1. Introduction
Although the need for tissue repair and regeneration continues to soar, there is still a lack of effective tissue repair and regeneration strategies in current clinical practices. Biomaterials serve an important role in tissue engineering and regenerative medicine, and a new class of ‘smart biomaterials’ has been developed in the past few decades, which could adapt their compositions, stuctures or properties in response to the changes in surroundings [[1], [2], [3], [4], [5], [6], [7]]. In principle, such smart biomaterials can respond to stimuli, either generated endogenously from the tissue (e.g., pH, temperature, shear stress, and so on) or imposed by external or exogenous sources (e.g., high-energy radiations, electric or magnetic fields, acoustic wave, and so on), in an instant or in a programmed manner that can be devised to promote tissue regeneration or repair. Among these stimuli, mechanical stimuli are probably the most ubiquitous and exist in the hierarchy of biological systems from molecules to organs [8,9]. Mechanical stimuli are also involved in almost all the physiological processes and play an important role in the development, homeostasis, and functions of tissues and organs [[8], [9], [10], [11], [12]].
Based on the increasing understandings on the mechanics at different hierarchy of biological systems, and the role of mechanics in physiological processes, a new type of smart biomaterials that offer the advantages of intrinsic mechanical stimuli for the purpose of tissue repair and regeneration has recently emerged. In this context, this article elucidates a new perspective of perfecting the repair or regeneration of tissues using such types of biomaterials that can actively respond to the mechanical stimuli in vivo and in situ, establishing a provisional concept of mechano-active biomaterials and highlighting their recent progresses in the repair of musculoskeletal and cardiovascular tissues.
2. Rationale of mechano-active biomaterials for tissue repair and regeneration
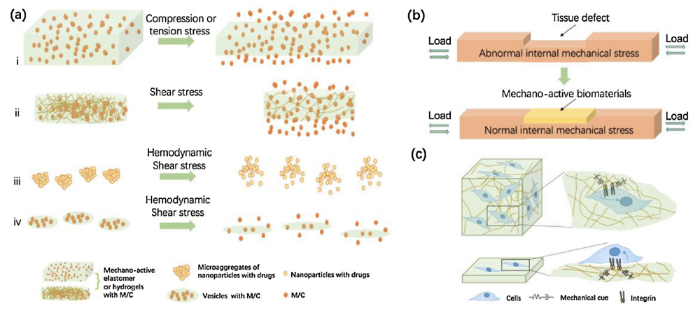
It is well established that mechanical stability is extremely important for tissue growth and regeneration, and aberrant mechanical environment have been found in the development and progression of diseases and tissue degeneration [[11], [12], [13], [14]]. There are also increasing number of studies demonstrating that biomaterials that can reconstruct the original or a favorable mechanical environment at a lesion, are highly effective for tissue repair or regeneration. Therefore, the rationale of enabling or improving tissue repair or regeneration by mechano-active biomaterials emerges on the basis of at least three mechanisms (Fig. 1):
(1) The important role of mechanics in tissue homeostasis: Given that tissue homeostasis is established upon the mechanical continuity and stability of the tissue, an important prerequisite for the recovery of injured or faulty tissues is restoration of the appropriate mechanical environment. One of the advantages of mechano-active biomaterials is that they can constantly adapt to local mechanical stimuli and can reconstruct the original or a favorable local mechanical environment for tissue restoration.
(2) Biomimetics of living tissues and cells: Living cells and tissues not only maintain their own mechanical balance (i.e., homeostasis) but also actively respond to various mechanical stimuli in vivo by instantly and constantly changing their geometry, structure or physiological properties. Many mechano-active biomaterials are inspired by this nature and have been devised to mimic such homeostatic and responsive abilities (e.g., varibale geometry, compliance, or structure as that of target tissue) and/or utilize the dynamic mechanical stimuli to realize specific purposes such as spatially-temporally controlled release or targeted delivery of molecules.
(3) Mechanobiological effects on tissue growth: Recent research progress in the field of mechanobiology has unveiled the great power and influence of mechanical cues on regulating cellular functions (including stem and functional cells) and tissue homeostasis (e.g., bone remodeling, joint cartilage degeneration, and so on) through mechano-transduction mechanisms at the molecular level [8,[15], [16], [17]]. Mechano-active biomaterials can be devised to dynamically and consistently regulate such important mechanical cues to cells or tissues and thus accelerate tissue remodeling or healing process via a mechanobiological effect.
Fig. 1.
Fig. 1.
Schematic illustration of the rationale and mechanisms of mechano-active biomaterials for tissue repair and regeneration. (a) Mechano-active biomaterials can dynamically utilize the mechanical stimuli to realize the spatially-temporally controlled release or the targeted delivery of molecules/cells (M/C) for tissue repair and regeneration. The main mechanisms include: (i) compression/tension-induced deformation of the bulk carriers, (ii) temporary loss of the hydrogel structure by rupturing the physical crosslinks, (iii) disintegration of the aggregates of nanoparticles with drugs into nanoscale components, (iv) deformation and disintegration of the drug-loaded vesicles. (b) Reconstruction of the mechanical environment at the tissue-level that are beneficial for tissue repair and regeneration. (c) Biomimetic mechano-active biomaterials can accommodate or utilize important mechanical cues to accelerate tissue remodeling or healing process via a mechanobiological effect.
Based on this rationale and numerous cutting-edge studies [5,[18], [19], [20]], the concept of mechano-active biomaterials can be summarized and provisionally defined as the biomaterial possessing specific functionality or performance that is purposely designed to be dynamically and consistently mediated by mechanical stimuli, preferably the endogeneous ones from the host biological systems. In this concept, the specific functionality refers to a wide spectrum of qualities attractive for biomaterial applications, including but not limited to the ability to precisely mimic homeostatic or dynamic native biomechanical environment at lesion site, the regenerative potential of damaged tissue, the controlled release of therapeutic agents and so on. The key of the concept, also the so-called “active”, is the mediation of the functionality in such a way that is designed to dynamically and consistently adapt to the mechanical stimuli. On the contrary, many clinically used biomaterials, such as titanium or stainless-steel implants or stents, have structural support function to host tissue and are designed to withstand mechanical loads. This functionality is, however, not designed to be mediated by the varied mechanical loads in vivo but to remain stable under different loads. Thus, these biomaterials are not mechano-active but mechano-passive. Some recent drug delivery materials, in which the release of cargo is triggered by force or strain, can only be considered mechanically active when the controlled release behavior dynamically and consistently alters with the varying extent of force or strain. The concept of mechano-active biomaterials will be elaborated more by reviewing the progress in this area as follows.
3. Recent advances in tissue repair and regeneration with mechano-active biomaterials
3.1. Precise drug/cell delivery enabled by mechano-active biomaterials
The first generation of force-activated drug/cell delivery systems is based on the biomaterials that passively respond to material deformation or disintegration [21,22]. These systems may realize a force-triggered or a sustained release but not the spatio-temporally precise release in prolonged time periods. In contrast, mechano-active biomaterials with elaborated active responses to mechanical cues may help realize the potential of precise drug/cell delivery. Drug delivery systems based on mechano-active biomaterials can be used for on-demand and precise delivery of antibacterial agents, anticancer drugs, and growth factors under repeated mechanical loading through a self-administrative manner. Some representative examples are summarized in Table 1.
Table 1 Examples of mechano-active biomaterials for precisely controlled delivery.
| Material systems | Mechanisms | Cargo release behavior | Potential applications | Ref. |
|---|---|---|---|---|
| Hyaluronic hydrogel covalently integrated with dexamethasone (DEX)-loaded block copolymer micelles (BCMs) | A force-induced reversible deformation of BCMs and the penetration of water into BCMs. | DEX release is controlled in a strain-dependent manner by external compression | Treatment of osteoarthritis-like symptoms with intra-articular hydrogel injections | [23] |
| Hydrogel physically and chemically attached with cargo-filled mechano-sensitive liposomes | Proper level of mechanical force applied to the hydrogel ruptures the liposomes and releases the cargo | Release increases with the increasing force (within 200 cycles, 0.016 % cargo release per cycle for 10 % strain and 0.034 % for 25 % strain, respectively) | A long-term intra-articular drug release | [24] |
| Polyacrylamide (PAAm) with pyrene-loaded BCMs | Stretch-induced reversible micelle deformation leads to a dynamic release of pyrene | In the presence of the selected stretching regimen, the amount of pyrene released during each stretch period at 60% strain was approximately two times higher than that at 30% strain, and 2.5 times higher than that by the static controls | Precise release of drug and therapeutic agents for tissue repair or regeneration | [25] |
| A highly stretchable elastomer imbedded with alginate microgels containing drug-encapsulated polymeric nanoparticles | The deformation of the enlarged surface area of microdepot under stretching of elastomer substrate facilitates the drug release | Tensile strain can effectively stimulate the release of DOX, with an increase in the release amount at a higher strain. An interval of 4 h was sufficient for the drug to “recharge” in the microdepots | • Skin-mountable device for treating skin diseases • Delivery of drugs to the body through transcutaneous administration • Integrated with | [26] |
| Highly textured superhydrophobic electrosprayed microparticle coatings with a cellulose/polyester core | Stretching induces formation of periodic parallel cracks in coating, leading to more exposure of the core surface to water | The release of entrapped cisplatin and 7-ethyl-10-hydroxycamptothecin was controlled by the magnitude of applied strain. Strain-dependent release rates depended on the lipophilicity of the drugs | Drug delivery applications to the tissue where periodic mechanical forces are generated | [27] |
| A drug-loaded polyurethane layer coated with a titanium layer | Under tensile strain, micro-sized cracks are generated and propagated in the titanium layer, acting as a channel for the drug molecules to diffuse into the surroundings | The amount of released drug increases with the applied strain. The drug release profile has a highly linear correlation with the ‘ratio of the exposed region under strain’ | Drug-releasing or -eluting stents. Self-administered drug delivery system for patients in emergency | [28] |
| Ceramic composite sponge (CCS) | The resilient nature and hierarchical pore structure allow liquids to flow in and out of CCS under cyclic compressive strains. Water content and strain are two logic control gates of release. | Achieved precise and logical release of both hydrophobic and hydrophilic molecules (about 100 nanogram/cycle) and fibroblasts (about 1400 cells/cycle) in proportion to the strains | Tissue engineering scaffold for bone, cartilage, blood vessel, muscle and myocardium repair by precise release of drugs or cells | [30] |
| Amide-bearing 1,3-dipalmitami dopropan-2-yl 2-(trimethylammonio)ethyl phosphate (Pad-PC-Pad) phospholipid vesicles | Under fluid shear stress, the lenticular shape of the liposomes leads to preferential breaking points along the vesicle’s equator | Pad-PC-Pad vesicles preferentially release drugs in response to high shear stress | Treating cardiovascular diseases in a targeted manner | [32] |
| Aggregates of multiple nanoparticles (NPs) immobilized with the tissue plasminogen activator | Microscale aggregates of nanoparticles break up into nanoscale components when exposed to abnormally high fluid shear stress | Pathological levels of shear caused large increase in the disintegration of the microscale aggregates into NPs compared to the physiological levels of shear | Thrombolytic therapies with high safety for administration of clot-busting drugs to patients with life-threatening clots | [34] |
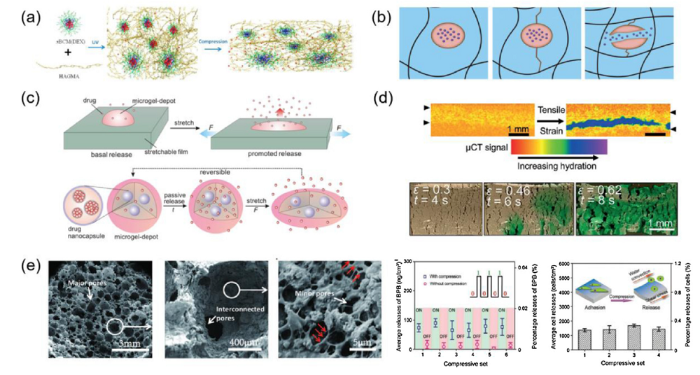
Hydrogels incorporated with mechano-sensitive components have been reported to realize an active, precise drug release. For instance, when the cartilage underwent compression during daily activities, a hyaluronic acid hydrogel with mechano-sensitive dexamethasone-loaded block copolymer micelles (BCMs) was able to consistently release dexamethasone in a controllable manner for pain management in osteoarthritic patients (Fig. 2(a)) [23]. Hydrogel covalently linked with the cargo-filled mechano-sensitive liposomes released the cargo under a compression force through the rupture of liposomes (Fig. 2(b)) [24]. Poly(acrylamide)-based networks covalently incorporated with the drug-laden mechano-sensitive BCMs achieved a dynamic tension-induced drug release through the recoverable deformation of BCMs [25]. Also, alginate microgels containing drug-encapsulated polymeric nanoparticles were imbedded in a highly stretchable elastomer to achieve a precise drug release proportional to the degree of the applied strain (Fig. 2(c)) [26].
Fig. 2.
Fig. 2.
Strain-sensitive mechano-active drug delivery systems. (a) Hyaluronic acid hydrogels with a compression-modulated dexamethasone release capacity (reprinted with permission from [23]); (b) A hydrogel trapped with drug-loaded liposomes, which releases drugs through the rupture of the liposomes under proper level of stress (reprinted with permission from [24]); (c) Stretching-triggered drug release from the nanoparticles into microdepots on an elastomeric substrate (reprinted with permission from [26]); (d) Crack development in the superhydrophobic barrier coating on a hydrophilic mesh core when stretched (reprinted with permission from [27]); (e) A ceramic-starch composite sponge (CCS) with a hierarchically porous structure, demonstrating a mechano-active delivery of molecules and cells with high precision (reprinted with permission from [30]).
The stress-induced crack propagation has also been utilized for spatially-controlled release of drugs or biologics. For instance, a mechano-active composite, consisting of a hydrophilic mesh core and superhydrophobic layers with wetting resistance, was capable of releasing drug upon a stretch-induced cracking (Fig. 2(d)) [27]. Similarly, a membrane that consisted of a drug-loaded polyurethane substrate and a titanium coating was able to actively release drugs through micro-sized cracks in the metallic layer under appropriate level of tensile strains [28]. Another approach for achieving tensile stress-induced release is to control the thickness of the barrier layer. By using this strategy, composites that were composed of an inelastic hydrophilic core and a superhydrophobic outer coating achieved a mechano-active agent release under tensile strains [29].
Recently, our group developed a porous elastic mechano-active platform, the ceramic-starch composite sponge (CCS) with a hierarchically porous structure mimicking sea sponge, to achieve precise and logical release of both hydrophobic and hydrophilic molecules (in a precision of about 100 ng) and cells (in a precision of about 1400 cells) proportional to the cyclic compressive strains (Fig. 2(e)) [30]. Water content and compressive strain works as two AND logic gates mediating the release of cargo preserved on the pore wall of CCS. While the logic condition is met, the precise release of these cargos can be repeatedly achived by controlling the cyclic compressive strains.
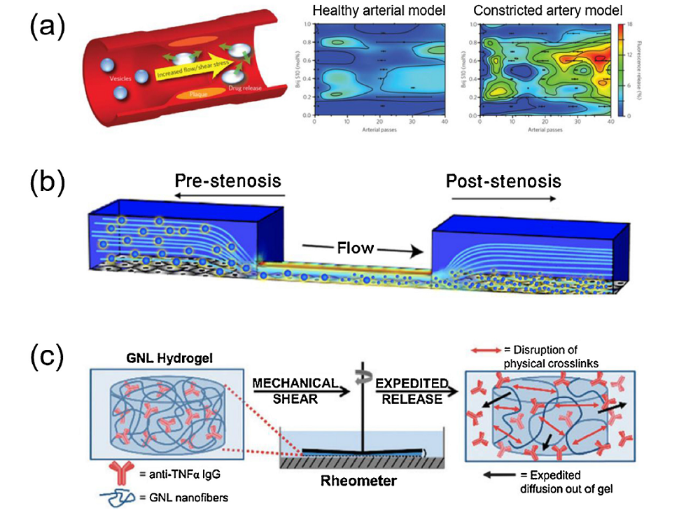
Atherosclerosis results in the narrowing of arterial blood vessels and causes significant increase in the endogenous shear stress [31], which has been found to be a periodic mechanical stimuli that can be used by shear-sensitive materials, such as nanoassemblies or microaggregates, for precise drug delivery (Fig. 3(a) and (b)) [32,33]. Microaggregates of nanoparticles which remained intact in a normal physiological flow can disintegrate into individual nanocomponents under high shear force and subsequently adhere to and accumulate at the stenotic regions (Fig. 3(b)) [33]. This shear-sensitive microaggregates have been combined with a temporary endovascular bypass for smart release of the recombinant tissue-type plasminogen activator (r-tPA). This approach can be used to concentrate r-tPA at the occlusion sites with stents, which achieves high rates of complete recanalization [34]. Hydrogels exhibiting a shear-thinning behavior and reversible mechanically-triggered sol-gel transitions have also been shown to realize a shear-sensitive release of anti-TNFα (Fig. 3(c)), bearing great potential for treating autoimmune diseases such as rheumatoid arthritis [35].
Fig. 3.
Fig. 3.
Shear-sensitive mechano-active drug delivery systems. (a) Shear-sensitive lenticular vesicles made from an artificial 1,3-diaminophospholipid which are stable under static conditions but release their contents at elevated shear stress via preferential breaking points along the vesicle’s equator (reprinted with permission from [32]). (b) Microaggregates of nanoparticles (large spheres) remain intact in the pre-stenotic region, but then disintegrate into nanoparticles (small spheres) when they flow through a constriction and accumulate in the endothelial cells (reprinted with permission from [33]); (c) A hydrogel with a shear-mediated release of anti-TNFα (reprinted with permission from [35]).
3.2. Mechano-active biomaterials for reconstructing tissue mechanical microenvironment
Biomaterial scaffolds have demonstrated a great potential for reconstruction of the mechanical environment at tissue and organ level. For instance, recent work has shown that the tissue-matched stiffness of a porous scaffold is important for maintaining the bone mechanical homeostasis and promoting the bone growth in the interior and at the periphery of implants [[36], [37], [38]]. However, the inert mechanical behavior of mechano-passive biomaterials cannot effectively restore the dynamic mechanical environment in tissues and may even cause a tissue injury. For instance, the mismatch of pressure-dependent mechanical properties between native arteries and mechano-passive artificial grafts have been shown to induce hydrodynamic flow disturbances and stress concentration [39], causing further tissue and cell damages [40]. In contrast, mechano-active biomaterials can be designed to constantly adapt to local mechanical stimuli and possess the functionality of reconstructing the original local mechanical environment at lesion site. For example, Matsuda et al. fabricated a bi-layer tubular mesh that could mechanically respond to pulsatile stress in a similar way to that by the native artery, which pulsates synchronously via responding to a pulsatile flow [41].
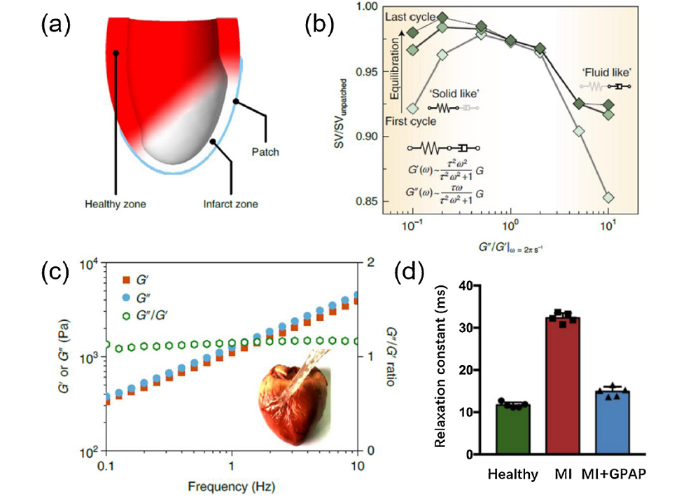
After myocardial infarction, the mechanical properties of the myocardium at the infarction zone change dramatically [42], and the affected ventricular wall enlarges and becomes thinner. Using biomaterials to mechanically strengthen the infarction zone have been attempted for decades [43]. However, most of the materials could only act as a passive barrier for preventing the dilation and had limited efficacy [44]. Our recent work suggests that an ideal cardiac patch for the reinforcement of the heart's outer surface may dynamically adjust its mechanical properties so as to adapt to varying deformation in the ventricular wall. Guided by computational modelling, a gel-point adhesive hydrogel patch (GPAP) possessing balanced viscoelasticity was developed (Fig. 4) [44]. The GPAP not only adapted to the heart’s beating but also actively interacted with the myocardium under the dynamic endogenous mechanical environment of heart. GPAP revealed a nearly optimal therapeutic efficacy for treating myocardial infarction compared to other acellular patches. Besides, recently developed bioinspired self-strengthening hydrogels under cyclic mechanical loading [45,46] also hold promise for reconstructing the dynamic mechanical microenvironment of muscles.
Fig. 4.
Fig. 4.
A finite-element simulation optimized gel-point adhesive starch hydrogel as a mechano-active epicardial patch for treating a myocardial infarction [44]. (a) Illustration of the finite-element simulation model for the epicardial patch; (b) Effect of viscous dissipation with a Maxwell viscoelastic patch on stroke volume, pointing the optimized effect when having G"/G′ ratio ∼ 1; (c) Dependence of G′, G′′ and the G"/G′ ratio of the gel-point adhesive epicardial patch (GPAP) on the frequency of oscillation, showing a gel-point viscoelastic characteristic. The GPAP not only accommodates the cyclic deformation of the myocardium but also actively interacted with it to reconstruct normal mechanical environment. Inset shows a stretched GPAP film adhering firmly on a pig epicardium; (d) GPAP significantly decreased the left ventricular relaxation time constant, indicating markedly improved biomechanical function of the left ventricle when the GPAP was applied after a myocardial infarction. (a, b, c and d reprinted with permission from [44]).
3.3. Accelerating tissue remodeling or healing process by mechanobiological effect
As the mechanobiological effect on tissue hemostasis and growth as well as related mechanisms have been recently revealed, biomimetic mechano-active biomaterials that can accommodate or utilize important mechanical cues have a high likelihood to establish a direct correlation with tissue remodeling or healing process via the mechanobiological effect [[47], [48], [49]]. Stress-relaxation and stress-stiffening biomaterials are two potential mechano-active candidates in this area and both have demonstrated regulative effects on the fate of stem cells [50,51]. For instance, migration, proliferation, and osteogenic differentiation of the mesenchymal stem cells cultured in gels have been reported to be mediated by the relaxation behavior of gel [50]. In another study, scaffolds with a non-linear elasticity could regulate the differentiation of human mesenchymal stem cells [51]. Several in vivo trials also demonstrated the promise of emerging mechano-active biomaterials for tissue repair and regeneration. For instance, a poly(L‐lactide‐co‐ε‐caprolactone) (PLCL) sponge with cartilage-mimetic viscoelastic properties in a dynamic mechanical environment was used to fabricated scaffold laden with bone marrow-derived mesenchymal stem cells (BMSCs). When filled in the large, confined osteochondral defects at weight-bearing sites of adult rabbit knee joints, the BMSC-seeded PLCL constructs could withstand physiologic stress loading and remodel over 6 months into osteochondral tissue with characteristically functionality [52]. Furthermore, an alginate scaffold with well-defined rheological properties induced an in-growth of the nucleus pulposus (NP) progenitor and provided an appropriate mechanobiological effect for an NP regeneration [53]. In addition, the emerging mechano-active topography and adhesive patterns [54,55] are also promising for promoting cellular reactions via mechanobiological effects. For example, by controlling crack formation with force, it is possible to generate ordered micro/nano patterns over large areas on a wide variety of materials from soft polymers to crystalline silicon [54], which mimics biological environments at cellular or even single-molecule scales and modulates cellular reaction via mechanobiological effects. When uniaxial stretching was applied on a poly(N-isopropylacrylamide) (PIPAAm) gel-grafted polydimethylsiloxane (PIPAAm-PDMS), the grafted PIPAAm gel surface was modulated to be thinner and the nanoscale aggregates of the grafted PIPAAm gel deformed [55], leading to that the stretched PIPAAm-PDMS became more cell adhesive than the unstretched PIPAAm-PDMS at 37 °C. Thus this PIPAAm-PDMS system could be used as a platform to promote cellular reaction via controlling mechanical cues to cells.
4. Summary and prospects
This article profiles a new type of smart biomaterials that can actively respond to the mechanical stimuli in vivo and elucidates the perspective and examples regarding the rationale of promoting tissue repair and regeneration by the so-called mechano-active biomaterials. In contrast to traditional biomaterials, which passively react to the mechanical environment of an implantation site, mechano-active biomaterials are able to consistently adapt to the local mechanical environment and have their functionality that is dynamically mediated by the mechanical environment. Mechano-active biomaterials have already demonstrated promising potential in the repair of musculoskeletal and cardiovascular tissues by enabling various functionalities to promote tissue repair or regeneration.
There are certainly challenges in this newly emerged area. First, the concept and rationale of mechano-active biomaterials for tissue engineering or repair needs further development and understanding, in which theoretical modeling and simulation approaches will be helpful [44]. Second, the underlying principles of combining mechanical mediation and biochemical strategies to enhance the mechano-active biomaterials’ capability for tissue repair needs to be elucidated. So far, only a few attempts have been reported, such as the mechano-active material system for reversibly controlling an enzyme-assisted biocatalysis activation [56,57]. Third, the design and fabrication of mechano-active biomaterials needs more accessible and feasible strategies or methods. Nevertheless, albeit outstanding challenges and unknowns, the emergence of mechano-active materials is opening up new strategic avenues for tissue engineering and repair.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (Nos. 81622032, 31801585, 81930070 and 51672184), Suzhou Science and Technology Project (No. SYS2019022), Natural Science Foundation of Jiangsu Province (Nos. BK20180837 and BK20181045), Postdoctoral Science Foundation (No. 2018T110546), Natural Science Research of Jiangsu Higher Education Institutions (No. 17KJA180011), and the Priority Academic Program Development of Jiangsu High Education Institutions (PAPD).
Reference
DOI
URL
PMID
[Cited within: 3]

A growing body of evidence suggests that mechanical signals emanating from the cell's microenvironment are fundamental regulators of cell behaviour. Moreover, at the macroscopic scale, the influence of forces, such as the forces generated by blood flow, muscle contraction, gravity and overall tissue rigidity (for example, inside of a tumour lump), is central to our understanding of physiology and disease pathogenesis. Still, how mechanical cues are sensed and transduced at the molecular level to regulate gene expression has long remained enigmatic. The identification of the transcription factors YAP and TAZ as mechanotransducers started to fill this gap. YAP and TAZ read a broad range of mechanical cues, from shear stress to cell shape and extracellular matrix rigidity, and translate them into cell-specific transcriptional programmes. YAP and TAZ mechanotransduction is critical for driving stem cell behaviour and regeneration, and it sheds new light on the mechanisms by which aberrant cell mechanics is instrumental for the onset of multiple diseases, such as atherosclerosis, fibrosis, pulmonary hypertension, inflammation, muscular dystrophy and cancer.
DOI
URL
PMID
[Cited within: 2]

15 dyne/cm2) induces endothelial quiescence and an atheroprotective gene expression profile, while low shear stress (
DOI URL PMID [Cited within: 1]
DOI
URL
PMID
[Cited within: 1]

Mechanically-activated delivery systems harness existing physiological and/or externally-applied forces to provide spatiotemporal control over the release of active agents. Current strategies to deliver therapeutic proteins and drugs use three types of mechanical stimuli: compression, tension, and shear. Based on the intended application, each stimulus requires specific material selection, in terms of substrate composition and size (e.g., macrostructured materials and nanomaterials), for optimal in vitro and in vivo performance. For example, compressive systems typically utilize hydrogels or elastomeric substrates that respond to and withstand cyclic compressive loading, whereas, tension-responsive systems use composites to compartmentalize payloads. Finally, shear-activated systems are based on nanoassemblies or microaggregates that respond to physiological or externally-applied shear stresses. In order to provide a comprehensive assessment of current research on mechanoresponsive drug delivery, the mechanical stimuli intrinsically present in the human body are first discussed, along with the mechanical forces typically applied during medical device interventions, followed by in-depth descriptions of compression, tension, and shear-mediated drug delivery devices. We conclude by summarizing the progress of current research aimed at integrating mechanoresponsive elements within these devices, identifying additional clinical opportunities for mechanically-activated systems, and discussing future prospects.
DOI
URL
PMID
[Cited within: 1]

Human tissues are sophisticated ensembles of various distinct cell types encapsulated in the biomechanical cues of the extracellular matrix. It has been known matrix stiffness plays a pivot role in cellular events and tissue-scale biological processes. Thus, materials that can mimic mechanical environments of tissues in vitro and possess wide, physiologically relevant elasticity are highly desirable. Hydrogels provide a good cell platform to mimic native cellular environment. However, the limited stiffness tunability, and hinders the efforts to reproduce the biomechanical microenvironment of many in vivo progresses. These problems have been addressed by the recently emerged great quantity of exquisitely designed smart hydrogels. Smart hydrogels that respond sensitively to external stimuli are good choices due to the convenience in regulating their mechanical properties. In this review, we summarize the latest progress in the development of stimuli-responsive hydrogels as a cell carrier (platform for cell culture) which spans a wide range of stiffness. Different kinds of smart hydrogels corresponding to various stimuli, including pH, temperature, light, metal ions, and forces, are introduced and their stiffness modulation through physicochemical procedures are reported.
DOI
URL
PMID
[Cited within: 3]

Synthetic hydrogels containing covalently integrated soft and deformable drug depots capable of releasing therapeutic molecules in response to mechanical forces are attractive candidates for the treatment of degenerated tissues that are normally load bearing. Herein, radically cross-linkable block copolymer micelles (xBCM) assembled from an amphiphilic block copolymer consisting of hydrophilic poly(acrylic acid) (PAA) partially modified with 2-hydroxyethyl acrylate, and hydrophobic poly(n-butyl acryclate) (PnBA) were employed as the drug depots and the microscopic cross-linkers for the preparation of hyaluronic acid (HA)-based, hydrogels. HA hydrogels containing covalently integrated micelles (HAxBCM) were prepared by radical polymerization of glycidyl methacrylate (GMA)-modified HA (HAGMA) in the presence of xBCMs. When micelles prepared from the parent PAA-b-PnBA without any polymerizable double bonds were used, hydrogels containing physically entrapped micelles (HApBCM) were obtained. The addition of xBCMs to a HAGMA precursor solution accelerated the gelation kinetics and altered the hydrogel mechanical properties. The resultant HAxBCM gels exhibit an elastic modulus of 847 +/- 43 Pa and a compressive modulus of 9.2 +/- 0.7 kPa. Diffusion analysis of Nile Red (NR)-labeled xBCMs employing fluorescence correlation spectroscopy confirmed the covalent immobilization of xBCMs in HA networks. Covalent integration of dexamethasone (DEX)-loaded xBCMs in HA gels significantly reduced the initial burst release and provided sustained release over a prolonged period. Importantly, DEX release from HAxBCM gels was accelerated by intermittently applied external compression in a strain-dependent manner. Culturing macrophages in the presence of DEX-releasing HAxBCM gels significantly reduced cellular production of inflammatory cytokines. Incorporating mechano-responsive modules in synthetic matrices offers a novel strategy to harvest mechanical stress present in the healing wounds to initiate tissue repair.
DOI
URL
PMID
[Cited within: 2]

Block copolymer micelles (BCMs) were prepared from amphiphilic diblock copolymers of poly(n-butyl acrylate) and poly(acrylic acid) partially modified with 2-hydroxyethyl acrylate. Radical polymerization of acrylamide in the presence of micellar crosslinkers gave rise to elastomeric hydrogels (BCM-PAAm) whose mechanical properties can be tuned by varying the BCM composition. Transmission electron microscopy (TEM) imaging revealed stretch-induced, reversible micelle deformation in BCM-PAAm gels. A model hydrophobic drug, pyrene, loaded into the micelle core prior to the formation of BCM-PAAm gels, was dynamically released in response to externally applied mechanical forces. The BCM-crosslinked hydrogels with combined strength and force-modulated drug release are attractive candidates for the repair and regeneration of mechanically-active tissues.
DOI
URL
PMID
[Cited within: 3]

Mechanical force-based stimulus provides a simple and easily accessible manner for spatiotemporally controlled drug delivery. Here we describe a wearable, tensile strain-triggered drug delivery device consisting of a stretchable elastomer and microgel depots containing drug loaded nanoparticles. By applying a tensile strain to the elastomer film, the release of drug from the microdepot is promoted due to the enlarged surface area for diffusion and Poisson's ratio-induced compression on the microdepot. Correspondingly, both sustained drug release by daily body motions and pulsatile release by intentional administration can be conveniently achieved. Our work demonstrated that the tensile strain, applied to the stretchable device, facilitated release of therapeutics from microdepots for anticancer and antibacterial treatments. Moreover, polymeric microneedles were further integrated with the stretch-responsive device for transcutaneous delivery of insulin and regulation of blood glucose levels of chemically induced type 1 diabetic mice.
DOI
URL
PMID
[Cited within: 1]

Platelet aggregation at sites of vascular injury is essential for hemostasis and arterial thrombosis. It has long been assumed that platelet aggregation and thrombus growth are initiated by soluble agonists generated at sites of vascular injury. By using high-resolution intravital imaging techniques and hydrodynamic analyses, we show that platelet aggregation is primarily driven by changes in blood flow parameters (rheology), with soluble agonists having a secondary role, stabilizing formed aggregates. We find that in response to vascular injury, thrombi initially develop through the progressive stabilization of discoid platelet aggregates. Analysis of blood flow dynamics revealed that discoid platelets preferentially adhere in low-shear zones at the downstream face of forming thrombi, with stabilization of aggregates dependent on the dynamic restructuring of membrane tethers. These findings provide insight into the prothrombotic effects of disturbed blood flow parameters and suggest a fundamental reinterpretation of the mechanisms driving platelet aggregation and thrombus growth.
DOI
URL
PMID
[Cited within: 3]

Atherosclerosis results in the narrowing of arterial blood vessels and this causes significant changes in the endogenous shear stress between healthy and constricted arteries. Nanocontainers that can release drugs locally with such rheological changes can be very useful. Here, we show that vesicles made from an artificial 1,3-diaminophospholipid are stable under static conditions but release their contents at elevated shear stress. These vesicles have a lenticular morphology, which potentially leads to instabilities along their equator. Using a model cardiovascular system based on polymer tubes and an external pump to represent shear stress in healthy and constricted vessels of the heart, we show that drugs preferentially release from the vesicles in constricted vessels that have high shear stress.
DOI
URL
PMID
[Cited within: 3]

Obstruction of critical blood vessels due to thrombosis or embolism is a leading cause of death worldwide. Here, we describe a biomimetic strategy that uses high shear stress caused by vascular narrowing as a targeting mechanism--in the same way platelets do--to deliver drugs to obstructed blood vessels. Microscale aggregates of nanoparticles were fabricated to break up into nanoscale components when exposed to abnormally high fluid shear stress. When coated with tissue plasminogen activator and administered intravenously in mice, these shear-activated nanotherapeutics induce rapid clot dissolution in a mesenteric injury model, restore normal flow dynamics, and increase survival in an otherwise fatal mouse pulmonary embolism model. This biophysical strategy for drug targeting, which lowers required doses and minimizes side effects while maximizing drug efficacy, offers a potential new approach for treatment of life-threatening diseases that result from acute vascular occlusion.
DOI
URL
PMID
[Cited within: 2]

1000; P=0.0231), intra-arterial r-tPA alone (odds ratio 65.019, 95% confidence interval 1.77, >1000; P=0.0231), or TEB with soluble r-tPA (2 mg; odds ratio 18.78, 95% confidence interval 1.28, 275.05; P=0.0322). Histological analysis showed circumferential loss of endothelium restricted to the area where the TEB was deployed; however, there was significantly less vascular injury using a TEB as compared with stent-retriever procedure (odds ratio 12.97, 95% confidence interval 8.01, 21.02; P
DOI
URL
PMID
[Cited within: 1]

Three-dimensional (3D) titanium-mesh scaffolds offer many advantages over autologous bone grafting for the regeneration of challenging large segmental bone defects. Our study supports the hypothesis that endogenous bone defect regeneration can be promoted by mechanobiologically optimized Ti-mesh scaffolds. Using finite element techniques, two mechanically distinct Ti-mesh scaffolds were designed in a honeycomb-like configuration to minimize stress shielding while ensuring resistance against mechanical failure. Scaffold stiffness was altered through small changes in the strut diameter only. Honeycombs were aligned to form three differently oriented channels (axial, perpendicular, and tilted) to guide the bone regeneration process. The soft scaffold (0.84 GPa stiffness) and a 3.5-fold stiffer scaffold (2.88 GPa) were tested in a critical size bone defect model in vivo in sheep. To verify that local scaffold stiffness could enhance healing, defects were stabilized with either a common locking compression plate that allowed dynamic loading of the 4-cm defect or a rigid custom-made plate that mechanically shielded the defect. Lower stress shielding led to earlier defect bridging, increased endochondral bone formation, and advanced bony regeneration of the critical size defect. This study demonstrates that mechanobiological optimization of 3D additive manufactured Ti-mesh scaffolds can enhance bone regeneration in a translational large animal study.
DOI
URL
PMID
[Cited within: 1]

This study explored the regenerative osteogenic response in the distal femur of sheep using scaffolds having stiffness values within, and above and below, the range of trabecular bone apparent modulus. Scaffolds 3D-printed from stiff titanium and compliant polyamide were implanted into a cylindrical metaphyseal defect 15x15mm. After six weeks, bone ingrowth varied between 7 and 21% of the scaffold pore volume and this was generally inversely proportional to scaffold stiffness. The individual reparative response considerably varied among the animals, which could be divided into weak and strong responders. Notably, bone regeneration specifically within the interior of the scaffold was inversely proportional to scaffold stiffness and was strain-driven in strongly-responding animals. Conversely, bone regeneration at the periphery of the defect was injury-driven and equal in all scaffolds and in all strongly- and weakly-responding animals. The observation of the strain-driven response in some, but not all, animals highlights that scaffold compliance is desirable for triggering host bone regeneration, but scaffold permanence is important for the load-bearing, structural role of the bone-replacing device. Indeed, scaffolds may benefit from being nonresorbable and mechanically reliable for those unforeseeable cases of weakly responding recipients.
DOI
URL
PMID
[Cited within: 1]

An increasing volume of work supports utilising the mechanobiology of bone for bone ingrowth into a porous scaffold. However, typically during in vivo testing of implants, the mechanical properties of the bone being replaced are not quantified. Consequently there remains inconsistencies in the literature regarding 'optimum' pore size and porosity for bone ingrowth. It is also difficult to compare ingrowth results between studies and to translate in vivo animal testing to human subjects without understanding the mechanical environment. This study presents a clinically applicable approach to determining local bone mechanical properties and design of a scaffold with similar properties. The performance of the scaffold was investigated in vivo in an ovine model. The density, modulus and strength of trabecular bone from the medial femoral condyle from ovine bones was characterised and power-law relationships were established. A porous titanium scaffold, intended to maintain bone mechanical homeostasis, was additively manufactured and implanted into the medial femoral condyle of 6 ewes. The stiffness of the scaffold varied throughout the heterogeneous structure and matched the stiffness variation of bone at the surgical site. Bone ingrowth into the scaffold was 10.73+/-2.97% after 6 weeks. Fine woven bone, in the interior of the scaffold, and intense formations of more developed woven bone overlaid with lamellar bone at the implant periphery were observed. The workflow presented will allow future in vivo testing to test specific bone strains on bone ingrowth in response to a scaffold and allow for better translation from in vivo testing to commercial implants.
DOI
URL
PMID
[Cited within: 1]

The development of intimal hyperplasia near the anastomosis of a vascular graft to an artery may be related to changes in the wall shear rate distribution. Mismatches in compliance and diameter at the end-to-end anastomosis of a compliant artery and a rigid graft cause shear rate disturbances that may induce intimal hyperplasia and ultimately graft failure. The goal of this study is to determine how compliance mismatch, diameter mismatch, and impedance phase angle affect the wall shear rate distribution in end-to-end anastomosis models under sinusoidal flow conditions. Wall shear rates are obtained through flow visualization using a photochromic dye. In a model with a well-matched graft diameter (6% undersized), the compliance mismatch causes low mean wall shear rates near the distal anastomosis. Considering diameter mismatch, the wall shear rate distributions in 6% undersized, 16% undersized, and 13% oversized graft models are markedly different at similar phase angles. In the two undersized graft models, the minimum mean shear rate occurs near the distal anastomosis, and this minimum is lower in the model with greater diameter mismatch. The oversized graft model has a minimum mean shear rate near the proximal anastomosis. Thus in all three models, the minimum mean wall shear rate is observed at the site of the divergent geometry. The impedance phase angle, which can be altered by disease states and vasoactive drugs, has a minor effect on the wall shear rate amplitude far from the anastomosis but a more pronounced effect closer to the anastomosis. Mean wall shear rates under sinusoidal flow conditions are significantly lower than under steady flow conditions at the same mean flow rate, but they are fairly insensitive to phase angle changes. In order to avoid the divergent geometry that may cause lower wall shear rates, we recommend that compliance mismatch be minimized whenever possible and that graft diameter be chosen to match the arterial diameter at the relevant physiologic pressure, not at the reduced pressure present when the graft is implanted.
DOI
URL
PMID
[Cited within: 1]

Clinical results from medium- and small-caliber arterial bypass grafts are unsatisfactory. Since elastic properties of grafts tested experimentally have been correlated with patency results, the compliance of the human femoral artery was compared with grafts currently in use: human saphenous vein (HSV), knitted Dacron (DAC), glutaraldehyde-treated umbilical cord vein (DBM), bovine heterograft, and expanded polytetrafluoroethylene (PTFE). This was correlated with clinical patency data for the different conduits in the femoropopliteal position. Increased patency correlated with a decreasing disparity between host artery and graft compliance. After two years, patency rates of the more compliant materials (HSV, DBM) exceeded 80%, while less than 45% of the incompliant grafts (DAC, PTFE) remained patent. Thus, clinical performance with synthetic grafts might be improved by use of prostheses in which the viscoelastic characteristics match those of arteries more closely.
DOI
URL
PMID
[Cited within: 1]

To design a
DOI
URL
PMID
[Cited within: 1]

Living tissues, such as muscle, autonomously grow and remodel themselves to adapt to their surrounding mechanical environment through metabolic processes. By contrast, typical synthetic materials cannot grow and reconstruct their structures once formed. We propose a strategy for developing
DOI
URL
PMID
[Cited within: 1]

Mechanical signals are increasingly recognized as overarching regulators of cell behaviour, controlling stemness, organoid biology, tissue development and regeneration. Moreover, aberrant mechanotransduction is a driver of disease, including cancer, fibrosis and cardiovascular defects. A central question remains how cells compute a host of biomechanical signals into meaningful biological behaviours. Biomaterials and microfabrication technologies are essential to address this issue. Here we review a large body of evidence that connects diverse biomaterial-based systems to the functions of YAP/TAZ, two highly related mechanosensitive transcriptional regulators. YAP/TAZ orchestrate the response to a suite of engineered microenviroments, emerging as a universal control system for cells in two and three dimensions, in static or dynamic fashions, over a range of elastic and viscoelastic stimuli, from solid to fluid states. This approach may guide the rational design of technological and material-based platforms with dramatically improved functionalities and inform the generation of new biomaterials for regenerative medicine applications.
DOI
URL
PMID
[Cited within: 1]

Synthetic hydrogels have ideal physiochemical properties to serve as reductionist mimics of the extracellular matrix (ECM) for studies on cellular mechanosensing. These studies range from basic observation of correlations between ECM mechanics and cell fate changes to molecular dissection of the underlying mechanisms. Despite intensive work on hydrogels to study mechanobiology, many fundamental questions regarding mechanosensing remain unanswered. In this review, I first discuss historical motivation for studying cellular mechanobiology, and challenges impeding this effort. I next overview recent efforts to engineer hydrogel properties to study cellular mechanosensing. Finally, I focus on in vitro modeling and cell-based therapies as applications of hydrogels that will exploit our ability to create micro-environments with physiologically relevant elasticity and viscoelasticity to control cell biology. These translational applications will not only use our current understanding of mechanobiology but will also bring new tools to study the fundamental problem of how cells sense their mechanical environment. STATEMENT OF SIGNIFICANCE: Hydrogels are an important tool for understanding how our cells can sense their mechanical environment, and to exploit that understanding in regenerative medicine. In the current review, I discuss historical work linking mechanics to cell behavior in vitro, and highlight the role hydrogels played in allowing us to understand how cells monitor mechanical cues. I then highlight potential translational applications of hydrogels with mechanical properties similar to those of the tissues where cells normally reside in our bodies, and discuss how these types of studies can provide clues to help us enhance our understanding of mechanosensing.
DOI
URL
PMID
[Cited within: 1]

Extracellular biophysical cues have a profound influence on a wide range of cell behaviors, including growth, motility, differentiation, apoptosis, gene expression, adhesion, and signal transduction. Cells not only respond to definitively mechanical cues from the extracellular matrix (ECM) but can also sometimes alter the mechanical properties of the matrix and hence influence subsequent matrix-based cues in both physiological and pathological processes. Interactions between cells and materials in vitro can modify cell phenotype and ECM structure, whether intentionally or inadvertently. Interactions between cell and matrix mechanics in vivo are of particular importance in a wide variety of disorders, including cancer, central nervous system injury, fibrotic diseases, and myocardial infarction. Both the in vitro and in vivo effects of this coupling between mechanics and biology hold important implications for clinical applications.
DOI
URL
PMID
[Cited within: 1]

Alginate is frequently studied as a scaffold for intervertebral disc (IVD) repair, since it closely mimics mechanical and cell-adhesive properties of the nucleus pulposus (NP) of the IVD. The aim of this study was to assess the relation between alginate concentration and scaffold stiffness and find preparation conditions where the viscoelastic behaviour mimics that of the NP. In addition, we measured the effect of variations in scaffold stiffness on the expression of extracellular matrix molecules specific to the NP (proteoglycans and collagen) by native NP cells. We prepared sample discs of different concentrations of alginate (1%-6%) by two different methods, diffusion and in situ gelation. The stiffness increased with increasing alginate concentration, while the loss tangent (dissipative behaviour) remained constant. The diffusion samples were ten-fold stiffer than samples prepared by in situ gelation. Sample discs prepared from 2% alginate by diffusion closely matched the stiffness and loss tangent of the NP. The stiffness of all samples declined upon prolonged incubation in medium, especially for samples prepared by diffusion. The biosynthetic phenotype of native cells isolated from NPs was preserved in alginate matrices up to 4 weeks of culturing. Gene expression levels of extracellular matrix components were insensitive to alginate concentration and corresponding matrix stiffness, likely due to the poor adhesiveness of the cells to alginate. In conclusion, alginate can mimic the viscoelastic properties of the NP and preserve the biosynthetic phenotype of NP cells but certain limitations like long-term stability still have to be addressed.
DOI
URL
PMID
[Cited within: 2]

While fracture is generally considered to be undesirable in various manufacturing processes, delicate control of fracture can be successfully implemented to generate structures at micro/nano length scales. Fracture-based fabrication techniques can serve as a template-free manufacturing method, and enables highly-ordered patterns or fluidic channels to be formed over large areas in a simple and cost-effective manner. Such technologies can be leveraged to address biologically-relevant problems, such as in the analysis of biomolecules or in the design of culture systems that imitate the cellular or molecular environment. This mini review provides an overview of current fracture-guided fabrication techniques and their biological applications. We first survey the mechanical principles of fracture-based approaches. Then we describe biological applications at the cellular and molecular levels. Finally, we discuss unique advantages of the different system for biological studies.
DOI
URL
PMID
[Cited within: 2]

Stretchable temperature-responsive cell culture surfaces composed of poly( N-isopropylacrylamide) (PIPAAm) gel-grafted polydimethylsiloxane (PIPAAm-PDMS) were prepared to demonstrate that dual stimulation of temperature and mechanical stress extensively altered graft polymer thickness, surface wettability, and cell detachment behavior. The PIPAAm-PDMS surface was hydrophilic and hydrophobic below and above the lower critical solution temperature, respectively, which was ascribed to the phase transition of PIPAAm chains. When uniaxial stretching was applied, the grafted PIPAAm gel surface was modulated to be more hydrophobic as shown by an increase in the contact angle. Atomic force microscopy observation revealed that uniaxial stretching made the grafted gel layer thinner and deformed the nanoscale aggregates of the grafted PIPAAm gel, implying extension of the PIPAAm chains. The stretched PIPAAm-PDMS became more cell adhesive than the unstretched PIPAAm-PDMS at 37 degrees C. Furthermore, dual stimulation, shrinking the already stretched PIPAAm-PDMS and decreasing the temperature, induced more rapid cell detachment than only a change in temperature did. Similarly, upon comparison with a single stimulation of a change in temperature or mechanical stress, dual stimulation accelerated cell sheet detachment and harvesting. This new stretchable and temperature-responsive culture surface can easily adjust the surface property to a different cell adhesiveness by appropriately combining each stimulus and enable the fabrication of cell sheets of various species.
DOI
URL
PMID
[Cited within: 1]

Fibronectin, like other proteins involved in mechanotransduction, has the ability to exhibit recognition sites under mechanical stretch. Such cryptic sites are buried inside the protein structure in the native fold and become exposed under an applied force, thereby activating specific signalling pathways. Here, we report the design of new active polymeric nanoassembled surfaces that show some similarities to these cryptic sites. These nanoassemblies consist of a first polyelectrolyte multilayer stratum loaded with enzymes and capped with a second polyelectrolyte multilayer acting as a mechanically sensitive nanobarrier. The biocatalytic activity of the film is switched on/off reversibly by mechanical stretching, which exposes enzymes through the capping barrier, similarly to mechanisms involved in proteins during mechanotransduction. This first example of a new class of biologically inspired surfaces should have great potential in the design of various devices aimed to trigger and modulate chemical reactions by mechanical action with applications in the field of microfluidic devices or mechanically controlled biopatches for example.




 WeChat
WeChat