1.Introduction
Corrosion of oil and natural gas pipelines has cost global industries billions of dollars and can contribute to large-scale environmental pollution. All materials are at risk for corrosion due to natural interactions with the environment. Therefore, an understanding of the factors involved in pipeline corrosion is essential for preventing future pipeline failures, providing safe and economical operation, and preserving the environment. Many studies investigating pipeline corrosion have focused on water chemistry and chemical interactions in pipelines. Factors including dissolved oxygen, pH, temperature, CO2 partial pressure, and flow rate of water in the pipeline may heavily influence corrosion [1,2].
Microorganisms may also accelerate the corrosion rate of metals, however microbiologically influenced corrosion (MIC) processes are often overlooked. Corrosive microorganisms form biofilms that adhere well on the inner surface of steel pipes, especially in the presence of nutrient-rich seawater, raw water, or sewage [3]. It has been estimated that MIC accounts for approximately 40% of corrosion-related failures in the gas industry [4]. MIC also has the potential to occur extremely rapidly. For example, a 3.5 mm thick duplex stainless-steel pipe in a new yacht rapidly corroded within three months likely due to sulfate reducing bacteria (SRB) and sulfur oxidizing bacteria (SOB) that attacked the welding zone [5]. In another case, a new 8-inch pipeline commissioned in an oil and gas field was subject to substantial internal corrosion and leakage after only 8 months due to SRB [6]. × 52 oil pipelines are also at risk for corrosion failure due to SRB [7].
One of the primary ways to evaluate the integrity of the pipelines is by hydrostatic testing, which involves testing with water pressurized to 125% of the maximum allowable operating pressure in order to detect flaws. Unfortunately, the water used during hydrostatic testing can inadvertently lead to pipe corrosion, especially if untreated water is used and left to sit in the pipe. For example, Alaska’s North Slope pipeline corroded after untreated lake water sat in the pipe for several months after hydrostatic testing [8]. Metabolically active microorganisms, particularly SRB, are thought to have played a major role in the corrosion damage [9,10]. To prevent microbial contamination during hydrostatic testing, hydrotest water may be treated with biocides, the pH may be adjusted, or water sources without sulfate may be used [11,12]. Unfortunately, these methods can be expensive, and microorganisms may still reside in treated water [13,14] or evolve mechanisms to resist biocidal agents [15].
Oil and gas pipelines are typically oxygen-free environments, therefore the microorganisms within them are predominantly anaerobes. Sulfate is a common electron acceptor in anoxic environments as well as water sources [16,17]. SRB are also naturally occurring in aquatic environments and promote corrosion by consuming hydrogen or organic compounds and reducing sulfate to hydrogen sulfide [[18], [19], [20]]. SRB are extremely resilient because they can grow or form endospores in low nutrient environments [13], form biofilms on metal surfaces [21,22], thrive in a wide range of pH and temperature conditions [11], and grow autotrophically. Although SRB are often regarded as the primary corrosive microorganisms, many other physiological groups including methanogenic archaea [[23], [24], [25], [26]], nitrate-reducing bacteria [[27], [28], [29]], acid producing bacteria [30,31], and acetogenic bacteria [32,33] are also capable of corrosion through extracellular electron transfer (EET) and/or corrosive metabolites.
It has become increasingly clear that MIC is not just due to one physiological group of microorganisms, but instead a diverse consortium which includes bacteria, archaea, and eukaryotic microbes. Yet, many MIC laboratory studies on metal alloys have been conducted using only single or slightly mixed bacterial strains [[34], [35], [36], [37], [38], [39]]. These culture studies do not reflect the complexity of the real environment [18,40], and do not provide any insight into some of the lesser understood mechanisms of biocorrosion. Development of high-throughput sequencing technologies has helped to identify microorganisms involved in corrosion processes [41,42], and these data will be invaluable for engineering new MIC prevention strategies.
The study outlined in this manuscript aimed to investigate the mechanism of corrosion in X52 oil pipelines used by the China National Petroleum Corporation (CNPC). These CNPC X52 oil pipelines had undergone hydrostatic testing with local untreated lake water. However, the pipelines corroded before they could be put into service, likely due to the corrosive water that was used and had not been removed from the pipe. To assess if this hydrostatic testing induced biocorrosion, X52 steel coupons were cut from uncorroded portions of the pipe, and treated with either groundwater (GW) and lake water (LW). MIC was screened for using surface analysis, weight loss, electrochemical measurements, and analysis of corrosion products. Microbial community composition was analyzed on the steel coupons exposed to lake water in order to gain insight into the physiological mechanisms involved in the corrosion process.
2. Experimental methods
2.1. Sample preparation
X52 steel coupons with 1 cm2 surface area were cut from an uncorroded portion of a corroded X52 pipe provided by the China National Petroleum Corporation (CNPC). Major elemental composition of the X52 steel was detected using a direct reading spectrometer (ARL 4460, Thermo Scientific™, Switzerland) according to the standard ASTM A751-14 as follows: 0.069% C, 0.190% Si, 1.150% Mn, 0.012% P, 0.004% S, 0.02% Cr and Fe for balance. The coupons were abraded to a grit of 1200 with a graded series of SiC papers.
Either groundwater or untreated lake water, taken from Shizuishan, Ningxia Hui Autonomous Region, China, were used in this study for testing. The chemical composition of GW and LW can be found in Table 1. Methods used to measure the chemical compositions of GW and LW are shown in Table S1 in Supplemental Materials.
Table 1 Chemical composition of groundwater (GW) and untreated lake water (LW) used in this study.
| Composition | Groundwater (GW) | Lake water (LW) | |
|---|---|---|---|
| Nitrate | mg/L | 0.030 | 0.036 |
| Fluoride | mg/L | 0.347 | 0.437 |
| Sulfate | mg/L | 44.4 | 236 |
| Chloride | mg/L | 84.0 | 0.09 |
| Phosphate | mg/L | 1.19 | 0.899 |
| Cadmium (Cd) | μg/L | <0.05 | 5.2 |
| Iron (Fe) | μg/L | 12.2 | 4.4 × 104 |
| Manganese (Mn) | μg/L | 2.3 | 95.6 |
| Lead (Pb) | μg/L | 1.1 | <0.09 |
| Copper (Cu) | μg/L | 2.2 | 1.65 |
| Zinc (Zn) | μg/L | 1.6 | 0.93 |
| Molybdenum (Mo) | μg/L | 0.436 | 65.3 |
| Aluminum (Al) | μg/L | 36.1 | 3.42 |
To assess if bacteria were present in GW and LW before exposure to steel coupons, bacteria were cultured using four different types of standard laboratory agar: (1) tryptic soy agar (TSA), (2) Reasoner’s 2A (R2A), (3) potato dextrose agar (PDA), and (4) rose Bengal. No isolates were obtained from GW on any of the agar plates, and the microbial concentration was below the limit of detection based on quantitative PCR, indicating the absence of microorganisms. Thus, GW was treated with antibiotics (300 μg/ml carbenicillin and 25 μg/ml irgasan) in order to prevent bacterial contamination. All corrosion tests were done at 30 °C, the first sets of coupons were immersed in either GW or LW for 14 days, and the second set for 30 days.
2.2. Microscopic analyses
Biofilms on the surface of the X52 steel coupons were visualized by field emission scanning electron microscopy (FESEM, Ultra-Plus, Zeiss, Germany). Sterile coupons were also analyzed for comparison. Coupon biofilms were fixed for 4 h in 4% (v/v) glutaraldehyde, followed by dehydration in 50%, 70%, 90%, 99%, and 100% ethanol solutions successively (10 min each). For FESEM visualization, the coupons were dried and then coated with gold. MIC morphology of coupons after removing the biofilms was also analyzed under FESEM. Biofilms were removed using Clarke’s solution (ASTM G1-90) consisting of 20 g Sb2O3, 50 g SnCl2, and 1 L 36% HCl, followed by washing with distilled water and absolute ethanol [19].
The elemental composition of corrosion products was measured by energy dispersive spectrometry (EDS). Corrosion products produced by the microbial community were also analyzed by X-ray photoelectron spectroscopy (XPS, Thermo VG, USA), using a previously described method [35]. S 2p and Fe 2p3/2 XPS were used to determine specific molecular species using the fitting parameters shown in Table S2.
2.3. Weight loss, pitting depth, and pH measurements
To determine the corrosion rates of X52 coupons exposed to GW or LW at 30 °C, weight loss was measured for the samples after incubation for 14 and 30 days with a high accuracy mass balance (ME104E, Mettler Toledo, readability 0.1 mg). Before testing, ten coupons for each assay were weighed. After immersion, attached biofilms as well as corrosion products were removed from the coupons using Clarke’s solution, and then cleaned with distilled water and absolute ethanol. The corrosion rate was calculated from weight loss based on the totally exposed surface area (specific weight loss-g/cm2) and time of exposure [43]. Morphology of the corroded coupon surface after removing the corrosion products was analyzed by confocal laser scanning microscopy (CLSM, LSM 700, Zeiss, Germany). The maximum pitting depth and average maximum pitting depth were obtained from five representative coupons. Variations in pH of test solutions were all measured before and after immersion. Initial pH of GW and LW were 7.2 ± 0.2 and 8.0 ± 0.3, respectively.
2.4. Electrochemical measurements
Electrochemical measurements were performed in a three-electrode system containing an X52 steel coupon (10 mm × 10 mm × 3 mm) as a working electrode, a platinum sheet of 1 cm2 as an auxiliary electrode, and a saturated calomel electrode (SCE) as a reference electrode [44,45]. All electrochemical measurements were taken using a Gamry Reference 600 potentiostat (Gamry Instruments Inc., USA). GW and LW corrosion tests were continuously monitored for 30 days at a temperature of 30 °C.
Open circuit potential (OCP) measurements were conducted daily until potential values stabilized. Linear polarization resistance (LPR) analysis was carried out at a potential of 15 mV vs. Eocp and a scan rate of 0.125 mV/s. To validate LPR results, electrochemical impedance spectroscopy (EIS) measurements were performed with perturbation amplitude of 5 mV vs. Eocp and a frequency between 100 kHz-0.01 Hz. Data was analyzed using ZSimpWin software (Princeton Applied Research, USA). Potential dynamic polarization measurements were used to investigate MIC of X52 steel coupons exposed to GW or LW. Polarization measurements were recorded at a scan rate of 0.5 mV/s between -0.5 to 1 V vs. Eocp.
2.5. Pyrosequencing and biofilm staining
Bacterial and archaeal communities were analyzed in the raw LW before hydrostatic testing, as well as biofilms attached to X52 steel coupon surfaces after 30 days of exposure to LW (LW1b), and biofilms attached to X52 steel coupon surfaces after 30 days of exposure to LW with addition of 2216E culture media (LW2b). Genomic DNA was extracted using the E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer’s instructions.
Bacterial 16S rRNA gene fragments in the hypervariable V3-V4 regions were amplified via polymerase chain reaction (PCR) with barcoded primer set 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC). The first PCR reaction was performed as follows: 94 °C for 3 min, followed by 5 cycles at 94 °C for 30 s, 45 °C for 20 s, and 65 °C for 30 s, then extended for 20 cycles at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, and finally 5 min at 72 °C. Total 50 μL PCR reactions consisted of: 5 μL 10 × PCR buffer, 0.5 μL dNTPs (10 mM each), 10 ng genomic DNA, 0.5 μL bar-PCR primer F (50 μM), 0.5 μL primer R (50 μM), 0.5 μL Platinum Taq DNA Polymerase (Thermo Fisher Scientific, Wilmington, DE, USA) (5 U/μL), and H2O for balance. For the second PCR reaction, an Illumina bridge PCR compatible primer was introduced followed by 95 °C for 30 s, and 5 cycles at 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s, and finally 5 min at 72 °C. The 50 μL PCR mixture consisted of 5 μL 10 × PCR buffer, 0.5 μL dNTPs (10 mM each), 20 ng genomic DNA, 0.5 μL primer F (50 μM), 0.5 μL primer R (50 μM), 0.5 μL Platinum Taq DNA Polymerase (5 U/μL), and H2O for balance.
Archaeal 16S rRNA gene fragments were amplified via PCR with barcoded primer set M-340 F (CCCTAYGGGGYGCASCAG) and GU1ST-1000R (GGCCATGCACYWCYTCTC). The first PCR reaction was performed as follows: 94 °C for 3 min, followed by 5 cycles at 94 °C for 30 s, 45 °C for 20 s, and 65 °C for 30 s, then extended for 20 cycles at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, and finally 5 min at 72 °C. 50 μL total PCR mixtures consisted of: 5 μL 10 × PCR buffer, 0.5 μL dNTPs (10 mM each), 10 ng genomic DNA, 0.5 μL bar-PCR primer F (50 μM), 0.5 μL primer R (50 μM), 0.5 μL Platinum Taq (5 U/μL), and H2O for balance. For the second PCR reaction, the first round of products in the hypervariable V3-V4 regions were amplified via PCR with barcoded primer set 349 F (GYGCASCAGKCGMGAAW) and 806R (GGACTACVSGGGTATCTAAT). The second PCR reaction was performed as follows: 94 °C for 3 min, followed by 5 cycles at 94 °C for 30 s, 45 °C for 20 s, and 65 °C for 30 s, then extended for 20 cycles at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, and finally 5 min at 72 °C. Total 50 μL PCR reactions consisted of: 5 μL 10 × PCR buffer, 0.5 μL dNTPs (10 mM each), 10 ng genomic DNA, 0.5 μL bar-PCR primer F (50 μM), 0.5 μL primer R (50 μM), 0.5 μL Platinum Taq (5 U/μL), and H2O for balance. For the third PCR reaction, an Illumina bridge PCR compatible primer was introduced followed by 95 °C for 30 s, and 5 cycles at 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s, and finally 5 min at 72 °C. The 50 μL PCR mixture consisted of 5 μL 10 × PCR buffer, 0.5 μL dNTPs (10 mM each), 20 ng genomic DNA, 0.5 μL primer F (50 μM), 0.5 μL primer R (50 μM), 0.5 μL Platinum Taq (5 U/μL), and H2O for balance.
Fungal analysis was also conducted by referring to previously published methods [46]. All PCR products were verified by agarose gel electrophoresis. High-throughput metagenomics was performed by Sangon Biotech (Shanghai) Co., Ltd, China.
2.6. Biofilm staining
After 14 and 30 days of exposure, biofilms on the LW1 and LW2 × 52 steel coupon surfaces were imaged with confocal laser scanning microscopy (C2 Plus, Nikon, Japan) using the LIVE/DEAD BacLight viability stain kit (Invitrogen, USA). Ten or more 3-D scan images were obtained for each sample in order to calculate biofilm thickness.
3. Results and discussion
3.1. Water analysis prior to experimentation
The chemical and biological compositions of GW and LW were analyzed prior to exposure to X52 steel coupons in order to assess differences between the two and evaluate factors that may induce corrosion. Major differences were found in the chemical composition of LW compared with GW (Table 1). Most notably, LW contained more than five times the amount of sulfate than that of GW. The high concentration of sulfate in LW (236 mg/L) provides a sufficient amount of electron acceptor for SRB and increasing sulfate concentrations have been shown to increase the abundance of SRB in lake water [47]. In addition, LW contained significantly more iron, manganese, and molybdenum than GW. High concentrations of these metals suggest that microorganisms capable of metal oxidation were likely present in the water, and these metal oxidizers may also be involved in MIC [21,48].
In addition to analyzing the overall chemistry of GW and LW, microbial composition was also assessed. In order to first gain some insight into the amounts of bacteria present, the water samples were inoculated on several types of general-purpose microbiological media. Many colonies with varying morphologies grew on the TSA and R2A agar plates cultured with LW, indicating an abundance of bacteria (Fig. S1(a)). Zero colonies grew on the plates inoculated with GW.
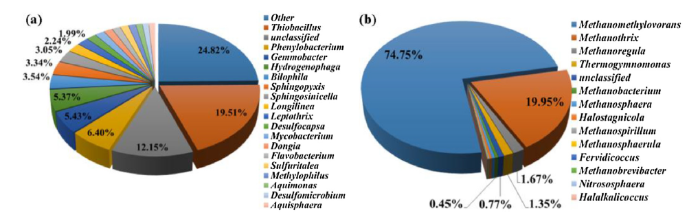
In order to determine initial microbial community composition of the untested LW, pyrosequencing of bacterial and archaeal 16S rRNA gene fragments was conducted (Fig. 1). ITS sequencing was also implemented in order to identify fungal species present in the raw LW (Fig. S1(b)). However, none of the fungi identified could be classified, therefore the role of MIC by fungi in this study could not be determined.
Fig. 1.
Fig. 1.
Relative distribution of (a) bacterial and (b) archaeal 16S rRNA gene sequences at the genus level in the raw LW used for this study.
Several bacterial genera known for MIC were identified in the raw water sample (Fig. 1(a)). For example, Thiobacillus species dominated the water and accounted for 19.51% of the bacterial population. Thiobacillus are aerobes that obtain energy via oxidation of sulfide or thiosulfate to sulfuric acid [49]. Members of this genus are frequently detected in MIC communities because they thrive well in environments with reduced sulfur compounds and do not require sources of organic carbon [[50], [51], [52]]. Hydrogenophaga was also abundant in LW, making up 5.37% of the overall community. This genus is capable of oxidizing hydrogen using nitrate as an electron acceptor [53]. Other biocorrosive species in the raw LW included SRB such as Desulfocapsa (1.68%) and Desulfomicrobium (1.09%).
The most abundant archaea detected in LW were methanogens from the genus Methanomethylovorans (74.75%), Methanothrix (19.95%), Methanoregula (1.67%), and Methanobacterium (0.45%) (Fig. 1(b)). Methanogens are known to contribute to MIC in anoxic pipeline systems and some can directly use electrons from elemental iron for methane production [54]. Overall, the LW microbial analysis provided evidence that hydrostatic testing using untreated water could introduce several biocorrosive species to the pipeline interior.
3.2. Biofilm visualization and corrosion chemistry of X52 steel coupons after laboratory hydrostatic testing with GW or LW
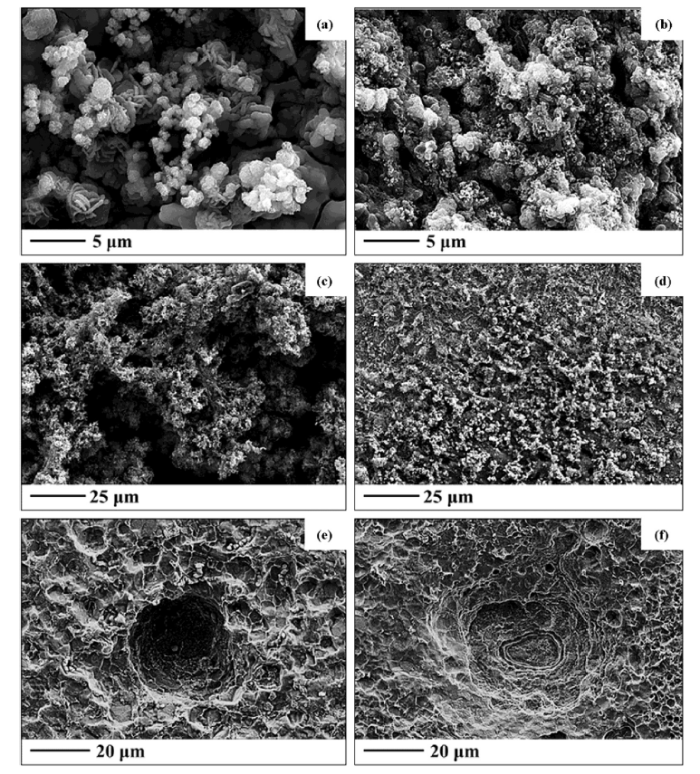
X52 steel coupons were cut from uncorroded portions of a CNPC oil pipeline and immersed in either GW or LW at 30 °C. A first batch of samples was incubated for 14 days, and a second batch for 30 days. During incubation, microorganisms formed notable biofilms on the surface of the coupons. FESEM was used to visualize the biofilms and embedded microorganisms (Fig. 2), and EDS spectrometry was conducted in parallel to analyze the chemistry of the exposed coupon corrosion due to biofilm activity (Table 2, Fig. S2). LW coupon biofilms were thick and dispersed, and clearly thickened after 30 days of exposure compared with 14 days (Fig. 2(a) and (b)). In contrast, GW biofilms were homogenous on the coupon surface and not as thick as LW biofilms (Fig. 2(c) and (d)). EDS spectrometry showed that Fe content significantly decreased in the presence of LW biofilms, whereas sulfur, phosphorous, oxygen and carbon were much higher (Table 2, Fig. S2).
Fig. 2.
Fig. 2.
FESEM images of X52 steel surfaces after (a) exposing to LW for 14 days, (b) exposing to LW for 30 days, (c) exposing to GW for 14 days, (d) exposing to GW for 30 days, (e) removal of the biofilm from coupons exposed to LW for 14 days, and (f) removal of the biofilm from coupons exposed to LW for 30 days.
Table 2 EDS analysis (wt.%) of exposed surfaces of the X52 steel coupons after exposure to LW or GW after 14 or 30 days.
| C | N | O | P | S | Na | Cl | Cr | Mn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| LW-14 days | 18.34 | 3.10 | 35.93 | 8.83 | 2.47 | 4.43 | — | — | 0.22 | 26.68 |
| LW-30 days | 33.43 | 5.66 | 27.74 | 1.62 | 7.32 | 1.18 | — | — | 0.18 | 22.87 |
| GW-14 days | 11.09 | 0.02 | 9.60 | 0.04 | 0.01 | — | 0.48 | 0.01 | 1.13 | 77.61 |
| GW-30 days | 7.69 | 0.03 | 5.11 | 0.30 | — | — | — | 0.30 | 1.00 | 85.56 |
| LW washed- 14 days | 5.75 | — | 1.92 | — | — | — | — | — | 1.26 | 91.07 |
| LW washed- 30 days | 4.41 | — | 2.66 | — | — | — | — | — | — | 92.93 |
In order to visualize effects the biofilms had on the X52 steel surfaces, biofilms were removed by washing with Clarke’s solution, and coupons were again viewed with FESEM. Deep pits were observed in the X52 steel coupons exposed to LW after both 14 and 30 days (Fig. 2(e) and (f)). Pitting corrosion is a type of MIC that occurs in a very limited area which leads to a visible deep dent or “pit” [55]. The average pit width was 40-50 μm after 30 days of exposure to LW (Fig. 2(f)). The X52 steel coupon matrix primarily consisted of Fe (> 90 wt.%) after removal of the LW biofilm (Table 2, Fig. S2).
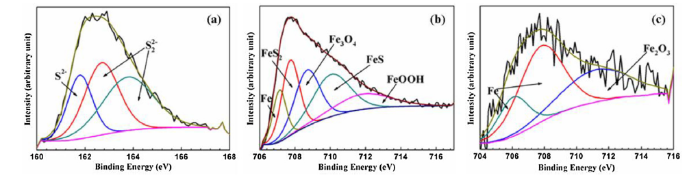
XPS was also used to measure the percent of various elements in the X52 steel coupons after hydrostatic testing (Table 3, Fig. S3). Sulfur was only detected on the coupons exposed to LW, and increased to nearly 10% of the elemental composition after 30 days (Table 3). This significant increase in sulfur detected in LW samples by EDS and XPS suggested microbial activity was occurring. Sulfur (S) 2p and iron (Fe) 2p3/2 XPS spectra of the X52 steel surfaces after 30 days of immersion in LW were analyzed in order to identify the chemical composition of the corroded surfaces (Fig. 3, Table S2). The S 2p spectrum revealed peaks at 161.9, 162.3, and 163.4 eV which corresponded with S2- and S22- compounds, likely indicating S-Fe bonds (Fig. 3(a)) [[56], [57], [58]]. Five different peaks were detected in the Fe 2p3/2 spectrum which corresponded with Fe (707.1 eV), FeS2 (707.5 eV), Fe3O4 (708.3 eV), FeS (710.3 eV), and FeOOH (711.8 eV) (Fig. 3(b)) [57,[59], [60], [61], [62], [63], [64]]. Visibly, the coupon exposed to LW appeared orange with an unstable rust layer (Fig. S4). When the rust layer was removed from the surface, a black layer composed of FeS and FeS2 was clear due to SRB activity. As a comparison, Fe 2p3/2 spectrum was also analyzed for the X52 steel coupon surface immersed in GW for 30 days (Fig. 3(c)). The GW coupon surface was dominated by iron oxides, and only Fe (BEs at 706.8 and 707.7 eV) and Fe2O3 (BE at 711.3 eV) were detected. In GW, oxygen reduction was likely the dominant reduction reaction, so a dense orange rust layer (Fe2O3) visibly formed on the steel coupon surfaces (Fig. S4).
Table 3 Element atomic percentages (%) measured by X-ray photoelectron spectroscopy (XPS) on coupons exposed to LW or GW for 14 and 30 days.
| Elements | Fe | Cr | P | Cl | S | N | O | C |
|---|---|---|---|---|---|---|---|---|
| 14 days | ||||||||
| LW | 23.57 | — | 1.17 | 0.70 | 0.93 | 2.25 | 42.72 | 28.66 |
| GW | 27.10 | 0.88 | — | — | — | — | 51.60 | 20.42 |
| 30 days | ||||||||
| LW | 11.21 | — | 1.72 | — | 9.92 | 7.85 | 10.36 | 58.94 |
| GW | 14.17 | — | — | — | — | 10.22 | 36.45 | 39.16 |
Fig. 3.
Fig. 3.
(a) S 2p peak of X52 steel coupon surfaces after exposure to LW solution for 30 days, (b) Fe 2p3/2 peak of X52 steel coupon surfaces after exposure to LW solution for 30 days, and (c) Fe 2p3/2 peak of X52 steel coupon surfaces after exposure to GW solution for 30 days.
Formation of iron sulfide in the LW coupons likely contributed to the severe pitting corrosion observed on the steel surfaces (Fig. 2(e) and (f)). Iron sulfide is a characteristic corrosion product of SRB, and iron sulfides have long been known to accelerate corrosion [[65], [66], [67]]. SRB reduce sulfate to H2S which can react rapidly with metallic iron by the following reaction:
As sulfide is produced by sulfate reducers, pH decreases leading to dissolution of iron [68].
Biogenic H2S production alone does not fully contribute to MIC caused by SRB. In fact, high concentrations of H2S may lead to acidic pH levels that are inhibitory for microbial growth. This was demonstrated in a previous study which showed that by providing a large headspace in which H2S could exit the liquid-phase, MIC was significantly enhanced due to an increase in SRB activity [69].
Methanogenic archaea are also capable of using iron as an electron donor in the absence of alternative substrates. For example, under starvation conditions, Methanosarcina barkeri accelerated corrosion of carbon steel [74]. A similar phenomenon was also observed in Methanosarcina maripaludis, which typically has no influence on corrosion when sufficient carbon source is available. Under starvation conditions, iron is the only available electron donor and methanogens must uptake electrons from iron in order to obtain maintenance energy.
3.3. Changes in pH, weight loss, and CLSM visualization of the X52 steel coupons after laboratory hydrostatic testing with GW or LW
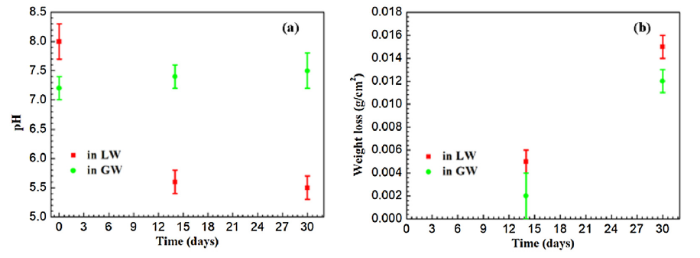
Microbial activity may cause variations in pH; therefore, pH levels were compared for GW and LW before exposure to the X52 steel coupons, at day 14, and at day 30 (Fig. 4(a)). The original pH of GW was 7.2 ± 0.2, and remained around this level for the complete 30-day duration. On the other hand, the pH of LW dropped from 8.0 ± 0.3 to 5.5 ± 0.2 over the course of the 30-day immersion. This decrease in pH for LW after exposure to the X52 steel coupons was likely due to aerobic SOB such as Thiobacillus, which were highly abundant in the raw LW (Fig. 1(a)). SOB couple the reduction of oxygen with the oxidation of H2S and other reduced sulfur compounds to sulfuric acid, thus lowering the pH [52]. The reduction of oxygen by SOB would create an anaerobic environment, particularly for deeper biofilm layers.
Fig. 4.
Fig. 4.
(a) Variation in pH of GW and LW after 14 and 30 days of laboratory hydrostatic testing. (b) Weight loss of triplicate X52 steel coupons after exposure to GW or LW after 14 and 30 days.
Weight loss of triplicate X52 steel coupons exposed to either GW or LW was also measured after 14 and 30 days of laboratory hydrostatic testing (Fig. 4(b)). Coupons exposed to GW and LW all decreased in weight over the duration of the experiment, however the weight loss was about 1.25 times more pronounced in the LW samples. After 14 days the weight of LW coupons decreased an average of 0.005 ± 0.001 g/cm2, while GW coupons decreased 0.002 ± 0.002 g/cm2. After 30 days the weight of LW coupons decreased an average of 0.015 ± 0.001 g/cm2 and GW coupons decreased 0.012 ± 0.001 g/cm2.
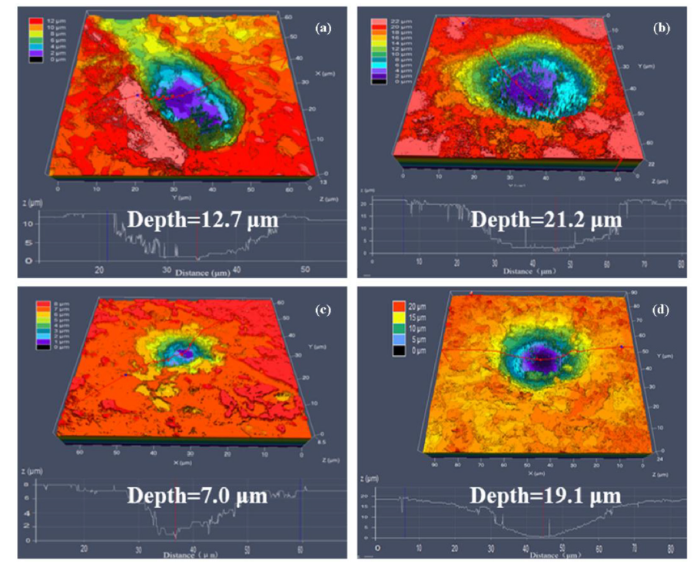
CLSM 3-D images were used to determine pit depths on X52 steel coupon surfaces after immersion in GW or LW (Fig. 5, Table S3). Pits were uniform, and the maximum pits were observed in coupons exposed to LW where pitting depth reached up to 26.1 μm after 30 days of exposure (Table S3). However, the depth of the maximum pit observed after 30 days of exposure in GW solution was only 19.1 μm, with an average pitting depth of about 12.4 ± 4.7 μm (Fig. 5(d), Table S3). The average maximum pit depth (20.7 ± 3.6 μm) of X52 steel pits after exposure to LW for 30 days was also higher than many previously reported pit-depths in corroded materials [19,75]. These results demonstrated severe bacterial attack on X52 steel surfaces after incubation in LW.
Fig. 5.
Fig. 5.
CLSM images of X52 steel coupon surfaces after exposing in (a, b) LW solution for 14 and 30 days, or (c, d) after exposing in GW solution for 14 and 30 days.
3.4. OCP and potentiodynamic polarization
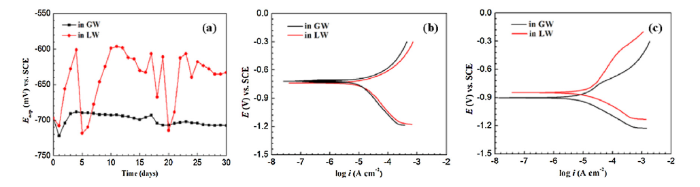
OCP variation was compared in order to assess corrosive behaviors of X52 steel surfaces exposed to GW or LW over the course of 30 days (Fig. 6(a)). EOCP of the GW samples stabilized at nearly -700 mV over the 30-day test. On the other hand, EOCP of the X52 steel coupons immersed in LW oscillated severely and never stabilized, indicating surface instability due to microbial activity.
Fig. 6.
Fig. 6.
(a) Variation in OCP with exposure time, and polarization curves of X52 steel coupons after exposure to LW or GW solutions for (b) 14 and (c) 30 days.
Potentiodynamic polarization curves were analyzed for X52 steel coupons immersed in LW or GW for 14 (Fig. 6(b)) and 30 (Fig. 6(c)) days. In both LW and GW solutions, corrosion potential of the X52 steel coupons shifted towards the negative direction after 30 days of exposure, indicating the surface was active. Electrochemical kinetic parameters, including corrosion current density (icorr) and corrosion potential (Ecorr) were also evaluated (Table 4). For coupons exposed to microbe-rich LW, the icorr significantly increased and reached its highest amount (17.4 μA/cm2) after 14 days of exposure. This was 8 times higher than the icorr of the X52 steel coupons in GW (2.58 μA/cm2). Acceleration of icorr in the X52 steel coupons exposed to LW after 14 days suggests that bacterial biofilms destroyed the passive film and increased the risk of localized attack [76]. After 30 days of exposure the icorr did not change considerably for LW coupons, but did increase to 6.56 μA/cm2 for GW coupons.
Table 4 Corrosion parameters obtained from dynamic potential polarization curves of X52 steel coupons exposed to GW or LW for 14 and 30 days, respectively.
| icorr (A/cm2) | Ecorr (V) vs. SCE | |
|---|---|---|
| 14 days | ||
| GW | 2.58 × 10-6 | -0.719 |
| LW | 1.74 × 10-5 | -0.738 |
| 30 days | ||
| GW | 6.56 × 10-6 | -0.903 |
| LW | 1.48 × 10-5 | -0.848 |
3.5. Biofilm formation
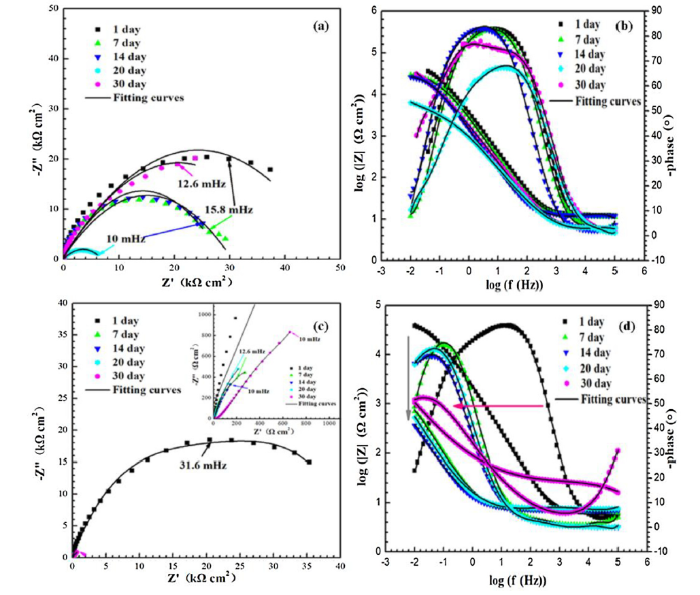
EIS is a technique that has been widely applied for monitoring biofilm growth on metal surfaces and biocorrosion [77,78]. Therefore, EIS was performed in order to monitor cell attachment and biofilm formation on X52 steel coupons exposed to LW or GW. EIS spectra were measured under stable EOCP at exposure times of 1, 7, 14, 20, and 30 days for both water samples. Time-dependent Nyquist plots are shown in Fig. 7(a) and (b), and the corresponding Bode plots are displayed in Fig. 7(c) and (d). All Nyquist impedance curves showed a semicircle pattern over the entire frequency range. Capacitive semicircles with larger diameters correlate with larger charge transfer resistance and lower rates of corrosion [79]. For both GW and LW, the semicircles decreased with exposure time. However, in the presence of microbe-rich LW, the diameter of the semicircles was considerably smaller than in GW, indicating an accelerated rate of corrosion in LW. Analysis of the Bode plots indicated that EIS values decreased over time (Fig. 7(c) and (d)), consistent with the potentiodynamic polarization measurements (Fig. 6(b) and (c)).
Fig. 7.
Fig. 7.
Nyquist and Bode plots of X52 steel coupons after 30 days of exposure to (a, b) GW and (c, d) LW, respectively.
The EIS data indicated that microorganisms introduced from LW aggregated and formed biofilms on the coupon surface. Biofilms contain a mixture of bacterial cells and extracellular polymeric substances (EPS), which contains mostly proteins and polysaccharides. As microorganisms directly obtain Fe by dissolution of the metal coupon, some of the corrosion products may also get stuck in the biofilm. Cells on the inside of the biofilm rely on diffusion because they have a difficult time accessing electron acceptor or obtaining nutrients from the outer solution [80]. This explains why the X52 steel coupons exposed to LW appeared to switch from capacitive behavior to diffusion behavior after 20 days of exposure (Fig. 7).
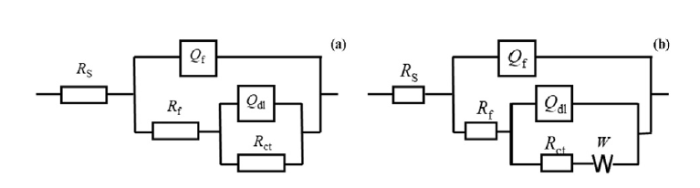
The EIS results were analyzed using ZSimDemo 3.22d software (Table S4), and electrochemical equivalent circuit modeling was used to explain the corrosion behavior of the X52 steel coupons after exposure to LW or GW (Fig. 8). In the models, Rs and Rct display the solution resistance and charge transfer resistance, respectively. Rf represents the film resistance of corrosion products in GW, and resistance of both corrosion products and bacterial biofilms in LW. In GW, Rct increased with exposure time during the first 7 days, and reached up to 73.7 kΩ cm2 due to the protective layer that developed at the metal/solution interface [81,82]. Rct then decreased to 53.5 kΩ cm2 after 30 days (Table S4). In LW, Rct also increased during the initial 4 days of exposure, probably due to biofilm formation and development of corrosion products on the metal surface. Thereafter, Rct markedly decreased to 7.7 kΩ cm2. Rf also decreased in LW, indicating a weakening in the protective film on the surface and acceleration of electron transfer between the matrix and biofilm [35].
Fig. 8.
Fig. 8.
Equivalent circuits used for simulating the impedance spectra (
3.6. Corrosion rate measurements
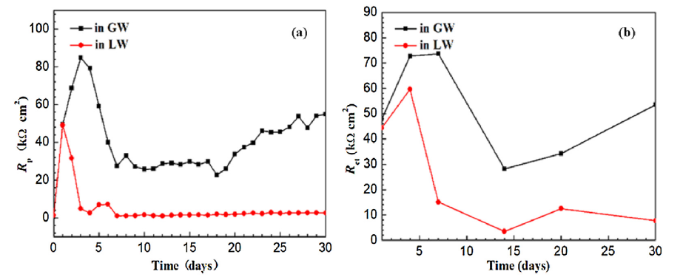
Polarization resistance (Rp) (Fig. 9(a)) and Rct (Fig. 9(b)) were also measured for X52 steel coupons exposed to GW or LW at different exposure times. The results verified that severe corrosion and pitting on the X52 steel coupons were caused by bacterial biofilms. The Rp trend was similar during the initial 7 days of exposure to GW and LW, due to similar surface activity and instability (Fig. 9(a)). Thereafter, Rp of the X52 steel coupons exposed to LW reached its lowest value and stabilized for the remaining 30-day duration. For the X52 steel coupons exposed to GW, Rp was stable at first and then increased due to formation of protective oxide layers on the steel surface. Rct variations over time showed a similar trend (Fig. 9(b)). Rct is 10 times higher in X52 steel coupons exposed to GW compared to LW, which suggests that the presence of bacteria accelerates electron transfer between X52 steel coupon surfaces and solution.
Fig. 9.
Fig. 9.
Corrosion rates of X52 steel coupons exposed to GW or LW for 30 days measured by (a) Rp obtained by LPR, and (b) Rct obtained by EIS.
3.7. Microbial community analysis
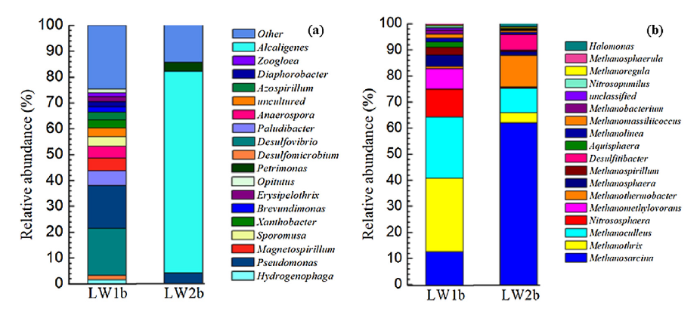
FESEM (Fig. 2(e)) and CLSM (Fig. 5(b)) images confirmed that microorganisms introduced from LW formed thick biofilms that induced pitting corrosion on the X52 steel coupon surfaces. In order to uncover the microorganisms involved in this MIC, 16S rRNA pyrosequencing was used for the following samples: (1) microorganisms that formed biofilms attached to the X52 steel coupon surfaces after exposure to LW for 30 days (LW1b) and (2) microorganisms that formed biofilms attached to the X52 steel coupon surfaces after exposure to LW and added 2216E culture media for 30 days (LW2b) (Fig. 10).
Fig. 10.
Fig. 10.
Relative distribution of (a) bacterial and (b) archaeal 16S rRNA gene sequences at the genus level. LW1b represents microorganisms in biofilms attached to X52 steel coupon surfaces after exposure to LW without added culture media; LW2b represents microorganisms in biofilms attached to X52 steel coupon surfaces after exposure to LW with added 2216E culture media.
Sulfate reducing bacterial genera, including Desulfovibrio (18.27%) and Desulfomicrobium (1.60%), dominated X52 biofilms exposed to LW alone (LW1b) (Fig. 10(a)). The LW1b sample did not contain any additional carbon or nutritional sources, so it is likely the bacteria present were starved of organic compounds. Desulfovibrio are common organotrophic hydrogen-utilizing SRB, but are also capable of directly taking up electrons from carbon steel by BCSR and inducing severe pitting corrosion in the absence of organic carbon [72]. Furthermore, pitting corrosion and BCSR can be accelerated when Desulfovibrio use electron-shuttling mediators, such as riboflavin and flavin adenine dinucleotide (FAD), that are released by bacteria in the biofilm [83]. Previous studies have also reported the involvement of SRB in MIC of steels [[84], [85], [86], [87], [88]]. For example, sulfate reducers corroded pipelines from the Halfdan oil field in the Danish Sector of the North Sea by producing H2S and decreasing pH [24].
Pseudomonas sp. were also heavily detected in the X52 steel coupon surfaces exposed to LW alone (16.61%), and to a lesser extent in the LW2b samples containing added culture media (3.97%). Pseudomonas aeruginosa is a facultative nitrate reducing bacterium (NRB) that like Desulfovibrio sp., have been shown to be more corrosive in the absence of organic carbon sources [89]. NRB are known to contribute to MIC attacks, but this mechanism has not been studied as well as SRB MIC [19]. NRB can use hydrogen released during biocorrosion as an electron donor, or may be capable of directly coupling iron oxidation with nitrate reduction [90].
The bacterial community of X52 steel biofilms exposed to LW with added 2216E culture media (LW2b) was significantly different than the LW1b community (Fig. 10). Addition of the carbon-rich media resulted in lower bacterial diversity, and SRB were not detected. Alcaligenes sp. almost completely dominated the LW2b biofilm, making up 78.01% of the overall community abundance. Alcaligenes are hydrocarbon-degrading facultative anaerobes that can grow by nitrate or nitrite reduction [91]. Alcaligenes faecalis has been shown to produce crude ethyl acetate, which is toxic to SRB [92], this may be one reason why there were few SRB in the biofilm. In addition, NRB have been shown to inhibit the growth of SRB by inhibiting production of the key SRB enzyme, sulfonic acid reductase [93].
Differences were also observed between the LW1b and LW2b archaeal communities (Fig. 10(b)). Both communities consisted mainly of methanogens, however the dominant genera varied. In LW1b, Methanothrix (28.14%), Methanoculleus (23.44%), Methanosarcina (12.58%), Nitrososphaera (10.73%), and Methanomethylovorans (7.93%) were the dominant strains. While, the dominant microorganisms in LW2b were Methanosarcina (62.02%), Methanothermobacter (12.08%), Methanoculleus (9.37%), and Desulfitibacter (5.88%). Strictly hydrogenotrophic methanogens, such as Methanoculleus and Methanomethylovorans, have widely been implicated in MIC. These methanogens consume hydrogen which may be derived from Fe° reacting with H+ from H2O [23]. Once electrons are derived via this cathodic depolarization, H2 combines with CO2 to produce CH4.
Methanothrix was the most abundant archaeal genera in the LW1b biofilms. Methanothrix are acetoclastic methanogens that also have the ability to accept electrons from neighboring species as the sole energy source to support growth via direct interspecies electron transfer (DIET) [94]. Similarly, Methanosarcina sp. are mixotrophic methanogens capable of DIET [95], and some are also incapable of using hydrogen [[96], [97], [98]]. It has been proposed that these methanogenic genera may be capable of directly taking up electrons from extracellular surfaces, including Fe0 [26]. Evidence supporting this comes from the finding that Geobacter sulfurreducens, another species capable of accepting electrons via DIET, is able to directly accept electrons from Fe0 using outer surface c-type cytochromes [32]. It was recently shown that Methanosarcina acetivorans uses a membrane-bound c-type cytochrome to directly transfer electrons onto extracellular electron acceptors to support growth [99]. In addition, Methanosarcina were also shown to accelerate corrosion in the absence of traditional methanogenic substrates such as hydrogen or methanol [74]. Further pure culture studies to elucidate the mechanisms of iron corrosion by Methanothrix and Methanosarcina species are warranted.
3.8. Biofilm measurements
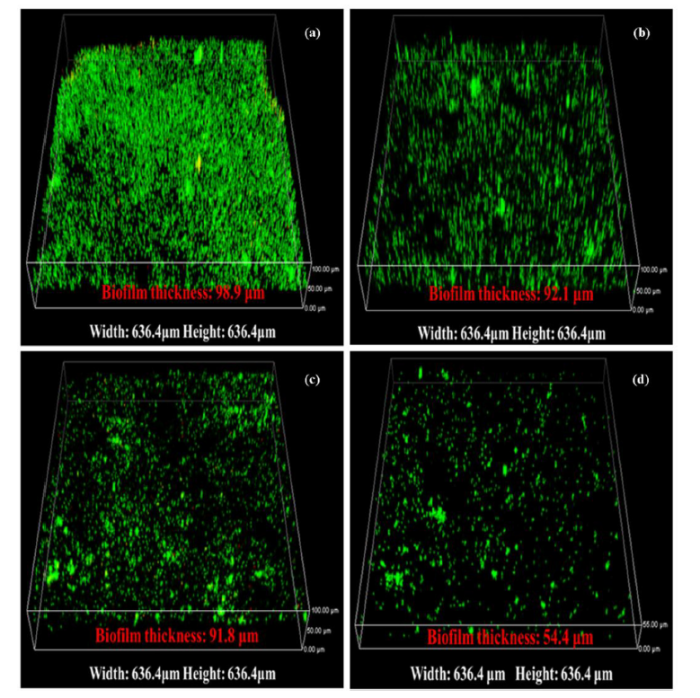
Biofilms attached to the X52 steel coupon surfaces after exposure to LW (LW1) or LW with added 2216E culture media (LW2) were examined using CLSM after 14 days (Fig. 11(a) and (b)) and 30 days (Fig. 11(c) and (d)) of incubation. After 14 days of incubation, thick biofilms attached to the coupon surfaces for both samples, however the average LW1 biofilm (108.6 ± 8.1 μm) (Fig. 11(a)) was slightly thicker than LW2 (97.0 ± 16.0 μm) (Fig. 11(b)). After 30 days of incubation, the biofilm became heterogeneous and average thickness decreased to 93.1 ± 4.9 μm in LW1 (Fig. 11(c)) and 56.6 ± 9.3 μm in LW2 (Fig. 11(d)). High levels of green fluorescence confirmed that the biofilms consisted of primarily living microorganisms. The differences in biofilm thickness between LW1 and LW2 were likely due to the microbial diversity, as shown in Fig. 10.
Fig. 11.
Fig. 11.
Live/dead CLSM 3-D images of biofilms and biofilm thickness from (a) X52 steel exposed to LW1 for 14 days, (b) X52 steel exposed to LW2 for 14 days, (c) X52 steel exposed to LW1 for 30 days, and (d) X52 steel exposed in LW2 for 30 days. LW1 represents incubation in lake water alone, and LW2 represent incubation in lake water with added 2216E culture media.
3.9. Overall predicted mechanism of MIC in X52 pipeline steel exposed to LW
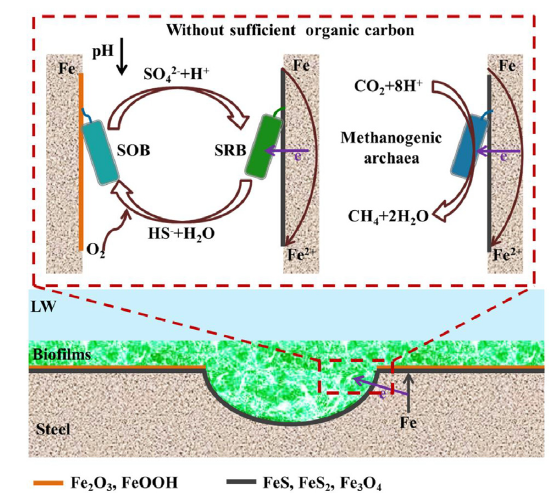
Proposed mechanisms for corrosion of the X52 steel coupons by SOB, SRB, and methanogenic archaea are represented in a schematic diagram shown in Fig. 12. SOB reduced oxygen, keeping conditions within the biofilm anaerobic, while at the same time oxidizing sulfide to sulfates. The sulfate produced provided sufficient electron acceptor for SRB which coupled sulfate reduction to oxidation of the X52 steel. SRB directly induced severe pitting corrosion via BCSR, sulfide production, and other metabolic activities within the biofilm.
Fig. 12.
Fig. 12.
Schematic diagrams of the proposed methanogenic and SRB corrosion mechanisms in low organic carbon environments.
In the presence of methanogens at pH 7.0 and 37 °C, steel corrosion accelerated via biocatalytic cathodic carbon dioxide reduction according to the following equation [100]:
Corrosion of X52 steel by these methanogenic species resulted in the production of methane, as well as FeOOH, Fe2O3, and Fe3O4.
4. Conclusion
This study presents suggested mechanisms of corrosion on X52 steel after hydrostatic testing using untreated lake water. Microorganisms present in the contaminated water attached and formed thick biofilms on the X52 steel surfaces, which could lead to pitting and perforation of oil pipelines. Analysis of bacterial and archaeal communities demonstrated that severe MIC could mainly be induced by methanogenic archaea and SRB. FeS and FeS2 precipitates collected on the X52 steel surfaces, confirming that biocatalysis by SRB accelerated corrosion. Methanogens on the coupon surface induced severe pitting by cathodic hydrogen depolarization. On the contrary, X52 steel coupons treated with groundwater showed significantly lower rates of corrosion based on weight loss, electrochemical measurements, and analysis of corrosion products. Results from this study should be taken into consideration in order to prevent MIC caused by hydrostatic testing.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 51871050), the Natural Science Foundation of Liaoning Province (No. 20180510041), the Liaoning Revitalization Talents Program (No. XLYC1907158) and the Fundamental Research Funds for the Central Universities of the Ministry of Education of China (N180205021 and N180203019).
Appendix A. Supplementary data
Supplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.jmst.2020.01.055.
Reference
DOI
URL
PMID
[Cited within: 1]

Microbiologically Influenced Corrosion (MIC) is a serious problem in many industries because it causes huge economic losses. Due to its excellent resistance to chemical corrosion, 2707 hyper duplex stainless steel (2707 HDSS) has been used in the marine environment. However, its resistance to MIC was not experimentally proven. In this study, the MIC behavior of 2707 HDSS caused by the marine aerobe Pseudomonas aeruginosa was investigated. Electrochemical analyses demonstrated a positive shift in the corrosion potential and an increase in the corrosion current density in the presence of the P. aeruginosa biofilm in the 2216E medium. X-ray photoelectron spectroscopy (XPS) analysis results showed a decrease in Cr content on the coupon surface beneath the biofilm. The pit imaging analysis showed that the P. aeruginosa biofilm caused a largest pit depth of 0.69 mum in 14 days of incubation. Although this was quite small, it indicated that 2707 HDSS was not completely immune to MIC by the P. aeruginosa biofilm.
DOI
URL
PMID
[Cited within: 1]

Increasing sulfate input has been seen as an issue in management of aquatic ecosystems, but its influences on eutrophic freshwater lakes is not clear. In this study, it was observed that increasing sulfate concentration without additional cyanobacterial bloom biomass (CBB) addition did not have an obvious effect on element cycling during 1-year continuous flow mesocosm experiments in which water and sediments were taken from a shallow eutrophic lake with sulfate levels near 1 mM. However, following addition of CBB to mesocosms, sulfate-reducing bacteria (SRB) were observed in the water column, and increasing numbers of SRB in the water column were associated with higher sulfate input. Sulfate amendment (0-70 mg L(-1)) also resulted in a larger amount of total dissolved sulfide (peak values of 5.90 +/- 0.36 to 7.60 +/- 0.12 mg L(-1)) in the water column and acid volatile sulfide (1081.71 +/- 69.91 to 1557.98 +/- 41.72 mg kg(-1)) in 0-1 cm surface sediments due to sulfate reduction. During the period of CBB decomposition, increasing sulfate levels in the water column were positively correlated with increasing diffusive phosphate fluxes of 1.23 +/- 0.32 to 2.17 +/- 0.01 mg m(-2) d(-1) at the water-sediment interface. As increases in sulfide and phosphate release rates deteriorated the water quality/ecosystem and even spurred the occurrence of a black water problem in lakes, the control of sulfate input level should be considered for shallow eutrophic lake management, especially during cyanobacterial bloom periods.
DOI
URL
PMID
[Cited within: 1]

Corrosion of iron presents a serious economic problem. Whereas aerobic corrosion is a chemical process, anaerobic corrosion is frequently linked to the activity of sulphate-reducing bacteria (SRB). SRB are supposed to act upon iron primarily by produced hydrogen sulphide as a corrosive agent and by consumption of 'cathodic hydrogen' formed on iron in contact with water. Among SRB, Desulfovibrio species--with their capacity to consume hydrogen effectively--are conventionally regarded as the main culprits of anaerobic corrosion; however, the underlying mechanisms are complex and insufficiently understood. Here we describe novel marine, corrosive types of SRB obtained via an isolation approach with metallic iron as the only electron donor. In particular, a Desulfobacterium-like isolate reduced sulphate with metallic iron much faster than conventional hydrogen-scavenging Desulfovibrio species, suggesting that the novel surface-attached cell type obtained electrons from metallic iron in a more direct manner than via free hydrogen. Similarly, a newly isolated Methanobacterium-like archaeon produced methane with iron faster than do known hydrogen-using methanogens, again suggesting a more direct access to electrons from iron than via hydrogen consumption.
DOI
URL
PMID
[Cited within: 1]

About a century ago, researchers first recognized a connection between the activity of environmental microorganisms and cases of anaerobic iron corrosion. Since then, such microbially influenced corrosion (MIC) has gained prominence and its technical and economic implications are now widely recognized. Under anoxic conditions (e.g., in oil and gas pipelines), sulfate-reducing bacteria (SRB) are commonly considered the main culprits of MIC. This perception largely stems from three recurrent observations. First, anoxic sulfate-rich environments (e.g., anoxic seawater) are particularly corrosive. Second, SRB and their characteristic corrosion product iron sulfide are ubiquitously associated with anaerobic corrosion damage, and third, no other physiological group produces comparably severe corrosion damage in laboratory-grown pure cultures. However, there remain many open questions as to the underlying mechanisms and their relative contributions to corrosion. On the one hand, SRB damage iron constructions indirectly through a corrosive chemical agent, hydrogen sulfide, formed by the organisms as a dissimilatory product from sulfate reduction with organic compounds or hydrogen (
DOI
URL
PMID
[Cited within: 1]

Impedance biosensor chips were developed for detection of Escherichia coli O157:H7 based on the surface immobilization of affinity-purified antibodies onto indium tin oxide (ITO) electrode chips. The immobilization of antibodies onto ITO chips was carried out using an epoxysilane monolayer to serve as a template for chemical anchoring of antibodies. The surface characteristics of chips before and after the binding reaction between the antibodies and antigens were characterized by atomic force microscopy (AFM). The patterns of the epoxysilanes monolayer, antibodies, and E. coli cells were clearly observed from the AFM images. Alkaline phosphatase as the labeled enzyme to anti-E. coli O157:H7 antibody was used to amplify the binding reaction of antibody-antigen on the chips. The biocatalyzed precipitation of 5-bromo-4-chloro-3-indolyl phosphate by alkaline phosphatase on the chips in pH 10 PBS buffer containing 0.1 M MgCl2 increased the electron-transfer resistance for a redox probe of Fe(CN)6(3-/4-) at the electrode-solution interface or the electrode resistance itself. Electrochemical impedance spectroscopy and cyclic voltammetric method were employed to follow the stepwise assembly of the systems and the electronic transduction for the detection of E. coli. The biosensor could detect the target bacteria with a detection limit of 6 x 10(3) cells/mL. A linear response in the electron-transfer resistance for the concentration of E. coli cells was found between 6 x 10(4) and 6 x 10(7) cells/mL.
DOI
URL
PMID
[Cited within: 1]

Electrochemical impedance spectroscopy was tested to monitor the cell attachment and the biofilm proliferation in order to identify characteristic events induced on the metal surface by Gram-negative (Pseudomonas aeruginosa PAO1) and Gram-positive (Bacillus subtilis) bacteria strains. Electrochemical impedance spectra of AISI 304 electrodes during cell attachment and initial biofilm growth for both strains were obtained. It can be observed that the resistance increases gradually with the culture time and decreases with the biofilm detachment. So, the applicability of electric cell-substrate impedance sensing (ECIS) for studying the attachment and spreading of cells on a metal surface has been demonstrated. The biofilm formation was also characterized by the use of scanning electron microscopy and confocal laser scanning microscopy and COMSTAT image analysis. The electrochemical results roughly agree with the microscope image observations. The ECIS technique used in this study was used for continuous real-time monitoring of the initial bacterial adhesion and the biofilm growth. It provides a simple and non-expensive electrochemical method for in vitro assessment of the presence of biofilms on metal surfaces.
DOI
URL
PMID
[Cited within: 1]

In the microbiologically influenced corrosion (MIC) caused by sulfate reducing bacteria (SRB), iron oxidation happens outside sessile cells while the utilization of the electrons released by the oxidation process for sulfate reduction occurs in the SRB cytoplasm. Thus, cross-cell wall electron transfer is needed. It can only be achieved by electrogenic biofilms. This work hypothesized that the electron transfer is a bottleneck in MIC by SRB. To prove this, MIC tests were carried out using 304 stainless steel coupons covered with the Desulfovibrio vulgaris (ATCC 7757) biofilm in the ATCC 1249 medium. It was found that both riboflavin and flavin adenine dinucleotide (FAD), two common electron mediators that enhance electron transfer, accelerated pitting corrosion and weight loss on the coupons when 10ppm (w/w) of either of them was added to the culture medium in 7-day anaerobic lab tests. This finding has important implications in MIC forensics and biofilm synergy in MIC that causes billions of dollars of damages to the US industry each year.
DOI
URL
PMID
[Cited within: 1]

Nitrates in different water and wastewater streams raised concerns due to severe impacts on human and animal health. Diverse methods are reported to remove nitrate from water streams which almost fail to entirely treat nitrate, except biological denitrification which is capable of reducing inorganic nitrate compounds to harmless nitrogen gas. Review of numerous studies in biological denitrification of nitrate containing water resources, aquaculture wastewaters and industrial wastewater confirmed the potential of this method and its flexibility towards the remediation of different concentrations of nitrate. The denitrifiers could be fed with organic and inorganic substrates which have different performances and subsequent advantages or disadvantages. Review of heterotrophic and autotrophic denitrifications with different food and energy sources concluded that autotrophic denitrifiers are more effective in denitrification. Autotrophs utilize carbon dioxide and hydrogen as the source of carbon substrate and electron donors, respectively. The application of this method in bio-electro reactors (BERs) has many advantages and is promising. However, this method is not so well established and documented. BERs provide proper environment for simultaneous hydrogen production on cathodes and appropriate consumption by immobilized autotrophs on these cathodes. This survey covers various designs and aspects of BERs and their performances.
DOI
URL
PMID
[Cited within: 1]

In the past decade, potential pathogens, including Alcaligenes species, have been increasingly recovered from cystic fibrosis (CF) patients. Accurate identification of multiply antibiotic-resistant gram-negative bacilli is critical to understanding the epidemiology and clinical implications of emerging pathogens in CF. We examined the frequency of correct identification of Alcaligenes spp. by microbiology laboratories affiliated with American CF patient care centers. Selective media, an exotoxin A probe for Pseudomonas aeruginosa, and a commercial identification assay, API 20 NE, were used for identification. The activity of antimicrobial agents against these clinical isolates was determined. A total of 106 strains from 78 patients from 49 CF centers in 22 states were studied. Most (89%) were correctly identified by the referring laboratories as Alcaligenes xylosoxidans. However, 12 (11%) strains were misidentified; these were found to be P. aeruginosa (n = 10), Stenotrophomonas maltophilia (n = 1), and Burkholderia cepacia (n = 1). Minocycline, imipenem, meropenem, piperacillin, and piperacillin-tazobactam were the most active since 51, 59, 51, 50, and 55% of strains, respectively, were inhibited. High concentrations of colistin (100 and 200 microg/ml) inhibited 92% of strains. Chloramphenicol paired with minocycline and ciprofloxacin paired with either imipenem or meropenem were the most active combinations and inhibited 40 and 32%, respectively, of strains. Selective media and biochemical identification proved to be useful strategies for distinguishing A. xylosoxidans from other CF pathogens. Standards for processing CF specimens should be developed, and the optimal method for antimicrobial susceptibility testing of A. xylosoxidans should be determined.
DOI
URL
PMID
[Cited within: 1]

Direct interspecies electron transfer (DIET) is potentially an effective form of syntrophy in methanogenic communities, but little is known about the diversity of methanogens capable of DIET. The ability of Methanosarcina barkeri to participate in DIET was evaluated in coculture with Geobacter metallireducens. Cocultures formed aggregates that shared electrons via DIET during the stoichiometric conversion of ethanol to methane. Cocultures could not be initiated with a pilin-deficient G. metallireducens strain, suggesting that long-range electron transfer along pili was important for DIET. Amendments of granular activated carbon permitted the pilin-deficient G. metallireducens isolates to share electrons with M. barkeri, demonstrating that this conductive material could substitute for pili in promoting DIET. When M. barkeri was grown in coculture with the H2-producing Pelobacter carbinolicus, incapable of DIET, M. barkeri utilized H2 as an electron donor but metabolized little of the acetate that P.carbinolicus produced. This suggested that H2, but not electrons derived from DIET, inhibited acetate metabolism. P. carbinolicus-M. barkeri cocultures did not aggregate, demonstrating that, unlike DIET, close physical contact was not necessary for interspecies H2 transfer. M. barkeri is the second methanogen found to accept electrons via DIET and the first methanogen known to be capable of using either H2 or electrons derived from DIET for CO2 reduction. Furthermore, M. barkeri is genetically tractable,making it a model organism for elucidating mechanisms by which methanogens make biological electrical connections with other cells.
A new acetotrophic marine methane-producing bacterium that was isolated from the methane-evolving sediments of a marine canyon is described. Exponential phase cultures grown with sodium acetate contained irregularly shaped cocci that aggregated in the early stationary phase and finally differentiated into communal cysts that released individual cocci when ruptured or transferred to fresh medium. The irregularly shaped cocci (1.9 +/- 0.2 mm in diameter) were gram negative and occurred singly or in pairs. Cells were nonmotile, but possessed a single fimbria-like structure. Micrographs of thin sections showed a monolayered cell wall approximately 10 nm thick that consisted of protein subunits. The cells in aggregates were separated by visible septation. The communal cysts contained several single cocci encased in a common envelope. An amorphous form of the communal cyst that had incomplete septation and internal membrane-like vesicles was also present in late exponential phase cultures. Sodium acetate, methanol, methylamine, dimethylamine, and trimethylamine were substrates for growth and methanogenesis; H(2)-CO(2) (80:20) and sodium formate were not. The optimal growth temperature was 35 to 40 degrees C. The optimal pH range was 6.5 to 7.0. Both NaCl and Mg were required for growth, with maximum growth rates at 0.2 M NaCl and 0.05 M MgSO(4). The DNA base composition was 41 +/- 1% guanine plus cytosine. Methanosarcina acetivorans is the proposed species. C2A is the type strain (DSM 2834, ATCC 35395).
DOI
URL
PMID
[Cited within: 1]

A methanogenic organism, designated strain HB-1(T), from the domain Archaea was isolated from groundwater sampled from a subsurface Miocene formation located in Horonobe, Hokkaido, Japan. The strain grew on methanol, dimethylamine, trimethylamine, dimethylsulfide and acetate but not on monomethylamine, H(2)/CO(2), formate, 2-propanol, 2-butanol or cyclopentanol. Cells were Gram-reaction-negative, non-motile, irregular cocci that were 1.4-2.9 microm in diameter and occurred singly or in pairs. The strain grew at 20-42 degrees C (optimum 37 degrees C), at pH 6.0-7.75 (optimum pH 7.0-7.25) and in 0-0.35 M NaCl (optimum 0.1 M). The G+C content of the genomic DNA was 41.4 mol%. 16S rRNA gene sequencing revealed that the strain was a member of the genus Methanosarcina but that it clearly differed from all recognized species of this genus (93.1-97.9 % sequence similarity). The phenotypic and phylogenetic features of strain HB-1(T) indicate that it represents a novel species of the genus Methanosarcina, for which the name Methanosarcina horonobensis sp. nov. is proposed. The type strain is HB-1(T) ( = DSM 21571(T) = JCM 15518(T) = NBRC 102577(T)).
e00789-19
DOI
URL
PMID
[Cited within: 1]

Toxin-antitoxin (TA) systems are broadly distributed modules whose biological roles remain mostly unknown. The mqsRA system is a noncanonical TA system in which the toxin and antitoxins genes are organized in operon but with the particularity that the toxin gene precedes that of the antitoxin. This system was shown to regulate global processes such as resistance to bile salts, motility, and biofilm formation. In addition, the MqsA antitoxin was shown to be a master regulator that represses the transcription of the csgD, cspD, and rpoS global regulator genes, thereby displaying a pleiotropic regulatory role. Here, we identified two promoters located in the toxin sequence driving the constitutive expression of mqsA, allowing thereby excess production of the MqsA antitoxin compared to the MqsR toxin. Our results show that both antitoxin-specific and operon promoters are not regulated by stresses such as amino acid starvation, oxidative shock, or bile salts. Moreover, we show that the MqsA antitoxin is not a global regulator as suggested, since the expression of csgD, cspD and rpoS is similar in wild-type and DeltamqsRA mutant strains. Moreover, these two strains behave similarly in terms of biofilm formation and sensitivity to oxidative stress or bile salts.IMPORTANCE There is growing controversy regarding the role of chromosomal toxin-antitoxin systems in bacterial physiology. mqsRA is a peculiar toxin-antitoxin system, as the gene encoding the toxin precedes that of the antitoxin. This system was previously shown to play a role in stress response and biofilm formation. In this work, we identified two promoters specifically driving the constitutive expression of the antitoxin, thereby decoupling the expression of antitoxin from the toxin. We also showed that mqsRA contributes neither to the regulation of biofilm formation nor to the sensitivity to oxidative stress and bile salts. Finally, we were unable to confirm that the MqsA antitoxin is a global regulator. Altogether, our data are ruling out the involvement of the mqsRA system in Escherichia coli regulatory networks.




 WeChat
WeChat