyaniline and graphene, can be incorporated into electrospun nanofibers to create conductive NGC that transmit electrical signals from the fibers to the neurons to promote neuronal migration, proliferation, and differentiation. In one example, Zhang et al. [61] prepared the composite fiber scaffold containing polyaniline for conduction and NGF by coaxial electrospinning and evaluated the synergistic effect of electrical stimulation and NGF on the potential for regeneration. The authors found that electrical stimulation combined with NGF promoted Schwann cell proliferation and the growth of long axonal extensions from pheochromocytoma cells, which suggests that the combined effects are beneficial for nerve regeneration. In another example, Sun et al. [62] used conductive polypyrrole to coat an electrospun scaffold by in situ oxidative polymerization. The polypyrrole coating not only imparted electrical conductivity, but also increased the hydrophilicity of the scaffold, which together had additive effects and resulted in higher rates of Schwann cell proliferation compared to control scaffolds. Similarly, the polypyrrole coating, in combination with electrical stimulation, was shown to induce pheochromocytoma cell differentiation and enhance axonal growth. These results indicate the promising potential of conductive polypyrrole-coated nanofibrous membranes for peripheral nerve repair and regeneration.
Graphene oxide has also been used as a coating to create conductive, electrospun NGC (Fig. 12) [63]. As shown by in vitro studies, the graphene oxide-coated scaffolds have enhanced the migration, proliferation, and myelin formation of Schwann cells and up-regulate the expression of focal adhesion kinase in pheochromocytoma cells, suggesting the scaffolds promote neurite outgrowth. Furthermore, when implanted in a 10-mm sciatic nerve defect in vivo, the NGC facilitated repair and regeneration, similar to that of autologous transplantation. In an additional study, Wang et al. [64] coated an electrospun scaffold with reduced graphene oxide and found similar results both in vitro and in vivo. Together, these results suggest that the conductive scaffolds positively impact the potential for peripheral nerve regeneration by providing electrical stimulation to the regenerating neurons.
Fig. 12.
Fig. 12.
Schematic of the graphene oxide-coated antheraea pernyi silk fibroin (ApF)/P(LLA-CL) scaffold preparation [63].
6.4. Filaments-containing NGC
To guide the migration and proliferation of nerve cells, NGCs have been fabricated containing filaments that function as cell highways. In one case, Li et al. [65] prepared poly (L-lactic acid) (PLLA) nanoyarns by a dual spinneret system and used them to fill the lumen of a P(LLA-CL) electrospun NGC (Fig. 13). In a series of in vitro experiments, Schwann cells demonstrated increased cell proliferation and enhanced elongation on the nanoyarns compared to PLLA-film, and further, migrate through the yarn structure with axons extended along the axes of the yarn. Similarly, Wu et al. [66] prepared a nanoyarn-filled NGC from electrospun PLGA and included a laminin coating to enhance cell adhesion. In vitro experiments showed that the laminin coating enhanced both Schwann cell proliferation and migration to compared to non-coated controls, demonstrating the importance of biological signals in addition to topographical features for regulating and enhancing peripheral nerve regeneration. In another study, polypyrrole was coated on electrospun polycaprolactone (PCL) nanoyarn filaments to create a conductive, filament-filled NGC (Fig. 14) [67]. Like the other examples, the coating enhanced Schwann cell proliferation, suggesting that conductive filaments may be beneficial for guiding peripheral nerve repair.
Fig. 13.
Fig. 13.
Mechanism of nanofiber yarn fabrication and schematic of incorporating the nanofiber yarn into the NGC [65].
Fig. 14.
Fig. 14.
Schematic of polypyrrole (Ppy)-coated PCL nanoyarn (NY) fabrication for the development of a conductive, filament-filled NGC [67].
6.5. Sponge-containing NGC
Like the filament-filled scaffolds, NGC filled with 3D sponges are more conducive to cell migration and axonal regeneration and encourage the distribution of axons within the lumen of the scaffolds. This has been demonstrated by Sun et al. [68] through the preparation of an electrospun NGC filled with a nanofiber sponge, containing large pores and high porosity (Fig. 15). When implanted into a 10-mm sciatic nerve defect in rats, the sponge of the NGC was infiltrated with Schwann cells and appeared similar to the internal structure of a nerve bundle as determined by histological analysis. Compared to a hollow NGC, the sponge-filled scaffold significantly improved functional nerve repair and performed similarly to an autograft control as indicated by gait analysis and muscle weight. This study suggests that sponge-containing NGCs have significant therapeutic potential for applications in peripheral nerve repair.
Fig. 15.
Fig. 15.
Schematic illustrating the fabrication of the electrospun 3D nanofiber sponges and the fabrication of a NGC by electrospinning [68].
7. Electrospun nanofibers for bone tissue regeneration
Due to the high incidence of bone defects, which are caused by bone infections, bone tumors, and bone loss by trauma, there is a tremendous clinical demand for bone grafts [69]. Autologous bone transplantation is considered the clinical “gold standard” for the repair of critical-sized bone defects due to its remarkable osteoinductivity and osteoconductivity without adverse immunoreactions [70]. However, both autologous bone and allogeneic bone use are limited clinically by their availability. Therefore, using tissue engineering to regenerate bone is a promising method to overcome the disadvantage of insufficient donors [71], [72].
A scaffold for bone tissue engineering should be biocompatible, biodegradable, bioactive, and have sufficient mechanical properties for the bone environment. To satisfy these requirements, nanofiber scaffolds based on composite materials, such as organic and inorganic hybrid nanofibers, and loaded with functional factors, such as bone morphogenetic proteins (BMP), transforming growth factor-β3 (TGF-β3), VEGF, and silver nanoparticles, have become the focus of current research [73]. Using electrospinning to create these scaffolds is advantageous because the nanofibers are biomimetic of the ECM in natural bone, which consists mainly of hierarchically organized, mineralized collagen fibers [74], [75]. For example, Ye et al. [76] created a composite scaffold containing nano-hydroxyapatite, the main mineral component of bone, from electrospun scaffolds that were minced into small fibers, freeze-dried, and thermally crosslinked to create a 3D bone-like scaffold. Synthetic BMP-2-derived peptides were then immobilized on the surface, and the scaffold was evaluated in vitro and in vivo in a rat cranial bone defect model (Fig. 16). The results of the experiments demonstrated that the presence of nano-hydroxyapatite and the BMP-2 increased gene expression related to the osteogenic differentiation of stem cells and that BMP-2 peptide release was maintained for 21 days. Compared to controls, the scaffolds had better osteoinductive activity, promoted the expression of type Ⅰ collagen and osteogenic markers, such as Runt-related transcription factor 2 and osteocalcin, and increased alkaline phosphatase activity, resulting in new bone growth in the center of the defect, which was absent in controls.
Fig. 16.
Fig. 16.
Schematic of 3D nanofibrous scaffold preparation incorporating nano-hydroxyapatite (nHA) and BMP-2 for testing in an in vivo, cranial defect regeneration model [76].
In an attempt to regulate the bone remodeling processes, Wang et al. [77] designed an electrospun scaffold for the dual delivery of MSNs, shown to accelerate bone formation, and alendronate, shown to suppress bone resorption (Fig. 17). This scaffold was found to reduce the bone healing time from 12 weeks to nearly 4 weeks based on bone maturity scores and vascularization, as indicated by CD31 staining. As healing continued to 12 weeks, the bone maturity scores for the scaffold were nearly double the control scaffolds without MSN and alendronate, and the scaffolds were significantly more vascularized. These results indicate that electrospun nanofibers loaded with silicate and alendronate modulate the bone remodeling process and facilitate robust vascularization, which is vital for adequate nutrient supply during bone regeneration.
Fig. 17.
Fig. 17.
Design of an scaffold containing MSN and alendronate (ALN) for the dual delivery of ALN and silicate to modulate bone resorption and formation for accelerating bone repair. The ALN pre-loaded within the MSNs is released from the nanofibers and inhibits the bone-resorption process by preventing GTP-related protein expression. The silicate produced by the hydrolysis of MSNs is released from the nanofibers and promotes the bone-forming process by improving vascularization and bone calcification [77].
To promote cell adhesion, Gutiérrez-Sánchez et al. [78] used electrospinning to prepare poly (lactic acid) (PLA) nanofibers containing starch and adsorbed arginine-glycine-aspartic acid (RGD) peptides to the surface. The scaffolds modified with RGD had better cell adhesion compared to controls, which shows potential for guided bone regeneration. To mimic the mineral composition of bone, hydroxyapatite has been incorporated into electrospun silk fibroin scaffolds through a two-stage process (Fig. 18) [79]. First, hydroxyapatite was included in the electrospinning solution to localize the particles within the fibers. Then, the surface of the fibers was modified with mussel adhesive-inspired polydopamine chemistry and deposited with hydroxyapatite, creating the second layer of particles. This two-stage functionalization was shown to improve the osteogenesis of human adipose-derived mesenchymal stem cells transfected with the transcriptional coactivator with PDZ-binding motif both in vitro and in vivo, demonstrating the potential use of these scaffold with stem cells for enhanced bone formation. Similarly, Natália Hadler Marins et al. [80] prepared a composite scaffold with hydroxyapatite and niobium pentoxide (Nb2O5) particles, which has been used as a bioactive component on metallic implants. These scaffolds were found to be non-cytotixc and promoted cell proliferation and cell adhesion, suggesting a potential use for this novel mineral additive in bone tissue engineering. Ultimately, electrospun biomaterials have attracted more and more attention in bone tissue engineering in recent years, and designing such to support osteoinduction and vascularization should enhance their performance in bone tissue engineering.
Fig. 18.
Fig. 18.
Electrospun silk fibroin nanofibrous scaffolds engineered with two-stage hydroxyapatite (HA) particle functionalization. These scaffolds were found to support the osteogenic differentiation of genetically-modified human adipose-derived mesenchymal stem cells after 21 days in vitro and enhance mineralized bone formation and collagen deposition in vivo in a critical-sized calvarial bone defect after 8 weeks [79].
8. Electrospun nanofibers for cartilage tissue regeneration
Damage to the articular cartilage, through sports-related injuries, trauma, and aging can lead to osteoarthritis and debilitating pain in the joints, eventually necessitating total joint replacements [81]. Although autografts can be used to treat minor damage, the availability of grafts is limited, and their implantation can lead to an adverse immune response. Furthermore, autografts are not efficacious for treating severe cartilage defects. Therefore, using a tissue engineering approach with electrospun scaffolds is a promising strategy for the treatment of minor and severe cartilage defects to reduce the occurrence of osteoarthritis and total joint replacements [82]. Because electrospun nanofiber scaffolds contain densely packed fibers with small pores, a current focus in cartilage tissue engineering is developing methods to process the pseudo-2D electrospun scaffolds into more 3D structures that are suitable for cartilage repair and mimicking of the native ECM.
Recently, Chen et al. [83] developed a thermally-crosslinked scaffold from a freeze-dried structure composed of short fibers derived from a nanofiber membrane (Fig. 19). The scaffold ontained was functionalized with hyaluronic acid, the protein that gives articular cartilage its lubricating and shock-absorbing properties, and evaluated in vitro and in vivo for its ability to stimulate cartilage regeneration. The resultant scaffold possessed high water adsorption and good mechanical and compressive strength. In the in vitro experiments, chondrocytes were found to adhere to the surface of the scaffold and proliferate along the nanofibers. Moreover, when implanted into an articular cartilage defect in vivo for 12 weeks, the scaffold was found to promote improved repair relative to the non-treated defect as well as a scaffold that was not functionalized with hyaluronic acid. These data indicate that the 3D scaffold generated from an electrospun membrane and functionalized with hyaluronic acid may have therapeutic potential.
Fig. 19.
Fig. 19.
Macroscopic images (a, d, and g) of the cartilage joints from the non-treated group, group treated with the non-functionalized scaffold (3DS-1), and the group treated with the hyaluronic acid-functionalized scaffold (3DS-2) at 12 weeks after surgery. Histological analysis of the cartilage defect area from the three groups, stained with Safranin O-fast green (b, e, and h) and H&E (c, f, and i), indicate that the functionalized scaffold enhanced repair. Arrows and dotted lines indicated the defect sites. OC: original cartilage tissue. RC: repaired cartilage tissue [83].
In addition to articular cartilage, regeneration of hyaline cartilage for the treatment of congenital defects, trauma, or disease is being investigated. Because hyaline cartilage has unique morphologies, such as in the ears and nose, combining electrospinning with 3D printing enables the construction of detailed shapes that may be beneficial for hyaline cartilage regeneration. For example, Chen et al. [84] developed an ink for 3D printing composed of electrospun PLGA/gelatin nanofibers from a minced membrane in a hyaluronic acid and polyethylene oxide (PEO) solution (Fig. 20). The hydrophilic scaffold was found to have large, regular pores between the fibers, high porosity, absorbed large volumes of water, and possessed water-induced shape memory (Fig. 21). The rapid recovery of shape, within 30 s, suggests that this 3D printable scaffold could be used to guide the regeneration of complex cartilage structures in vivo.
Fig. 20.
Fig. 20.
Schematic of various electrospun fiber scaffolds [84]. (a) Traditional fiber scaffold-electrospun fiber membrane. (b) 3D fibrous scaffold constructed by dispersed Electrospun, short fibers via freeze-shaping. (c) The synthetic steps of a 3D-printed fiber-based scaffold.
Fig. 21.
Fig. 21.
Water-induced shape memory of 3D printed scaffolds made from an ink containing electrospun nanofibers [84]. (a) Square scaffold. (b) In a wet state, a tubular-shaped scaffold was obtained by folding the opposite angle of the square, and the scaffold was freeze-dried to maintain its deformed shape. (c) Shape recovery process of square scaffold after absorption of water for 12 s. (d) Square scaffold completely recovered its original shape within 20 s of water absorption. (e) Rectangular scaffold. (f) Wet scaffold was folded into a wave shape and freeze-dried to maintain its shape. (g) Shape recovery process of rectangular scaffold after water absorption for 12 s. (h) Rectangular scaffold completely recovered its original shape within 30 s of water absorption.
In another study, Zhang et al. [82] created a bi-layered scaffold with a layer of electrospun PLA nanofibers and a layer of compressed, type I collagen for repairing an osteochondral defect (Fig. 22). Compared to a control scaffold of collagen alone, the bi-layer scaffold was found to promote osteogenic differentiation in vitro and induce rapid subchondral bone formation in vivo in a rabbit model of an osteochondral defect (Fig. 23).
Fig. 22.
Fig. 22.
Fabrication and characterization of collagen control scaffolds and bi-layered scaffolds of electrospun PLA and collagen [82]. The fabrication process of collagen scaffolds (A) and bi-layered scaffolds (B) and their microstructures.
Fig. 23.
Fig. 23.
Macroscopic images of the cartilage joints from three groups at 6 (upper panel) and 12 (lower panel) weeks after surgery [82]. (A, D) Non-treated group, (B, E) collagen control group, and (C, F) bi-layered scaffold group.
Like the ears and nose, the cartilage of the trachea has a unique shape and is in close proximity to additional tissues, such as muscle, mucous membranes, and other connective tissues. When formed into a tubular structure, an electrospun tissue engineering scaffold could be used to guide tracheal cartilage regeneration. For example, one group created a scaffold by coaxial electrospinning with a P(LLA-CL) and collagen shell and kartogenin solution as the core fluid [85]. Kartogenin, which induces chondrogenesis in mesenchymal stem cells, was released over a period of two months from the nanofibers and shown to induce the proliferation and chondrogenic differentiation of rabbit bone-marrow derived mesenchymal stem cells by morphological analysis and PCR. These results suggest that the core-shell nanofibrous scaffold could be an effective delivery system for kartogenin and could be used as a tissue engineered scaffold for tracheal cartilage regeneration. Using the same shell, Wang et al. [86] substituted bovine serum albumin plus recombinant TGF-β3 into the core to create another core-shell nanofiber scaffold. The TGF-β3 was released over a two-month period and was shown to be bioactive as determined by type II collagen and glycosaminoglycan production by chondrocytes. Additionally, the proliferation, morphological analyses, and differentiation of mesenchymal stems cells derived from Wharton's jelly and seeded on the scaffolds indicated that the scaffold was biocompatible and promoted chondrogenic differentiation. These data suggest that the TGF-β3 releasing scaffold in combination with mesenchymal stem cells could be used in the construction of tissue-engineered tracheal cartilage.
9. Electrospun nanofibers for tendon/ligament tissue regeneration
9.1. Electrospun nanofibers for tendon tissue regeneration
Tendon and ligament injuries include inflammation, tears, and ruptures that result in severe pain and account for roughly 50 million related surgeries annually around the world [87], [88]. Currently, standard surgical treatments use autografts, allografts, or artificial prostheses. Although considered the “gold standard” because of their good remodeling and lack of immune response, autografts are limited by donor site availability and lead to extended operating times and donor site morbidity [89]. Alternatively, allografts can be used, but allogenic materials are associated with a risk of disease transmission, immune rejection, and high re-rupture rates due to mismatches of gender, age, and body weight between donors and recipients. To deal with these shortcomings, tissue engineering scaffolds based on electrospun fibers provide a possible alternative for the treatment and regeneration of damaged tendons and ligaments.
The major challenge in tendon tissue engineering is the low self-regenerative capacity associated with the hierarchically organized, dense collagen ECM. Uniaxially aligned nanofibers can be used to mimic the organization of collagen fibers in tendons and can be incorporated with biochemical features to stimulate tissue regeneration. For example, Yang et al. [90] developed a novel, multilayer composite scaffold composed of fibrous PCL and methacrylated gelatin, interspersed by dual-electrospinning, and incorporated human-adipose derived stem cells (Fig. 24(A)). The scaffold was composed of five sheets that were crosslinked together and reinforced with a methacrylated gelatin layer carrying the stem cells (Fig. 24(B)). The stem cells were treated with TGF-β3 for 7 days to promote differentiation into tenocytes, and real-time PCR showed pronounced up-regulation of tendon markers scleraxis and tenascin-C, indicating that the encapsulated cells remained responsive to soluble tenogenic factors, and that the constructs were porous enough for the diffusion of exogenous biochemical cues. This novel cell-scaffold construct combines the mechanical advantages of PCL nanofibrous scaffolds and gelatin to mimic the mechanical features and structure of tendons while promoting the native tendon cell phenotype. Likewise, Rinoldi et al. [91] fabricated an electrospun nanocomposite system for tendon tissue engineering. The authors created a bead-on-string fibrous structure and incorporated silica particles to improve the biological activity of the constructs and modify their topography, wettability, stiffness, and degradation rate. The results from their studies also indicate that the bead-on-string fibrous nonwoven composite scaffold is an attractive candidate that may be suitable for guided tendon regeneration.
Fig. 24.
Fig. 24.
(A) Composite scaffold preparation. Dual electrospinning was employed to fabricate a scaffold containing PCL and methacrylated gelatin (mGLT) fibers (Insert 1). The dry scaffold was wetted with an aqueous photo-initiator solution (Insert 2) and then photo-crosslinked by visible light to retain the gelatin (Insert 3). (B) Scaffold sheets were wetted, stacked, and exposed to visible light for crosslink formation between adjacent scaffold layers to create a complex multi-layered structure [90].
Several groups have been working on improving the three-dimensionality of electrospun scaffolds for tendon and ligament engineering by creating new processing methods for enhanced porosity. Laranjeira et al. [92] proposed the production of continuous aligned nanofiber threads from blends of PCL, chitosan, and cellulose nanocrystals and explored their assembly into 3D scaffolds using different textile techniques (Fig. 25). The authors demonstrated that the dimensions of the scaffold could be tailored through varying the braiding technique and manipulated to produce structures that matched the specific sizes of tendon or ligament defects. In addition, the scaffolds were found to have stress-strain curves that mimicked the characteristic nonlinear deformation behavior of tendons and ligaments without the typical plastic deformation observed in tendons and ligaments at high strain. Also, the scaffolds were shown to up-regulate the deposition of ECM from human adipose-derived stem cells in vitro and promote the differentiation towards a tenogenic-like phenotype. Given the morphological similarities, excellent mechanical properties, and the ability to promote differentiation, the 3D woven scaffolds have potential for tendon and ligament tissue engineering.
Fig. 25.
Fig. 25.
Schematic representation of electrospinning setup and hierarchical assembling of continuous aligned nanofiber threads (CANT). (A) Continuous electrospinning system to produce CANT; (B) CANT as the elementary unit of the 3D assembly, mimicking the collagen fibers in native tendon; (C) Yarns composed of twisted CANT represent tendon fascicles: i) Yarn 6, ii) Yarn 9, and iii) Yarn 12; (D) Braided 3D scaffold produced using Yarns; (E) Weaving process using Yarns: (i) arrays of 1 mm pins, (ii) Yarns weaving, and (iii) final 3D Woven scaffold. Both textile scaffolds represent the tendon unit [92].
In another study, Zhang et al. [93] created an electrospun scaffold with aligned PLLA fibers and trichostatin A, a histone deacetylase inhibitor, and evaluated the role of the inhibitor in tenocyte differentiation. Trichostatin A was shown to significantly up-regulate the expression of tendon markers compared to controls without the signaling molecule or scaffolds composed of random fibers, suggesting that the topographical cues of the aligned fibers in combination with trichostatin A could be used to promote teno-lineage differentiation and tendon defect repair.
In a recent study, researchers used electrospinning to build hierarchical, multiscale assemblies to mimic the hierarchical structure of tendons and ligaments [94]. To fabricate the scaffold, several fascicle-inspired PLLA bundles, composed of nanofibers, were grouped together by an electrospun PLLA sheath to mimic the epitenon ligament membrane. The hierarchical scaffold had a similar stiffness to a natural tendon and exhibited similar deformation to tendons and ligaments. These data indicate that the multiscale features of the electrospun scaffold were beneficial in creating the mechanical properties needed for a tendon or ligament scaffold.
9.2. Electrospun nanofibers for tendon-to-bone interface tissue regeneration
Engineering the tendon-to-bone interface (enthesis) is exceptionally challenging because it is a complex gradient of multiple tissues with unique compositions and cells that further requires the mechanical strength to avoid repair site elongation or rupture. Current anchor sutures that are used for tendon-to-bone repairs create stress concentrations that limit attachment strength, suggesting the need for a tissue-engineered solution [95]. Madhurakkat et al. [96] created an electrospun scaffold for the tendon-to-bone interface and immobilized platelet-derived growth factor-BB (PDGF-BB) on its aligned fibers in a gradient to promote the tenogenic differentiation of adipose-derived stem cells (Fig. 26). Their results suggest that the PDGF-BB gradient on the aligned nanofibers acted synergistically with topographical cues to spatially control cell differentiation, leading to high cytoskeleton elongation and anisotropic organization resembling the tendon-bone insertion site. Moreover, the scaffold increased the expression of tenogenic markers, such as type I and III collagen, tenascin-C, and scleraxis over a 14-day period. These data suggest the PDGF-BB gradient on aligned nanofibers may be useful for engineering the bone-tendon interface.
Fig. 26.
Fig. 26.
Schematic showing the structure of a native bone-tendon insertion site and fabrication of an aligned gradient platform with immobilized PDGF-BB that could mimic the bone-tendon insertion site [96].
In another approach, Li et al. [97] developed a double-layer scaffold with a PLLA layer and PLLA layer loaded with nanohydroxyapatite, mimicking the non-mineralized fibrocartilage and mineralized fibrocartilage at the enthesis (Fig. 27). In an in vivo study, the scaffold was found to significantly increase glycosaminoglycan staining at the tendon-bone interface and improve collagen organization when compared to a single layer of an electrospun PLLA control. These data indicate that the double-layer scaffold provides spatial control for the repair of mineralized and non-mineralized tissue at the tendon-bone interface, which may be beneficial for tissue engineering.
Fig. 27.
Fig. 27.
Schematic illustration of the application of the double-layer membrane. (a and b) The nanofibrous membrane is placed at the site of tendon-to-bone insertion. (c) The structure and composition of the scaffold mimic the normal fibrocartilage enthesis. (d) Illustration of hydroxyapatite (HA) growth on PLLA fibers [97].
10. Commercialization
Electrospinning nanofibers for biomedical applications is not just research and publications, it has real current and potential commercialization. For example, Guangzhou Medprin Regenerative Medical Technologies Co., Ltd developed a PLA nanofiber Dura product to regenerate the human dura after. njury, The Dura product has received European CE mark clearance and China Food and Drug Administration (CFDA) certificate many years before. Shanghai Songli Biotechnology Co. Ltd developed a fibrinogen/PLCL nanofiber Hernia Patch product for hernia treatment, and received the CFDA certificate in 2018. Shangdong Hanfang Pharmaceutical Co. Ltd developed a silk fibroin/PLCL nanofiber membrane for use as a wound dressing and also received the CFDA certificate in 2019. While there have been some commercial successes, there are still many biomedical companies on the way to develop nanofiber scaffolds for other biomedical application, thus in time we will see more commercialized, nanofiber medical products in the near future.
11. Conclusion
Electrospinning provides a simple and highly tailorable platform for the fabrication of ECM-mimicking tissue engineering scaffolds. Over the last several decades, progress in the field has resulted in new variations of the electrospinning process, including coaxial, emulsion, and dynamic liquid electrospinning, enabling the inclusion of bioactive agents and signaling molecules. Moreover, electrospinning can be combined with other processes, such as freeze-drying and 3D printing, to create nanofibrous scaffolds with complex 3D features. A wide variety of materials, including natural polymers, synthetic polymers, and their blends, have been used to fabricate nanofibrous, electrospun scaffolds and investigated for use in skin, bone, cartilage, nerve, blood vessel and tendon tissue engineering. Such studies have led to the commercialization of several electrospun products for treating a variety of clinical needs. Ultimately, the further development of electrospinning and electrospun nanofibrous scaffolds holds great promise for a variety of tissue engineering applications and may improve the quality of life for patients around the world.
Acknowledgements
This work was financially surpported by the Fundamental Research Funds for the Central Universities (No. 2232019A3-07), the National Key Research Program of China (Nos. 2016YFA0201702 of 2016YFA0201700), the National Nature Science Foundation of China (No. 31771023), the Science and Technology Commission of Shanghai Municipality (No. 19441902600), the Fundamental Research Funds for the Central Universities and Graduate Student Innovation Fund of Donghua University (No. CUSF-DH-D-2020061). The authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this work through Researchers Supporting Project number (RSP-2020/30).
Reference
DOI
URL
PMID
[Cited within: 2]

Electrospinning is a versatile and viable technique for generating ultrathin fibers. Remarkable progress has been made with regard to the development of electrospinning methods and engineering of electrospun nanofibers to suit or enable various applications. We aim to provide a comprehensive overview of electrospinning, including the principle, methods, materials, and applications. We begin with a brief introduction to the early history of electrospinning, followed by discussion of its principle and typical apparatus. We then discuss its renaissance over the past two decades as a powerful technology for the production of nanofibers with diversified compositions, structures, and properties. Afterward, we discuss the applications of electrospun nanofibers, including their use as
DOI
URL
PMID
[Cited within: 1]

Nerve tissue engineering (NTE) is one of the most promising methods to restore central nerve systems in human health care. Three-dimensional distribution and growth of cells within the porous scaffold are of clinical significance for NTE. In this study, an attempt was made to develop porous polymeric nano-fibrous scaffold using a biodegradable poly(L-lactic acid) (PLLA) for in vitro culture of nerve stem cells (NSCs). The processing of PLLA scaffold has been carried out by liquid-liquid phase separation method. The physico-chemical properties of the scaffold were fully characterized by using differential scanning calorimetry and scanning electron microscopy. These results confirmed that the prepared scaffold is highly porous and fibrous with diameters down to nanometer scale. As our nano-structured PLLA scaffold mimics natural extracellular matrix, we have intended this biodegradable scaffold as cell carrier in NTE. The in vitro performance of NSCs seeded on nano-fibrous scaffold is addressed in this study. The cell cultural tests showed that the NSCs could differentiate on the nano-structured scaffold and the scaffold acted as a positive cue to support neurite outgrowth. These results suggested that the nano-structured porous PLLA scaffold is a potential cell carrier in NTE.
DOI
URL
PMID
[Cited within: 1]

Highly periodic, geometrically directed, anisotropic Se-Pb films have been synthesized at room temperature from an isotropic aqueous solution without the use of physical templates by photoelectrodeposition using a series of discrete input illumination polarizations and wavelengths from an unstructured, uncorrelated, incoherent light source. Dark growth did not generate deposits with substantial long-range order, but growth using unpolarized illumination resulted in an ordered, nanoscale, mesh-type morphology. Linearly polarized illumination generated Se-Pb deposits that displayed an ordered, highly anisotropic lamellar pattern wherein the long axes of the lamellae were aligned parallel to the light polarization vector. The pitch of the lamellar features was proportional to the input light wavelength, as confirmed by Fourier analysis. Full-wave electromagnetic and Monte Carlo growth simulations that incorporated only the fundamental light-matter interactions during growth successfully reproduced the experimentally observed morphologies and quantitatively matched the pattern periodicities. Electrochemical postprocessing of the as-deposited Se-Pb structures resulted in the generation of stoichiometric, crystalline PbSe while preserving the nanopatterned morphology, thus broadening the genus of materials that can be prepared with controlled three-dimensional morphologies through maskless photoelectrodeposition.
DOI
URL
PMID
[Cited within: 1]

With the emergence of nanotechnology, researchers become more interested in studying the unique properties of nanoscale materials. Electrospinning, an electrostatic fiber fabrication technique has evinced more interest and attention in recent years due to its versatility and potential for applications in diverse fields. The notable applications include in tissue engineering, biosensors, filtration, wound dressings, drug delivery, and enzyme immobilization. The nanoscale fibers are generated by the application of strong electric field on polymer solution or melt. The non-wovens nanofibrous mats produced by this technique mimics extracellular matrix components much closely as compared to the conventional techniques. The sub-micron range spun fibers produced by this process, offer various advantages like high surface area to volume ratio, tunable porosity and the ability to manipulate nanofiber composition in order to get desired properties and function. Over the years, more than 200 polymers have been electrospun for various applications and the number is still increasing gradually with time. With these in perspectives, we aim to present in this review, an overview of the electrospinning technique with its promising advantages and potential applications. We have discussed the electrospinning theory, spinnable polymers, parameters (solution and processing), which significantly affect the fiber morphology, solvent properties and melt electrospinning (alternative to solution electrospinning). Finally, we have focused on varied applications of electrospun fibers in different fields and concluded with the future prospects of this efficient technology.
DOI
URL
PMID
[Cited within: 1]

Due to their size and tailorable physicochemical properties, nanomaterials are an emerging class of structures utilized in biomedical applications. There are now many prominent examples of nanomaterials being used to improve human health, in areas ranging from imaging and diagnostics to therapeutics and regenerative medicine. An overview of these examples reveals several common areas of synergy and future challenges. This Nano Focus discusses the current status and future potential of promising nanomaterials and their translation from the laboratory to the clinic, by highlighting a handful of successful examples.
DOI
URL
PMID
[Cited within: 1]

UNLABELLED: The evolution of synthetic RNAi faces the paradox of interfering with the human biological environment. Due to the fact that all cell physiological processes can be target candidates, silencing a precise biological pathway could be challenging if target selectivity is not properly addressed. Molecular biology has provided scientific tools to suppress some of the most critical issues in gene therapy, while setting the standards for siRNA clinical application. However, the protein down-regulation through the mRNA silencing is intimately related to the sequence-specific siRNA ability to interact accurately with the potential target. Moreover, its in vivo biological fate is highly dependent on the successful design of a vehicle able to overcome both extracellular and intracellular barriers. Anticipating a great deal of innovation, crucial to meet the challenges involved in the RNAi therapeutics, the present review intends to build up a synopsis on the delivery strategies currently developed. FROM THE CLINICAL EDITOR: This review discusses recent progress and pertinent limiting factors related to the use of siRNA-s as efficient protein-specific
DOI
URL
PMID
[Cited within: 1]

Biomaterials have played an increasingly prominent role in the success of biomedical devices and in the development of tissue engineering, which seeks to unlock the regenerative potential innate to human tissues/organs in a state of deterioration and to restore or reestablish normal bodily function. Advances in our understanding of regenerative biomaterials and their roles in new tissue formation can potentially open a new frontier in the fast-growing field of regenerative medicine. Taking inspiration from the role and multi-component construction of native extracellular matrices (ECMs) for cell accommodation, the synthetic biomaterials produced today routinely incorporate biologically active components to define an artificial in vivo milieu with complex and dynamic interactions that foster and regulate stem cells, similar to the events occurring in a natural cellular microenvironment. The range and degree of biomaterial sophistication have also dramatically increased as more knowledge has accumulated through materials science, matrix biology and tissue engineering. However, achieving clinical translation and commercial success requires regenerative biomaterials to be not only efficacious and safe but also cost-effective and convenient for use and production. Utilizing biomaterials of human origin as building blocks for therapeutic purposes has provided a facilitated approach that closely mimics the critical aspects of natural tissue with regard to its physical and chemical properties for the orchestration of wound healing and tissue regeneration. In addition to directly using tissue transfers and transplants for repair, new applications of human-derived biomaterials are now focusing on the use of naturally occurring biomacromolecules, decellularized ECM scaffolds and autologous preparations rich in growth factors/non-expanded stem cells to either target acceleration/magnification of the body's own repair capacity or use nature's paradigms to create new tissues for restoration. In particular, there is increasing interest in separating ECMs into simplified functional domains and/or biopolymeric assemblies so that these components/constituents can be discretely exploited and manipulated for the production of bioscaffolds and new biomimetic biomaterials. Here, following an overview of tissue auto-/allo-transplantation, we discuss the recent trends and advances as well as the challenges and future directions in the evolution and application of human-derived biomaterials for reconstructive surgery and tissue engineering. In particular, we focus on an exploration of the structural, mechanical, biochemical and biological information present in native human tissue for bioengineering applications and to provide inspiration for the design of future biomaterials.
DOI
URL
PMID
[Cited within: 1]

The rising incidence of bone disorders has resulted in the need for more effective therapies to meet this demand, exacerbated by an increasing ageing population. Bone tissue engineering is seen as a means of developing alternatives to conventional bone grafts for repairing or reconstructing bone defects by combining biomaterials, cells and signalling factors. However, skeletal tissue engineering has not yet achieved full translation into clinical practice as a consequence of several challenges. The use of additive manufacturing techniques for bone biofabrication is seen as a potential solution, with its inherent capability for reproducibility, accuracy and customisation of scaffolds as well as cell and signalling factor delivery. This review highlights the current research in bone biofabrication, the necessary factors for successful bone biofabrication, in addition to the current limitations affecting biofabrication, some of which are a consequence of the limitations of the additive manufacturing technology itself.
DOI
URL
PMID
[Cited within: 1]

Three-dimensional nanofibrous aerogels (NFAs) that are both highly compressible and resilient would have broad technological implications for areas ranging from electrical devices and bioengineering to damping materials; however, creating such NFAs has proven extremely challenging. Here we report a novel strategy to create fibrous, isotropically bonded elastic reconstructed (FIBER) NFAs with a hierarchical cellular structure and superelasticity by combining electrospun nanofibres and the fibrous freeze-shaping technique. Our approach causes the intrinsically lamellar deposited electrospun nanofibres to assemble into elastic bulk aerogels with tunable densities and desirable shapes on a large scale. The resulting FIBER NFAs exhibit densities of >0.12 mg cm(-3), rapid recovery from deformation, efficient energy absorption and multifunctionality in terms of the combination of thermal insulation, sound absorption, emulsion separation and elasticity-responsive electric conduction. The successful synthesis of such fascinating materials may provide new insights into the design and development of multifunctional NFAs for various applications.
DOI
URL
PMID
[Cited within: 1]

A novel electrospun nanoyarn scaffold, aimed to improve cell infiltration and vascularization, as well as guide cell behaviors by its biomimetic structure, was fabricated for tissue engineering. Electrospun nanofibers were deposited and twisted into yarns in a water vortex before collecting on a rotating mandrel to form a nanoyarn scaffold. Field emission-scanning electronic microscope (FE-SEM) images revealed that the scaffold, composed of aligned nanoyarns (24 micro m) which were composed of a bundle of nanofibers, created a porous structure which may be conducive to cellular infiltration. Thus, we hypothesized that the biomimetic nanoyarn will have a positive influence on cell proliferation and morphology. Pig iliac endothelial cells (PIECs) and MC3T3-E1 pre-osteoblastic cells cultured on the nanoyarn scaffolds showed significantly higher proliferation rates than that on traditional electrospun nanofiber scaffolds. Histological analysis demonstrated that cells infiltrate throughout the nanoyarn scaffolds over a 10-day period, however, no cell infiltration was observed on the nanofiber scaffolds. Moreover, confocal microscopy images indicated that both PIECs and MC3T3-E1 pre-osteoblastic cells cultured on the nanoyarn scaffolds exhibit an extremely elongated morphology compared to the flattened morphology when cells were cultured on electrospun nanofiber scaffolds or tissue culture plates. Furthermore, complex capillary-like structures were observed when PIECs cultured on the nanoyarn scaffold for 7 days, indicating that the nanoyarns provide templates and topographical cues for the assembly of PIECs and the promotion of a capillary network in vitro. In conclusion, the positive cellular interactions on the nanoyarn scaffold demonstrate potential application for use in tissue engineering.
DOI
URL
PMID
[Cited within: 1]

We have developed a novel spray gelation-based method to synthesize a new series of magnetically responsive hydrogel nanoparticles for biomedical and drug delivery applications. The method is based on the production of hydrogel nanoparticles from sprayed polymeric microdroplets obtained by an air-jet nebulization process that is immediately followed by gelation in a crosslinking fluid. Oligoguluronate (G-blocks) was prepared through the partial acid hydrolysis of sodium alginate. PEG-grafted chitosan was also synthesized and characterized (FTIR, EA, and DSC). Then, magnetically responsive hydrogel nanoparticles based on alginate and alginate/G-blocks were synthesized via aerosolization followed by either ionotropic gelation or both ionotropic and polyelectrolyte complexation using CaCl(2) or PEG-g-chitosan/CaCl(2) as crosslinking agents, respectively. Particle size and dynamic swelling were determined using dynamic light scattering (DLS) and microscopy. Surface morphology of the nanoparticles was examined using SEM. The distribution of magnetic cores within the hydrogels nanoparticles was also examined using TEM. In addition, the iron and calcium contents of the particles were estimated using EDS. Spherical magnetic hydrogel nanoparticles with average particle size of 811 +/- 162 to 941 +/- 2 nm were obtained. This study showed that the developed method is promising for the manufacture of hydrogel nanoparticles, and it represents a relatively simple and potential low-cost system.
DOI
URL
PMID
[Cited within: 1]

In total, approximately 400 million people worldwide suffer from urinary bladder cancer (Nat Biotechnol 17 (1999) 149). When radical cysectomy is required as treatment, a replacement material is clearly necessitated. For this purpose, three-dimensional poly(lactic-co-glycolic acid) (PLGA) scaffolds were constructed using solvent casting and salt leaching processes. These scaffolds were manipulated to possess nano-dimensional surface features by soaking in sodium hydroxide at select concentrations and for various periods of time. Human bladder smooth muscle cells were then seeded onto these nano-dimensional scaffolds; adhesion and longer-term cell growth experiments were performed for either 4 h, or 1, 3, and 5 days, respectively. Additionally, collagen and elastin production was quantified following each experiment. In all cases, control cells were placed in an incubator and subjected to normal atmospheric pressure, while experimental cells were placed in a pressure chamber and subjected to a sustained pressure of 10 cm H(2)O. Results of this study provided evidence that porous, nano-dimensional polymeric scaffolds enhanced cell adhesion and growth, while also promoting increased elastin and collagen production. Moreover, in general, exposure to pressure did not alter cellular adhesion, growth, or extracellular matrix protein production, which suggests that the scaffolds and their resident cells will fair well in the complex mechanical environment of the bladder wall. In combination, these results provide evidence that the nano-dimensional PLGA scaffolds created in this research are promising as the next generation of bladder wall replacement materials.
DOI
URL
PMID
[Cited within: 1]

The present in vitro study investigated select functions (specifically, proliferation, synthesis of intracellular proteins, alkaline phosphatase activity, and deposition of calcium-containing mineral) of osteoblasts (the bone-forming cells) cultured on carbon fibers with nanometer dimensions. Carbon fiber compacts were synthesized to possess either nanophase (i.e., dimensions 100 nm or less) or conventional (i.e., dimensions larger than 100 nm) fiber diameters. Osteoblast proliferation increased with decreasing carbon fiber diameters after 3 and 7 days of culture. Moreover, compared to larger-diameter carbon fibers, osteoblasts synthesized more alkaline phosphatase and deposited more extracellular calcium on nanometer-diameter carbon fibers after 7, 14, and 21 days of culture. The results of the present study provided the first evidence of enhanced long-term (in the order of days to weeks) functions of osteoblasts cultured on nanometer-diameter carbon fibers; in this manner, carbon nanofibers clearly represent a unique and promising class of orthopedic/dental implant formulations with improved osseointegrative properties.
DOI
URL
PMID
[Cited within: 2]

Cells are inherently sensitive to local mesoscale, microscale, and nanoscale patterns of chemistry and topography. We review current approaches to control cell behavior through the nanoscale engineering of materials surfaces. Far-reaching implications are emerging for applications including medical implants, cell supports, and materials that can be used as instructive three-dimensional environments for tissue regeneration.
DOI
URL
PMID
[Cited within: 1]

Microporous, non-woven poly( epsilon -caprolactone) (PCL) scaffolds were made by electrostatic fiber spinning. In this process, polymer fibers with diameters down to the nanometer range, or nanofibers, are formed by subjecting a fluid jet to a high electric field. Mesenchymal stem cells (MSCs) derived from the bone marrow of neonatal rats were cultured, expanded and seeded on electrospun PCL scaffolds. The cell-polymer constructs were cultured with osteogenic supplements under dynamic culture conditions for up to 4 weeks. The cell-polymer constructs maintained the size and shape of the original scaffolds. Scanning electron microscopy (SEM), histological and immunohistochemical examinations were performed. Penetration of cells and abundant extracellular matrix were observed in the cell-polymer constructs after 1 week. SEM showed that the surfaces of the cell-polymer constructs were covered with cell multilayers at 4 weeks. In addition, mineralization and type I collagen were observed at 4 weeks. This suggests that electrospun PCL is a potential candidate scaffold for bone tissue engineering.
DOI
URL
PMID
[Cited within: 1]

A unique biodegradable nanofibrous structure, aligned poly(L-lactid-co-epsilon-caprolactone) [P(LLA-CL)] (75:25) copolymer nanofibrous scaffold was produced by electrospinning. The diameter of the generated fibers was around 500 nm with an aligned topography which mimics the circumferential orientation of cells and fibrils found in the medial layer of a native artery. A favorable interaction between this scaffold with human coronary artery smooth muscle cells (SMCs) was demonstrated via MTS assay, phase contrast light microscopy, scanning electron microscopy, immunohistology assay and laser scanning confocal microscopy separately. Tissue culture polystyrene and plane solvent-cast P(LLA-CL) film were used as controls. The results showed that, the SMCs attached and migrated along the axis of the aligned nanofibers and expressed a spindle-like contractile phenotype; the distribution and organization of smooth muscle cytoskeleton proteins inside SMCs were parallel to the direction of the nanofibers; the adhesion and proliferation rate of SMCs on the aligned nanofibrous scaffold was significantly improved than on the plane polymer films. The above results strongly suggest that this synthetic aligned matrix combines with the advantages of synthetic biodegradable polymers, nanometer-scale dimension mimicking the natural ECM and a defined architecture replicating the in vivo-like vascular structure, may represent an ideal tissue engineering scaffold, especially for blood vessel engineering.
DOI
URL
PMID
[Cited within: 1]

Basic fibroblast growth factor (bFGF) can stimulate wound healing. However, consistent delivery of bFGF has many disadvantages. To decrease their instability and diffusible, we introduced chitin-binding domain (ChtBD) into bFGF. Two expression plasimids were constructed. The first one (named bFGF) contained bFGF (154 amino acids), the second (named ChtBD-bFGF) contained bFGF and the ChtBD (54 amino acids). ChtBD was derived from chitinase A1 (ChiA1) of Bacillus circulans WL-12. The recombinant protein ChtBD-bFGF had the same biological activity as bFGF in promoting fibroblast proliferation. Chitin powder was dissolved in 11wt% NaOH and 4wt% urea aqueous solution via the freezing/thawing method. A chitin solution was spread on a glass plate and coagulated with anhydrous alcohol. The chitin binding ability of ChtBD-bFGF was 11.4-fold higher (up to 286mug/cm(2)) than bFGF in vitro. The immunofluorescence data indicated that the ChtBD-bFGF@chitin film promoted cell adhesion and proliferation. The ChtBD-bFGF@chitin film and bFGF@chitin films were implanted subcutaneously. Histological analysis showed that ChtBD-bFGF promoted vascularization at the implanted site more effectively than bFGF. These results suggest that the ChtBD-bFGF@chitin film is a stabile delivery vehicle for accelerating wound healing.
DOI
URL
PMID
[Cited within: 1]

Agar, a highly hydrophilic polymer, has a special gel property and favorable biocompatibility, but moderate intension strength in an aqueous condition and a low degradation rate. In order to tailor both properties of mechanical intension and degradation, type I collagen was composited with agar in a certain ratio by drying at 50 degrees C or by a freeze-dry process. Glutaraldehyde was chosen as a crosslinking agent, and the most favorable condition for crosslinking was that the weight ratio of agar to glutaraldehyde was 66.7 and the pH value about 5. Dynamic mechanical analysis results showed that the single agar membrane had a modulus value between 640 MPa and 1064 MPa, but it was between 340 MPa and 819 MPa after being composited with type I collagen. It was discovered under an optical microscope that the pores were interconnected in the composite scaffolds instead of the honeycomb-like pores in a single type I collagen scaffold or the laminated gaps in a single agar scaffold. The results of an acute toxicity test disclosed that the composites were not toxic to mice although the composites were crosslinked with a certain concentration of glutaraldehyde. The results of gross examinations showed that when the composite membranes or scaffolds were applied to a repair rabbit skin lesion, the composites had a good repair effect without infection, liquid exudation or visible scar in the lesion covered with them. But in the control group, the autologous skin showed necrosis and there were a lot of scar tissues in the lesion site. H&E staining results showed that the repair tissue was similar to the normal one and very few scaffolds or membranes were left without degradation after 2 or 3 weeks. In conclusion, it is proved that type I collagen increases the toughness of the agar membrane, and the agar/type I collagen composites are promising biomaterials as wound dressings for healing burns or ulcers.
DOI
URL
PMID
[Cited within: 1]

Although different kinds of antibacterial formulas have been explored against bacterial infections, the development of both biocompatible and efficiently antibacterial matrices is still a challenge. In this study, we report a novel HPRP-A2 antimicrobial peptide/silk fibroin (SF) composite nanofibrous matrix fabricated by an all-aqueous electrospinning process (HPRP-A2 is an antibacterial peptide originated from Helicobacter pylori). The HPRP-A2/SF composite nanofibers had a round and smooth morphology. The incorporation of HPRP-A2 had little influence on both the morphology and biocompatibility of the SF nanofibers. Interestingly, the composite nanofibrous matrices showed an impressive antimicrobial activity against both Gram-positive and Gram-negative bacteria. Furthermore, the HPRP-A2/SF composite nanofibers showed excellent performance on accelerating healing of wound according to the data of animal experiment. Considering the facile and all-aqueous process, the HPRP-A2/SF composite nanofibrous matrices could be a promising candidate for antibacterial or wound management applications.
DOI
URL
PMID
[Cited within: 1]

Type I collagen, used as a raw material, plays a pivotal role in the development of medical devices and tissue engineering. Due to the risk of zoonotic transmission and religious constraints for mammalian collagen, fish collagen gains increased attention and is widely seen as an alternative. In this study, two collagen micro/nanofiber materials, self-assembled collagen nanofiber and electrospun collagen nanofiber, were prepared by tilapia skin collagen and their biocompatibility and immunogenicity was thoroughly investigated. The result revealed that the state of tilapia skin collagen in self-assembled collagen nanofiber and electrospun collagen nanofiber was different. The circular dichroism spectrum indicated that collagen in self-assembled collagen nanofiber retained the triple helical structure of the native collagen, while collagen in electrospun collagen nanofiber was denatured into gelatin. Nevertheless, the evaluation according to ISO10993, including tests of cytotoxicity, hemolysis, skin sensitization, acute systemic toxicity, mouse immunization and lymphocyte proliferation, demonstrated good biocompatibility and low immunogenicity for both self-assembled and electrospun collagen nanofiber materials. Overall, the present study highlighted that type I collagen from tilapia skin would be a promising biomaterial for the development of regenerate medical products.
DOI
URL
PMID
[Cited within: 1]

An ideal wound dressing should not only promote rapid hemostasis and wound healing but also have good biocompatibility, antimicrobial activities, and should mimic the skin's physiological function. In the present study, five O-quaternized chitosan (QAS-CS) materials with satisfactory antibacterial activity and no cytotoxicity were successfully synthesized. Furthermore, we reported the synthesis and characterization of the two novel composite nanofibrous scaffolds consisting of selfdeveloped collagen (COL), quaternary ammonium salt (QAS) or QAS-CS and polycaprolactone (PCL) or polyvinyl alcohol (PVA) with an electrospinning approach. The PCL/COL/QAS (PCQ3) and PVA/COL/QAS-CS (PCQC5) materials exhibited uniform, three-dimensional interconnected pore structure, high porosity, and large specific surface area, which can mimic the architecture and biological functions of the native extracellular matrix microenvironment and provide suitable conditions for cell adhesion, proliferation, and differentiation. The growth and proliferation of human immortalized epidermal (HaCaT) cells on PCQ3 and PCQC5 materials was good, and the result of blood compatibility and the skin irritant test indicated that the scaffolds had good blood compatibility and did not exhibit significant irritability. Importantly, PCQ3 and PCQC5 have notable effects on rapid hemostasis, antibacterial activity, and anti-inflammation, promote wound healing, and display other superior benefits in comparison to available products. Overall, PCQ3 and PCQC5 are proposed to be good candidates for skin tissue engineering applications and can be used on different types of wounds.
DOI
URL
PMID
[Cited within: 1]

We herein proved that the two commonly used antithrombotic methods, heparin loading and pre-endothelialization could both greatly enhance the patency rate of a small-diameter graft in a canine model. Tubular grafts having an inner diameter of 4 mm were prepared by electrospinning poly(l-lactide-co-epsilon-caprolactone) (P(LLA-CL)) and heparin through a coaxial electrospinning technique. Seventy-two percent of heparin was found to be released sustainably from the graft within 14 days. To prepare the pre-endothelialized grafts, we seeded endothelial cells isolated from the femoral artery and cultured then dynamically on the lumen until a cell monolayer was formed. Digital subtraction angiography (DSA) and color Doppler flow imaging (CDFI) were used to monitor the patency without sacrificing the animals. Histological analyses revealed that following the direction of blood flow, a cell monolayer was formed at the proximal end of the heparin-loaded grafts, but such a monolayer could be found in the middle or distal region of the grafts. In contrast, the whole luminal surface of the pre-endothelialized graft was covered by a cell monolayer, suggesting the in vivo survival of the preseeded cells. This demonstrated that heparin was a comparatively simple method to achieve good patency, but the pre-endothelialization had better mechanical properties and cellular compatibility.
DOI
URL
PMID
[Cited within: 1]

Emulsion electrospinning is a convenient and promising method for incorporating proteins and drugs into nanofiber scaffolds. The aim of this study was to fabricate a nanofiber scaffold for anticoagulation and rapid endothelialization. For this purpose, we encapsulated heparin and vascular endothelial growth factor (VEGF) into the core of poly(L-lactic acid-co-varepsilon-caprolactone) (P(LLA-CL)) core-shell nanofibers via emulsion electrospinning. The fiber morphology, core-shell structure and hydrophilicity of the nanofiber mats were analyzed by scanning electron microscopy, transmission electron microscopy and water contact angle. The blood compatibility was measured by hemolysis and anticoagulation testing. A CCK-8 assay was performed to study the promotion of endothelial progenitor cell (EPC) growth and was complemented by immunofluorescent staining and SEM. Our study demonstrates that heparin and VEGF can be incorporated into P(LLA-CL) nanofibers via emulsion. The released heparin performed well as an anticoagulant, and the released VEGF promoted EPC growth on the fiber scaffolds. These results imply that electrospun P(LLA-CL) nanofibers containing heparin and VEGF have great potential in the development of vascular grafts in cases where antithrombogenicity and accelerated endothelialization are desirable.
DOI
URL
PMID
[Cited within: 2]

Poly(l-lactide-co-caprolactone)-collagen-chitosan (P(LLA-CL)-COL-CS) composite grafts were electrospun in this study. Based on the test results for mechanical properties, biodegradability and in vitro cellular compatibility, the optimal weight ratio of P(LLA-CL) to COL/CS was set as 3 : 1. In vivo study was further performed in a canine femoral artery model. The results showed that the 3 : 1 grafts possessed excellent structural integrity, higher patency rate, better endothelial cell (EC) and smooth muscle cells (SMC) growth, as well as higher levels of gene and protein expression of angiogenesis-related cues than those of grafts based on P(LLA-CL). The findings confirmed that the addition of natural materials, such as collagen and chitosan, could effectively improve endothelialization, SMC incursion into the tunica media, and vascular remodeling for tissue engineering.
DOI
URL
PMID
[Cited within: 2]

Studies have shown that salvianolic acid B (SAB), which is derived from Chinese salvia ( Salvia miltiorrhiza), a plant used in traditional Chinese medicine, can promote the proliferation and migration of endothelial cells. The inner layer of an artificial vascular graft was fabricated using the coaxial electrospinning method and was loaded with the anticoagulant heparin and SAB. The release of heparin and SAB was sustained for almost 30 days and without an initial burst release of SAB. Furthermore, the combined effect of SAB and heparin contributed to promoting human umbilical vein endothelial cell (HUVEC) growth and improved the blood compatibility of the graft. In addition, upregulation of GRP78 by SAB protected human endothelial cells from oxidative stress-induced cellular damage. In vivo evaluation through Masson's trichrome and H&E staining was performed after the graft was subcutaneously embedded in SD rats for 2 weeks and indicated that the graft possessed satisfactory biocompatibility and did not cause a significant immune response. Hence, the functional inner layer is promising for preventing acute thrombosis and promotes rapid endothelialization of artificial vascular grafts.
DOI
URL
PMID
[Cited within: 1]

Simulating the modeling of smooth muscle layer in the vascular structure makes a great difference for vascular tissue regeneration. A functional tissue engineered vascular media shall promote the aligned organization and three-dimensional penetration of smooth muscle cells (SMCs) into the scaffold. To this aim, dynamic liquid and conjugated nanoyarns based on poly(L-lactide-co-caprolactone) (P(LLA-CL)) and collagen (COL) with a weight ratio at 3:1 were fabricated by electrospinning methods, with random and aligned nanofibers as control groups. The Fourier transform infrared spectroscopy and X-ray diffraction analyses confirmed the preservation of P(LLA-CL)/COL components and structure. Scanning electron microscope (SEM) results indicated a significant increase of yarn diameters at 19.27 +/- 6.16 mum (dynamic liquid) and 10.24 +/- 3.09 mum (conjugated), and both of the nanoyarns had improved mechanical tensile properties than the random nanofibers. Compared with random and aligned nanofibers, the nanoyarns presented significant higher porosity and larger pore diameter, leading to a decrease of water contact angle and a promotion of SMCs proliferation and migration. Better SMCs orientation was observed on the conjugated nanoyarns, while superior SMCs penetration was achieved on the dynamic liquid nanoyarns, owing to the differences in yarns microstructure. Herein, this study demonstrated that the aligned and porous P(LLA-CL)/COL nanoyarns fabricated by dynamic liquid and conjugated electrospinning were beneficial to regulating vascular SMCs outgrowth, which had important implications for functional reconstruction of vascular media.
DOI
URL
PMID
[Cited within: 1]

Electrospinning using natural proteins and synthetic polymers offers an attractive technique for producing fibrous scaffolds with potential for tissue regeneration and repair. Nanofibrous scaffolds of silk fibroin (SF) and poly(L-lactic acid-co-epsilon-caprolactone) (P(LLA-CL)) blends were fabricated using 1,1,1,3,3,3-hexafluoro-2-propanol as a solvent via electrospinning. The average nanofibrous diameter increased with increasing polymer concentration and decreasing the blend ratio of SF to P(LLA-CL). Characterizations of XPS and (13)C NMR clarified the presence of SF on their surfaces and no obvious chemical bond reaction between SF with P(LLA-CL) and SF in SF/P(LLA-CL) nanofibers was present in a random coil conformation, SF conformation transformed from random coil to beta-sheet when treated with water vapor. Whereas water contact angle measurements conformed greater hydrophilicity than P(LLA-CL). Both the tensile strength and elongation at break increased with the content increasing of P(LLA-CL). Cell viability studies with pig iliac endothelial cells demonstrated that SF/P(LLA-CL) blended nanofibrous scaffolds significantly promoted cell growth in comparison with P(LLA-CL), especially when the weight ratio of SF to P(LLA-CL) was 25:75. These results suggested that SF/P(LLA-CL) blended nanofibrous scaffolds might be potential candidates for vascular tissue engineering.
DOI
URL
PMID
[Cited within: 1]

Peripheral nerve regeneration remains a significant clinical challenge to researchers. Progress in the design of tissue engineering scaffolds provides an alternative approach for neural regeneration. In this study aligned silk fibroin (SF) blended poly(L-lactic acid-co-epsilon-caprolactone) (P(LLA-CL)) nanofibrous scaffolds were fabricated by electrospinning methods and then reeled into aligned nerve guidance conduits (NGC) to promote nerve regeneration. The aligned SF/P(LLA-CL) NGC was used as a bridge implanted across a 10mm defect in the sciatic nerve of rats and the outcome in terms of of regenerated nerve at 4 and 8 weeks was evaluated by a combination of electrophysiological assessment and histological and immunohistological analysis, as well as electron microscopy. The electrophysiological examination showed that functional recovery of the regenerated nerve in the SF/P(LLA-CL) NGC group was superior to that in the P(LLA-CL) NGC group. The morphological analysis also indicated that the regenerated nerve in the SF/P(LLA-CL) NGC was more mature. All the results demonstrated that the aligned SF/P(LLA-CL) NGC promoted peripheral nerve regeneration significantly better in comparison with the aligned P(LLA-CL) NGC, thus suggesting a potential application in nerve regeneration.
DOI
URL
PMID
[Cited within: 1]

Artificial nerve guidance conduits (NGCs) containing bioactive neurotrophic factors and topographical structure to biomimic native tissues are essential for efficient regeneration of nerve gaps. In this study, aligned SF/P(LLA-CL) nanofibers encapsulating nerve growth factor (NGF), which was stabilized by SF in core, were fabricated via a coaxial electrospinning technique. The controlled release of NGF from the nanofibers was evaluated using enzyme-linked immune sorbent assay (ELISA) and PC12 cell-based bioassay over a 60-day time period. The results demonstrated that NGF presented a sustained release and remained biological activity over 60 days. Nerve guidance conduits (NGCs) were fabricated by reeling the aligned SF/P(LLA-CL) nanofibrous scaffolds encapsulating NGF and then used as a bridge implanted across a 15-mm defect in the sciatic nerve of rats to promote nerve regeneration. The outcome in terms of regenerated nerve at 12 weeks was evaluated by a combination of electrophysiological assessment, histochemistry, and electron microscopy. All results clarified that the NGF-encapsulated-aligned SF/P(LLA-CL) NGCs promoted peripheral nerve regeneration significantly better than the aligned SF/P(LLA-CL) NGCs, suggesting that the released NGF from nanofibers could effectively promote the regeneration of peripheral nerve.
DOI
URL
PMID
[Cited within: 1]

Recent bioengineering strategies for peripheral nerve regeneration have been focusing on the development of alternative treatments for nerve repair. In this study, we incorporated nerve growth factor (NGF) into aligned core-shell nanofibres by coaxial electrospinning, and reeled the scaffold into aligned fibrous nerve guidance conduits (NGCs) for nerve regeneration study. This aligned PLGA/NGF NGC combined physical guidance cues and biomolecular signals to closely mimic the native extracellular matrix (ECM). The effect of this aligned PLGA/NGF NGC on the promotion of nerve regeneration was evaluated in a 13-mm rat sciatic nerve defect using functional and morphological analysis. After 12 weeks implantation, the results of electrophysiological and muscle weight examination demonstrated that the functional recovery of the regenerated nerve in the PLGA/NGF NGC group was significantly better than that in the PLGA group, yet had no significant difference compared with the autograft group. The toluidine blue staining study showed that more nerve fibres were regenerated in the PLGA/NGF group, while the electron microscopy study indicated that the regenerated nerve in the PLGA/NGF group was more mature than that in the PLGA group. This study demonstrated that the aligned PLGA/NGF could greatly promote peripheral nerve regeneration and have a potential application in nerve regeneration.
DOI
URL
PMID
[Cited within: 1]

Currently, electroactive biomaterials have often been fabricated as tissue engineering scaffolds to provide electrical stimulation for neural tissue engineering. The goal of this work was to study the synergistic effect of electrical stimulation and nerve growth factor (NGF) on neuron growth. The composite meshes of polyaniline (PANi) and well-blended poly(l-lactic acid-co-epsilon-caprolactone)/silk fibroin (PS) incorporated with nerve growth factor (NGF) were prepared by coaxial electrospinning. The results showed that the increased concentration of PANi had a large effect on the fiber diameter, which was significantly reduced from 683 +/- 138 nm to 411 +/- 98 nm and then increased to 498 +/- 100 nm. The contact angles and Young's modulus decreased to 28.3 degrees +/- 5.4 degrees and 7.2 +/- 1.2 MPa, respectively, and the conductance increased to 30.5 +/- 3.1 mS cm(-1). The results of the viability and morphology of mouse Schwann cells on the nanofibrous meshes showed that PS-PANi-1 loaded with NGF exhibited the highest cell number after 5 days culture and the aligned nanofibers could guide cell orientation. The synergistic effects of electrical stimulation and NGF were also investigated via the growth and differentiation of rat pheochromocytoma 12 (PC12) cells. The scaffolds loaded with NGF under electrical stimulation could effectively support PC12 neurite outgrowth and increase the percentage of neurite-bearing cells as well as the median neurite length. More importantly, the NGF release from the conductive core-shell structure nanofiber could be increased by electrical stimulation. These promising results demonstrated that there was a potential use of this functional scaffold for nerve tissue regeneration.
DOI
URL
PMID
[Cited within: 1]

Polypyrrole (Ppy), as a conductive polymer, is commonly used for nerve tissue engineering because of its good conductivity and non-cytotoxicity. To avoid the inconvenience of Ppy processing, it was coated on electrospun poly(l-lactic acid-co-epsilon-caprolactone)/silk fibroin (PLCL/SF) nanofibers via the in situ oxidative polymerization of pyrrole monomers in this study. Ppy-coated PLCL/SF membranes were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and thermogravimetric (TG) analysis. The results confirmed the disposition of Ppy on the PLCL/SF nanofibers, and the nanofibers kept their nanofibrous morphology and thermal stability, in comparison to the untreated ones. The conductivities and water contact angles were evaluated as well, and indicated that the conductivity and hydrophilicity of Ppy-coated nanofibers were increased. Furthermore, this study showed that electrical stimulation (ES) promoted PC12 cell differentiation and axonal extension on Ppy-coated nanofibers. The MTT assay suggested that both Ppy and ES could promote Schwann cell (SC) proliferation. Immunofluorescence staining and real time-qPCR (RT-qPCR) testing demonstrated that ES could induce PC12 cell differentiation even without nerve growth factor (NGF) treatment, and moreover, Ppy coating increased the inducing effects on PC12 cell differentiation. The overall results indicated the promising potential of Ppy-coated PLCL/SF nanofibrous membranes for peripheral nerve repair and regeneration.
DOI
URL
PMID
[Cited within: 2]

Injuries of the peripheral nerve occur commonly in various people of different ages and backgrounds. Generally, surgical repairing, such as suturing the transected nerve stumps and transplanting an autologous nerve graft, is the only choice. However, tissue engineering provides an alternative strategy for regeneration of neural context. Functional nerve conduits with three dimensional (3D) support and guidance structure are badly in need. Herein, a uniform PLLA nanofiber yarn constructed by unidirectionally aligned nanofibers was fabricated via a dual spinneret system, which was subsequently incorporated into a hollow poly(l-lactide-co-caprolactone) (P(LLA-CL)) tube to form a nerve conduit with inner aligned texture. The biocompatibility of the poly(l-lactic acid) (PLLA) yarn was assessed by in vitro experiments. Schwann cells (SCs) presented a better proliferation rate and spread morphology of the PLLA yarn than that of PLLA film. Confocal images indicated that the axon spreads along the length of the yarn. SCs were also cultured in the conduit. The data indicated that SCs proliferated well in the conduit and distributed dispersedly throughout the entire lumen. These results demonstrated the potential of the PLLA nanofiber yarn conduit in nerve regeneration.
DOI
URL
PMID
[Cited within: 1]

The treatment of long bone defects and non-unions is still a major clinical and socio-economical problem. In addition to the non-operative therapeutic options, such as the application of various forms of electricity, extracorporeal shock wave therapy and ultrasound therapy, which are still in clinical use, several operative treatment methods are available. No consensus guidelines are available and the treatments of such defects differ greatly. Therefore, clinicians and researchers are presently investigating ways to treat large bone defects based on tissue engineering approaches. Tissue engineering strategies for bone regeneration seem to be a promising option in regenerative medicine. Several in vitro and in vivo studies in small and large animal models have been conducted to establish the efficiency of various tissue engineering approaches. Neverthelsss, the literature still lacks controlled studies that compare the different clinical treatment strategies currently in use. However, based on the results obtained so far in diverse animal studies, bone tissue engineering approaches need further validation in more clinically relevant animal models and in clinical pilot studies for the translation of bone tissue engineering approaches into clinical practice.
DOI
URL
PMID
[Cited within: 1]

The loss or failure of an organ or tissue is one of the most frequent, devastating, and costly problems in human health care. A new field, tissue engineering, applies the principles of biology and engineering to the development of functional substitutes for damaged tissue. This article discusses the foundations and challenges of this interdisciplinary field and its attempts to provide solutions to tissue creation and repair.
DOI
URL
PMID
[Cited within: 1]

The electrospinning process was utilized successfully to fabricate the random oriented and aligned electrically conductive nanofibers of biodegradable poly-DL-lactide (PLA) in which multiwalled carbon nanotubes (MWCNTs) were embedded. The topographical features of the composite nanofibers were characterized by SEM. The dispersion and alignment of MWCNTs in nanofiber matrix were observed by TEM. The in vitro degradation was characterized in terms of the morphological change, the mass loss and the reduction of polymer molecular weight as well as the decrease of pH value of degradation media. In particular, these conductive nanofiber meshes offered a unique system to study the synergistic effect of topographic cues and electrical stimulation on osteoblasts outgrowth as a way of exploring their potential application in bone tissue engineering. The results of obsteoblasts assay unstimulated showed that the aligned nanofibers as topographic cues could enhance the extension and direct the outgrowth of obsteoblasts better than random fibers. In the presence of direct current (DC) of 100 muA, the obsteoblasts on all samples grew along the electrical current direction. The cellular elongation and proliferation were mainly dependent on the electrical stimulation whereas the topographical features played a minor role in them. Therefore, electrical stimulation with an appropriate DC value imparted on conductive substrate had great potential in application of bone tissue engineering.
DOI
URL
PMID
[Cited within: 2]

Developing a highly bioactive bone tissue engineering scaffold that can modulate the bone remodeling process for promoting bone regeneration is a great challenge. In order to tackle this issue, inspired by the balance between bone resorption and formation in the bone remodeling process, here we developed a mesoporous silicate nanoparticle (MSN)-based electrospun polycaprolactone (PCL)/gelatin nanofibrous scaffold to achieve dual delivery of alendronate (ALN) and silicate for a synergetic effect in modulating bone remodeling, where ALN inhibited the bone-resorbing process via preventing guanosine triphosphate-related protein expression, and silicate promoted the bone-forming process via improving vascularization and bone calcification. The scaffold was successfully prepared by encapsulation of ALN into MSNs (ALN@MSNs) and co-electrospinning of an acetic acid-mediated PCL/gelatin homogeneous solution with well-dispersed ALN@MSNs. The results of ALN and Si element release profiles indicated that the ALN@MSN-loaded nanofibers achieved dual release of ALN and silicate (produced due to the hydrolysis of MSNs) simultaneously. The bone repair data from a rat critical-sized cranial defect model revealed that the developed strategy accelerated the healing time from 12 weeks to 4 weeks, almost three times faster, while the other nanofiber groups only had limited bone regeneration at 4 weeks. In addition, we used interactive double-factor analysis of variance for the data of bone volume and maturity to evaluate the synergetic effect of ALN and silicate in promoting bone regeneration, and the result clearly proved our original design and hypothesis. In summary, the presented bone remodeling-inspired electrospun nanofibers with dual delivery of ALN and silicate may be highly promising for bone repair in the clinic.
DOI
URL
PMID
[Cited within: 2]

The development of functional scaffolds with improved osteogenic potential is important for successful bone formation and mineralization in bone tissue engineering. In this study, we developed a functional electrospun silk fibroin (SF) nanofibrous scaffold functionalized with two-stage hydroxyapatite (HAp) particles, using mussel adhesive-inspired polydopamine (PDA) chemistry. HAp particles were first incorporated into SF scaffolds during the electrospinning process, and then immobilized onto the electrospun SF nanofibrous scaffolds containing HAp via PDA-mediated adhesive chemistry. We obtained two-stage HAp-functionalized SF nanofibrous scaffolds with improved mechanical properties and capable of providing a bone-specific physiological microenvironment. The developed scaffolds were tested for their ability to enhance the osteogenic differentiation of human adipose-derived mesenchymal stem cells (hADMSCs) in vitro and repair bone defect in vivo. To boost their ability for bone repair, we genetically modified hADMSCs with the transcriptional coactivator with PDZ-binding motif (TAZ) via polymer nanoparticle-mediated gene delivery. TAZ is a well-known transcriptional modulator that activates the osteogenic differentiation of mesenchymal stem cells (MSCs). Two-stage HAp-functionalized SF scaffolds significantly promoted the osteogenic differentiation of TAZ-transfected hADMSCs in vitro and enhanced mineralized bone formation in a critical-sized calvarial bone defect model. Our study shows the potential utility of SF scaffolds with nanofibrous structures and enriched inorganic components in bone tissue engineering.
DOI
URL
PMID
[Cited within: 1]

Effective methods of accelerating the bone regeneration healing process are in demand for a number of bone-related diseases and trauma. This work developed scaffolds with improved properties for bone tissue engineering by electrospinning composite polycaprolactone-gelatin-hydroxyapatite-niobium pentoxide (PGHANb) membranes. Composite membranes, with average fiber diameters ranging from 123 to 156nm, were produced by adding hydroxyapatite (HA) and varying concentrations of niobium pentoxide (Nb2O5) particles (0, 3, 7, and 10wt%) to a polycaprolactone (PCL) and gelatin (GL) matrix prior to electrospinning. The morphology, mechanical, chemical and biological properties of resultant membranes were evaluated. Bioactivity was assessed using simulated body fluid (SBF) and it confirmed that the presence of particles induced the formation of hydroxyapatite crystals on the surface of the membranes. Samples were hydrophilic and cell metabolism results showed that the niobium-containing membranes were non-toxic while improving cell proliferation and differentiation compared to controls. This study demonstrated that electrospun membranes containing HA and Nb2O5 particles have potential to promote cell adhesion and proliferation while exhibiting bioactive properties. PGHANb membranes are promising candidates for bone tissue engineering applications.
DOI
URL
PMID
[Cited within: 1]

Osteochondral defect management and repair remain a significant challenge in orthopedic surgery. Osteochondral defects contain damage to both the articular cartilage as well as the underlying subchondral bone. In order to repair an osteochondral defect the needs of the bone, cartilage and the bone-cartilage interface must be taken into account. Current clinical treatments for the repair of osteochondral defects have only been palliative, not curative. Tissue engineering has emerged as a potential alternative as it can be effectively used to regenerate bone, cartilage and the bone-cartilage interface. Several scaffold strategies, such as single phase, layered, and recently graded structures have been developed and evaluated for osteochondral defect repair. Also, as a potential cell source, tissue specific cells and progenitor cells are widely studied in cell culture models, as well with the osteochondral scaffolds in vitro and in vivo. Novel factor strategies being developed, including single factor, multi-factor, or controlled factor release in a graded fashion, not only assist bone and cartilage regeneration, but also establish osteochondral interface formation. The field of tissue engineering has made great strides, however further research needs to be carried out to make this strategy a clinical reality. In this review, we summarize current tissue engineering strategies, including scaffold design, bioreactor use, as well as cell and factor based approaches and recent developments for osteochondral defect repair. In addition, we discuss various challenges that need to be addressed in years to come.
DOI
URL
PMID
[Cited within: 4]

An optimal scaffold is crucial for osteochondral regeneration. Collagen and electrospun nanofibers have been demonstrated to facilitate cartilage and bone regeneration, respectively. However, the effect of combining collagen and electrospun nanofibers on osteochondral regeneration has yet to be evaluated. Here, we report that the combination of collagen and electrospun poly-l-lactic acid nanofibers synergistically promotes osteochondral regeneration. We first fabricated bi-layer microporous scaffold with collagen and electrospun poly-l-lactic acid nanofibers (COL-nanofiber). Mesenchymal stem cells were cultured on the bi-layer scaffold and their adhesion, proliferation and differentiation were examined. Moreover, osteochondral defects were created in rabbits and implanted with COL-nanofiber scaffold. Cartilage and subchondral bone regeneration were evaluated at 6 and 12weeks after surgery. Compared with COL scaffold, cells on COL-nanofiber scaffold exhibited more robust osteogenic differentiation, indicated by higher expression levels of OCN and runx2 genes as well as the accumulation of calcium nodules. Furthermore, implantation of COL-nanofiber scaffold seeded with cells induced more rapid subchondral bone emergence, and better cartilage formation, which led to better functional repair of osteochondral defects as manifested by histological staining, biomechanical test and micro-computed tomography data. Our study underscores the potential of using the bi-layer microporous COL-nanofiber scaffold for the treatment of deep osteochondral defects.
DOI
URL
PMID
[Cited within: 2]

Electrospun nanofibers have been used for various biomedical applications. However, electrospinning commonly produces two-dimensional (2D) membranes, which limits the application of nanofibers for the 3D tissue engineering scaffold. In the present study, a porous 3D scaffold (3DS-1) based on electrospun gelatin/PLA nanofibers has been prepared for cartilage tissue regeneration. To further improve the repairing effect of cartilage, a modified scaffold (3DS-2) cross-linked with hyaluronic acid (HA) was also successfully fabricated. The nanofibrous structure, water absorption, and compressive mechanical properties of 3D scaffold were studied. Chondrocytes were cultured on 3D scaffold, and their viability and morphology were examined. 3D scaffolds were also subjected to an in vivo cartilage regeneration study on rabbits using an articular cartilage injury model. The results indicated that 3DS-1 and 3DS-2 exhibited superabsorbent property and excellent cytocompatibility. Both these scaffolds present elastic property in the wet state. An in vivo study showed that 3DS-2 could enhance the repair of cartilage. The present 3D nanofibrous scaffold (3DS-2) would be promising for cartilage tissue engineering application.
DOI
URL
PMID
[Cited within: 1]

Tracheal stenosis is one of major challenging issues in clinical medicine because of the poor intrinsic ability of tracheal cartilage for repair. Tissue engineering provides an alternative method for the treatment of tracheal defects by generating replacement tracheal structures. In this study, we fabricated coaxial electrospun fibers using poly(L-lactic acid-co-caprolactone) and collagen solution as shell fluid and kartogenin solution as core fluid. Scanning electron microscope and transmission electron microscope images demonstrated that nanofibers had uniform and smooth structure. The kartogenin released from the scaffolds in a sustained and stable manner for about 2 months. The bioactivity of released kartogenin was evaluated by its effect on maintain the synthesis of type II collagen and glycosaminoglycans by chondrocytes. The proliferation and morphology analyses of mesenchymal stems cells derived from bone marrow of rabbits indicated the good biocompatibility of the fabricated nanofibrous scaffold. Meanwhile, the chondrogenic differentiation of bone marrow mesenchymal stem cells cultured on core-shell nanofibrous scaffold was evaluated by real-time polymerase chain reaction. The results suggested that the core-shell nanofibrous scaffold with kartogenin could promote the chondrogenic differentiation ability of bone marrow mesenchymal stem cells. Overall, the core-shell nanofibrous scaffold could be an effective delivery system for kartogenin and served as a promising tissue engineered scaffold for tracheal cartilage regeneration.
DOI
URL
PMID
[Cited within: 1]

Tendon and ligament injures cause significant loss of performance in sport and decreased functional capacity in the workplace. Many of these injures remain difficult to treat, and many individuals have long-term pain and discomfort. Animal studies of growth factor and cell-based therapies have shown promising results, but these treatments also can be misused to enhance athletic performance. The International Olympic Committee (IOC) now has high-level scientific advisors who can advise the IOC as to the use and abuse of these technologies.
DOI
URL
PMID
[Cited within: 1]

BACKGROUND: Biostatic (nonvital) tissue allografts have been used for temporary replacement as well as to trigger, stimulate, and ensure space for the regeneration of a recipient's own tissues. Examples of biostatic allografts routinely used in clinic are bone, tendons, skin, and amniotic membrane. A characteristic feature of biostatic allografts is the lack of living cells. In the recipient's body, biostatic allografts function as scaffolds as well as sources of growth, differentiation, and chemotactic factors. After implantation, recipient cells migrate onto the graft, colonize it, and initiate synthesis of extracellular matrix, thereby regenerating the structure of the lost or damaged tissue. The allograft gradually degrades before being remodeled and substituted by the recipient's new tissue. However, this process is not always effective due to a lack of reaction by recipient cells. New concepts have proposed seeding recipient cells onto the allograft prior to implantation, that is, biostatic allografts that are revitalized ex vivo. The aim of this presentation was to review scientific publications to provide essential information on the revitalization of biostatic allografts, as a rising trend in tissue transplantology. RESULTS: Biostatic allografts show the following advantages: they are human-derived, nontoxic, biocompatible, and, in some cases, already display the desired shape. The process of introducing cells into the biostatic graft is described as
DOI
URL
PMID
[Cited within: 2]

UNLABELLED: Regeneration of injured tendon and ligament (T&L) remains a clinical challenge due to their poor intrinsic healing capacity. Tissue engineering provides a promising alternative treatment approach to facilitate T&L healing and regeneration. Successful tendon tissue engineering requires the use of three-dimensional (3D) biomimetic scaffolds that possess the physical and biochemical features of native tendon tissue. We report here the development and characterization of a novel composite scaffold fabricated by co-electrospinning of poly-epsilon-caprolactone (PCL) and methacrylated gelatin (mGLT). We found that photocrosslinking retained mGLT, resulted in a uniform distribution of mGLT throughout the depth of scaffold and also preserved scaffold mechanical strength. Moreover, photocrosslinking was able to integrate stacked scaffold sheets to form multilayered constructs that mimic the structure of native tendon tissues. Importantly, cells impregnated into the constructs remained responsive to topographical cues and exogenous tenogenic factors, such as TGF-beta3. The excellent biocompatibility and highly integrated structure of the scaffold developed in this study will allow the creation of a more advanced tendon graft that possesses the architecture and cell phenotype of native tendon tissues. STATEMENT OF SIGNIFICANCE: The clinical challenges in tendon repair have spurred the development of tendon tissue engineering approaches to create functional tissue replacements. In this study, we have developed a novel composite scaffold as a tendon graft consisting of aligned poly-epsilon-caprolactone (PCL) microfibers and methacrylated gelatin (mGLT). Cell seeding and photocrosslinking between scaffold layers can be performed simultaneously to create cell impregnated multilayered constructs. This cell-scaffold construct combines the advantages of PCL nanofibrous scaffolds and photocrosslinked gelatin hydrogels to mimic the structure, mechanical anisotropy, and cell phenotype of native tendon tissue. The scaffold engineered here as a building block for multilayer constructs should have applications beyond tendon tissue engineering in the fabrication of tissue grafts that consist of both fibrous and hydrogel components.
DOI
URL
PMID
[Cited within: 1]

Tissue engineering holds great potential in the production of functional substitutes to restore, maintain or improve the functionality in defective or lost tissues. So far, a great variety of techniques and approaches for fabrication of scaffolds have been developed and evaluated, allowing researchers to tailor precisely the morphological, chemical and mechanical features of the final constructs. Electrospinning of biocompatible and biodegradable polymers is a popular method for producing homogeneous nanofibrous structures, which might reproduce the nanosized organization of the tendons. Moreover, composite scaffolds obtained by incorporating nanoparticles within electrospun fibers have been lately explored in order to enhance the properties and the functionalities of the pristine polymeric constructs. The present study is focused on the design and fabrication of biocompatible electrospun nanocomposite fibrous scaffolds for tendon regeneration. A mixture of poly(amide 6) and poly(caprolactone) is electrospun to generate constructs with mechanical properties comparable to that of native tendons. To improve the biological activity of the constructs and modify their topography, wettability, stiffness and degradation rate, we incorporated silica particles into the electrospun substrates. The use of nanosize silica particles enables us to form bead-on-fiber topography, allowing the better exposure of ceramic particles to better profit their beneficial characteristics. In vitro biocompatibility studies using L929 fibroblasts demonstrated that the presence of 20 wt% of silica nanoparticles in the engineered scaffolds enhanced cell spreading and proliferation as well as extracellular matrix deposition. The results reveal that the electrospun nanocomposite scaffold represents an interesting candidate for tendon tissue engineering.
DOI
URL
PMID
[Cited within: 1]

Poor tendon repair is often a clinical challenge due to the lack of ideal biomaterials. Electrospun aligned fibers, resembling the ultrastructure of tendon, have been previously reported to promote tenogenesis. However, the underlying mechanism is unclear and the aligned fibers alone are not capable enough to commit teno-differentiation of stem cells. Here, based on our observation of reduced expression of histone deacetylases (HDACs) in tendon stem/progenitor cells (TSPCs) cultured on aligned fibers, we proposed a strategy to enhance the tenogenesis effect of aligned fibers by using a small molecule Trichostatin A (TSA), an HDAC inhibitor. Such a TSA-laden poly (l-lactic acid) (PLLA) aligned fiber (A-TSA) scaffold was successfully fabricated by a stable jet electrospinning method, and demonstrated its sustained capability in releasing TSA. We found that TSA incorporated aligned fibers of PLLA had an additive effect in directing tenogenic differentiation. Moreover, the in situ implantation study in rat model further confirmed that A-TSA scaffold promoted the structural and mechanical properties of the regenerated Achilles tendon. This study demonstrated that HDAC was involved in the teno-differentiation with aligned fiber topography, and the combination of HDAC with aligned topography might be a more efficient strategy to promote tenogenesis of stem cells. STATEMENT OF SIGNIFICANCE: Electrospun aligned fibers, resembling the ultrastructure of tendon, have been previously reported to promote tenogenesis. However, the underlying mechanism is unclear and the aligned fibers alone are not capable enough to commit teno-differentiation of stem cells. The uniqueness of our studies are as follows, based on our observation of reduced expression of histone deacetylases (HDACs) in tendon stem/progenitor cells (TSPCs) cultured on aligned fibers, we proposed a strategy to enhance the tenogenesis effect of aligned fibers by using a small molecule Trichostatin A (TSA), a HDAC inhibitor. Such a TSA-laden poly (l-lactic acid) (PLLA) aligned fiber (A-TSA) scaffold was successfully fabricated by a stable jet electrospinning method, and demonstrated its sustained capability in releasing TSA. The incorporation and subsequent release of bioactive small molecule TSA into electrospun aligned fibers allows a controllable manner for both biochemical and physical regulation of tenogenesis of stem cells both in vitro and in vivo. Collectively, the present study provides a model of
DOI
URL
PMID
[Cited within: 1]

Tendon-to-bone surgical repairs have unacceptably high failure rates, possibly due to their inability to recreate the load transfer mechanisms of the native enthesis. Instead of distributing load across a wide attachment footprint area, surgical repairs concentrate shear stress on a small number of suture anchor points. This motivates development of technologies that distribute shear stresses away from suture anchors and across the enthesis footprint. Here, we present predictions and proof-of-concept experiments showing that mechanically-optimized adhesive films can mimic the natural load transfer mechanisms of the healthy attachment and increase the load tolerance of a repair. Mechanical optimization, based upon a shear lag model corroborated by a finite element analysis, revealed that adhesives with relatively high strength and low stiffness can, theoretically, strengthen tendon-to-bone repairs by over 10-fold. Lap shear testing using tendon and bone planks validated the mechanical models for a range of adhesive stiffnesses and strengths. Ex vivo human supraspinatus repairs of cadaveric tissues using multipartite adhesives showed substantial increase in strength. Results suggest that adhesive-enhanced repair can improve repair strength, and motivate a search for optimal adhesives. STATEMENT OF SIGNIFICANCE: Current surgical techniques for tendon-to-bone repair have unacceptably high failure rates, indicating that the initial repair strength is insufficient to prevent gapping or rupture. In the rotator cuff, repair techniques apply compression over the repair interface to achieve contact healing between tendon and bone, but transfer almost all force in shear across only a few points where sutures puncture the tendon. Therefore, we evaluated the ability of an adhesive film, implanted between tendon and bone, to enhance repair strength and minimize the likelihood of rupture. Mechanical models demonstrated that optimally designed adhesives would improve repair strength by over 10-fold. Experiments using idealized and clinically-relevant repairs validated these models. This work demonstrates an opportunity to dramatically improve tendon-to-bone repair strength using adhesive films with appropriate material properties.
DOI
URL
PMID
[Cited within: 2]

Tendon-bone interface tissue is extremely challenging to engineer because it exhibits complex gradients of structure, composition, biologics, and cellular phenotypes. As a step toward engineering these transitional zones, we initially analyzed how different (topographical or biological) cues affect tenogenic differentiation of adipose-derived stem cells (ADSCs). We immobilized platelet-derived growth factor - BB (PDGF-BB) using polydopamine (PD) chemistry on random and aligned nanofibers and investigated ADSC proliferation and tenogenic differentiation. Immobilized PDGF greatly enhanced the proliferation and tenogenic differentiation of ADSCs; however, nanofiber alignment had no effect. Interestingly, the PDGF immobilized aligned nanofiber group showed a synergistic effect with maximum expression of tenogenic markers for 14 days. We also generated a nanofiber surface with spatially controlled presentation of immobilized PDGF on an aligned architecture, mimicking native tendon tissue. A gradient of immobilized PDGF was able to control the phenotypic differentiation of ADSCs into tenocytes in a spatially controlled manner, as confirmed by analysis of the expression of tenogenic markers and immunofluorescence staining. We further explored the gradient formation strategy by generation of a symmetrical gradient on the nanofiber surface for the generation of a structure mimicking bone-patellar-tendon-bone with provision for gradient immobilization of PDGF and controlled mineralization. Our study reveals that, together with biochemical cues, favorable topographical cues are important for tenogenic differentiation of ADSCs, and gradient presentation of PDGF can be used as a tool for engineering stem cell-based bone-tendon interface tissues.



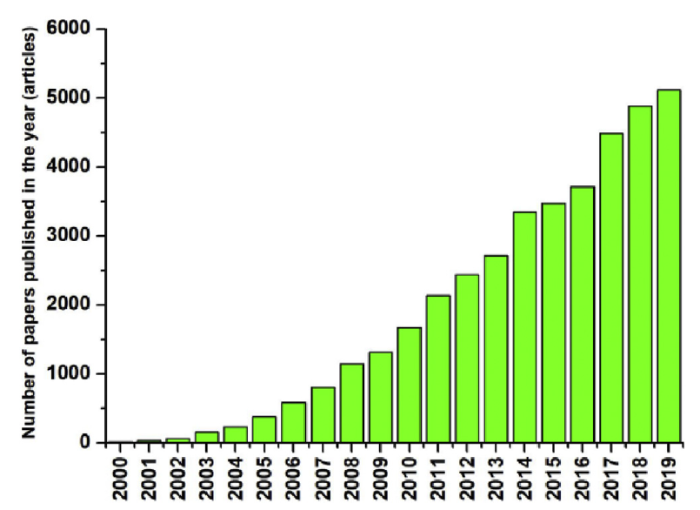




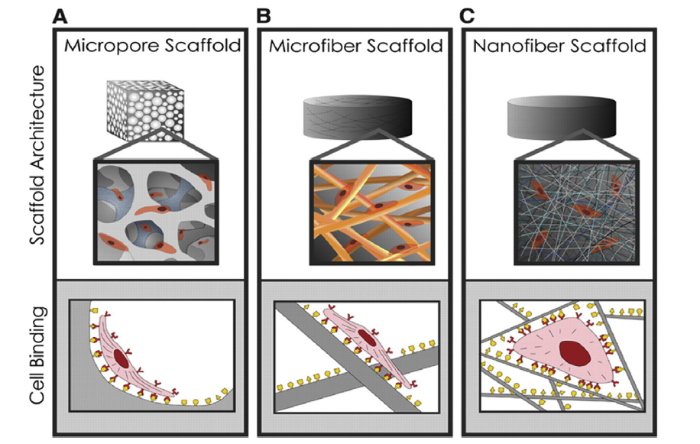
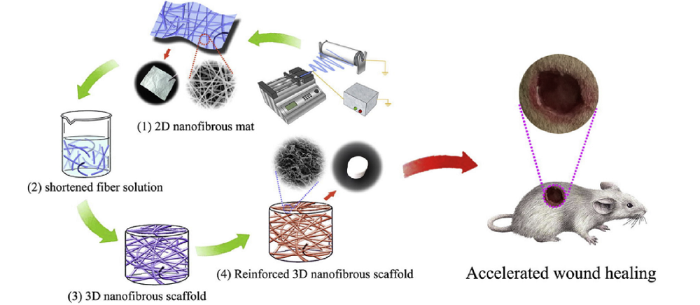


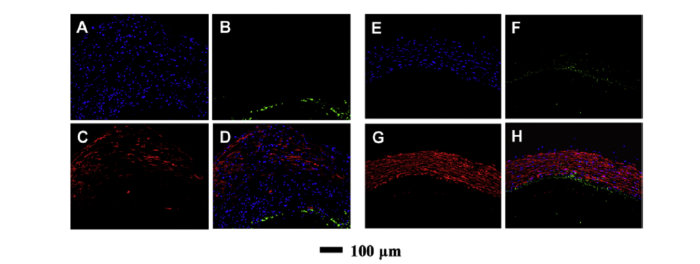


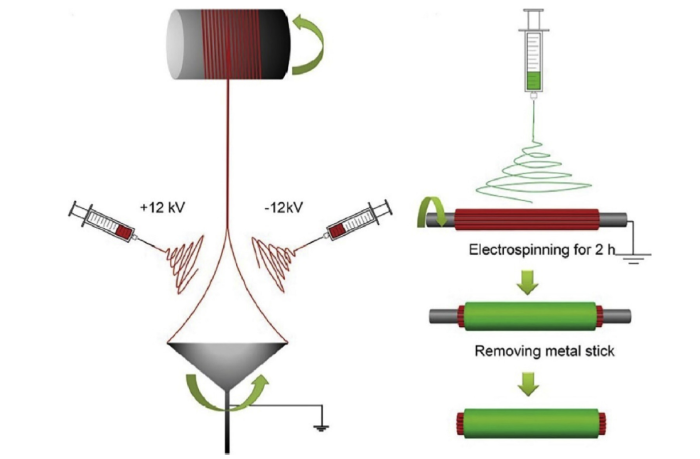

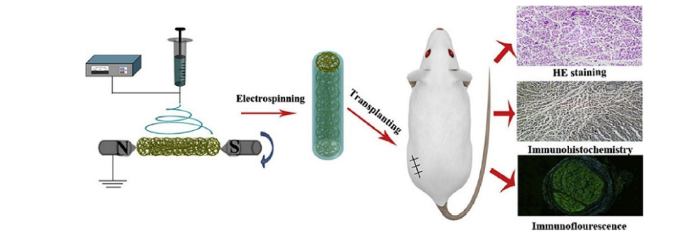








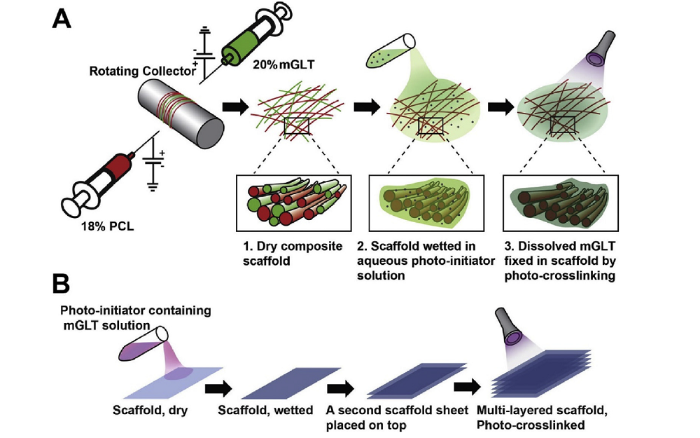




 WeChat
WeChat