1. Introduction
Drinking water shortage has caused serious concerns with increasing population and industrialization [1,2]. Solely relying on labor to deliver freshwater from water-rich areas to water-deprived areas is unrealistic. Seawater desalination, the technology to turn seawater into fresh drinking water, has been successfully adopted to provide drinking water in a number of countries. However, this technology remains costly and thus insufficient to supply water for all the water-stressed regions in the world. Over 700 million people still live under water shortage conditions [3,4]. Fog, which contains a large number of micron-sized water droplets, is ubiquitous and has attracted great amount of attention recently as a potential source for fresh water. Towards this aim, many creatures have demonstrated excellent ability to harvest water from the fog even in a very dry ambience, including Namib Desert beetle [5,6] (patterned back), cactus [7,8] (conical spine), spider silk [[9], [10], [11]] (spindle-knots structure), etc. Inspired by these creatures, various advanced fog collection technologies have been designed to combat the water shortage problem [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]].
In nature, superhydrophobic-superhydrophilic patterned surface possesses a great potential to effectively collect water from fog. In 2001, Parker and Lawrence first discovered that Namib desert beetle captures water by using its complementary superhydrophobic-superhydrophilic skeleton on the back. Every morning when the fog is relatively dense, the fog droplets are condensed on the hydrophilic area of the desert beetle back and then roll along the waxy hydrophobic crevices until falling down to beetle’s mouth [24]. Inspired by this interesting strategy, scientists have successfully constructed water-trapping tent and water condensers. Through the past research, it is recognized that in order to achieve a continuous and efficient fog harvesting process, the fog collector must satisfy three key criteria: (1) good condensate performance, (2) rapid transport of the condensed water on the surface, (3) continuous generation of fresh water on the surface [25,26]. Moreover, Cao et al. [13] have proved that boundary layer on the surface of fog collector can further affect the collection efficiency.
Despite all these efforts, realizing industrial scale production remains a challenge due to cost of materials and the complicated processing techniques involved. For example, some lab scale fog collectors used UV irradiation, inkjet printing, or lithography process to prepare fine micro-nano surface structures, which hinders the up-scaling of the technology [18,19,[27], [28], [29], [30], [31], [32], [33]]. Inspired by the Namib Desert beetle, we introduced a superhydrophobic-superhydrophilic (SHB-SHL) patterned low-cost polyester fabric by a facile textile weaving method as the fog collector. High thermal conductivity is necessary for efficient fog collection [[34], [35], [36]], materials such as Cu [6,20,37], Al [[38], [39], [40]] and Fe [41] have been used before. Here, we used a one-step copper mirror reaction to selectively deposit copper on the patterned fabrics (denoted as Cu-SHB-SHL). Effect of the physical structure, wettability, and thermal conductivity of the material surface on the superhydrophilic-superhydrophobic composite polyester fabric was investigated. By comparison with other fabrics, this Cu-SHB-SHL patterned fabric showed an extremely efficient water-harvesting rate (1432.7 mg/h/cm2). This demonstrated technology is facile, inexpensive, and highly efficient in water collection from fog. It brings us one step closer towards low-cost large scale provision of fresh water to the needy region in the world.
2. Experimental
2.1. Fabrication of desert beetle-inspired superwettable fabrics
2.1.1. Pre-treatment of polyester yarns
Pristine polyester yarns were cleaned with ethanol solution and deionized water then dried in an oven at 60 ℃. The as-cleaned polyester yarns displays a water contact angle around 0°, and thus are denoted as superhydrophilic yarns thereafter.
2.1.2. Preparation of superhydrophobic polyester yarns
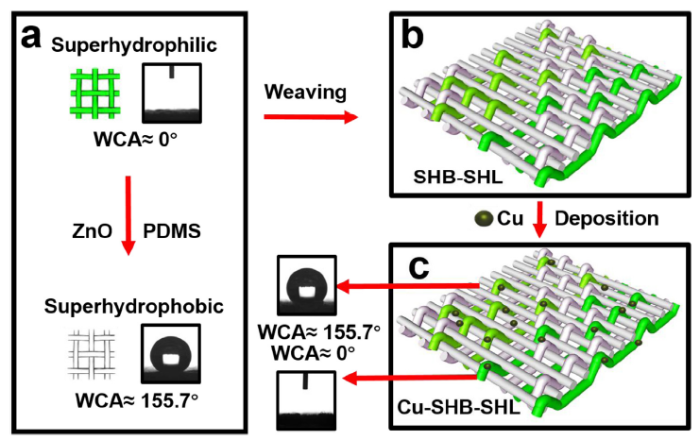
Superhydrophobic solution was prepared as follows: 2 g of PDMS (mpre-polymer : mcuring agent = 10 : 1) was completely dissolved in 60 mL n-hexane. Then 0.2 g of ZnO nano-particles were dispersed in the above solution. The superhydrophobic polyester yarns were obtained by dipping the dried polyester yarns into the prepared solution for 20 min and dried at 60 °C for 30 min.
2.1.3. Preparation of superwettable patterned woven fabric
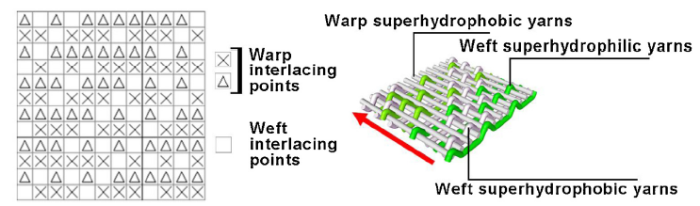
To mimic the structure of Namib Desert beetle, we adopted a commercial weaving method in textile industry. Polyester was used as the material of the fog collector due to its durability, low cost, light weight, and flexibility. First, the yarns were soaked into the PDMS solution to obtain the superhydrophobic yarns as described above. As shown in Scheme 1, the warp direction on the weaved surface comprises superhydrophobic polyester yarns only, while the weft direction comprises alternating superhydrophobic / superhydrophilic polyester yarns. If the weave point, the uppermost point exposed on the fabric surface, is formed by superhydrophilic weft yarn, the point will exhibit superhydrophilicity. Otherwise, it will display superhydrophobicity. All the weave points together constitute a patterned surface. When the warp yarns are superhydrophobic and weft direction consist of alternating superhydrophobic and superhydrophilic yarns (as shown in Scheme 1), the weaved fabric is denoted as SHB-SHL. On the other hand, when the warp yarns are superhydrophilic and the weft yarns are superhydrophobic and superhydrophilic, the weaved fabric is called as SHL-SHB. For comparison, we have prepared fabrics using superhydrophobic polyester yarns (SHB) or superhydrophilic polyester yarns (SHL) in both warp and weft directions. We have also designed several different weave structures, such as twill weave and double weaves. Scheme 2 shows the weaving process of the patterned surfaces. The reed number used for weaving all samples is 80#. All the samples are weaved with a semiautomatic loom.
Scheme 1.
Scheme 1.
Schematic diagram of weaved fabric structure for a minimum unit (left) and illustration of SHB-SHL fabric (right).
Scheme 2.
Scheme 2.
This schematic diagram showed the whole preperation process for various weaved fabric including (a) the prepation of superhydrophobic yarns, (b) fabrication of various types of fabric as well as (c) copper particles selective deposition on weaved fabric.
2.2. In-situ copper deposition
The thermal conductivity of polyester fibers is poor, which would hamper the fog collection efficiency. Coating with copper on the fiber surface could enhance their thermal conductivity. We have in-situ deposited copper particles onto the fabric surface via a simple copper mirror reaction process (the whole reaction just takes 20 min). Firstly, the fabrics were dipped into tin chloride dihydrate solution (1 wt%) for 5 min and then dried at 70 °C. This sensitization process ensures that copper can be then deposited on the fabrics. After that, 8 g of CuSO4 was added into 100 mL of deionized water, then ammonium hydroxide solution was dropwise added into CuSO4 solution. This whole reaction process produced precipitate at first (Equ. 1), but after further addition of ammonium hydroxide solution, the precipitate were completely disappeared (Equ. 2). Extra ammonium hydroxide would cause the occurrence of copper mirror reaction, which induces reduction of Cu2+ to Cu° (Equ. 3). The as-prepared solution described above is labelled as solution A. Separately, 0.5 g of potassium sodium tartrate tetrahydrate and 5 g of sodium hydrosulfite was dissolved into 100 mL of water to form solution B. The fabric was immersed in solution A first, then solution B waadded to the solution A for another 5 min at 40 °C. The fabric was then removed from the solution and dried at 70 °C. The whole deposition process is described by Eqs. (1), (2), (3).
2.3. Fog collecting measurement
The laboratory-made fog collection device (size: 60 × 60 × 60 cm3) is used to evaluate the fog harvesting rate of the as-prepared fabric samples. It consists of a humidifier, an iron support stand and a beaker. As shown in Fig. S1, the diameter of the droplets generated by the humidifier is 3~10 μm, which is similar to the fog droplets in the real world (5~15 μm). The continuous mist flow in the experiment is provided by the humidifier at a flow rate of ~300 mL/h onto the fabrics held by the support stand, located 20 cm away from the humidifier. When the droplets gravity is greater than the adhesion force of the fog collector, the fog droplets can be captured by the beaker under the fog collector. To determine the water harvesting rate, the amount of collected water in the beaker was weighted at one-hour intervals. Six measurements were carried for each sample.
2.4. Characterization
The morphology of the fibers were examined by a field emission scanning electron microscope (FESEM, Hitachi S-4800). Before the observation, the fibers were coated with a thin layer of Au to ensure the electrical conductivity of the samples. Energy-dispersive X-ray spectrometer (EDS) was used to map the surface elemental distribution. The water contact angle (WCA) and sliding angle (SA) of the prepared samples was tested using an optical contact angle meter system (Krüss DSA 100, Germany). The detailed processes of fog harvesting were recorded with Phantom VEO410 L high-speed camera. A flowmeter (Testo 450-V1, Germany) was employed to measure the fog flow rate.
3. Results and discussion
To further improve the efficiency of fog collection, some key factors have been studied. In the process of capturing water droplets from the fog, the size of droplets in the fog flow is typically less than 100 μm, thus air resistance can be significant. The boundary layer theory is used to explain this phenomenon: the velocity of the fog flow close to the sample can be affected by the boundary layer [13,42]. The thicker the boundary layer of an object, the slower the velocity of the fog flow passing through the surface of the object. The fog capturing process begins with a collision of the droplets toward the substrate. Only when the kinetic energy of droplet is fully consumed, the droplet can then be captured by the fog collector. Therefore, a higher collision frequency between the droplets and the collector surface leads to a better fog capturing ability. Since the water droplets move along with the fog flow, the speed of the fog flow can be approximated as the speed of the airflow (~70 cm/s). The Reynolds number (Re) is given by the Eq. (4):
where ρ, u and μ are the density, velocity and viscosity of the fog flow, respectively. d is the standard length equal to 1 for a plain smooth plate surface. By comparing the Re number obtained after a simple calculation (~5 *104) with the standard values (5 *105), it can be considered that the fog flow is regarded as laminar flow. Therefore, the thickness of boundary layer for a plain plate can be calculated with Eq. (5):
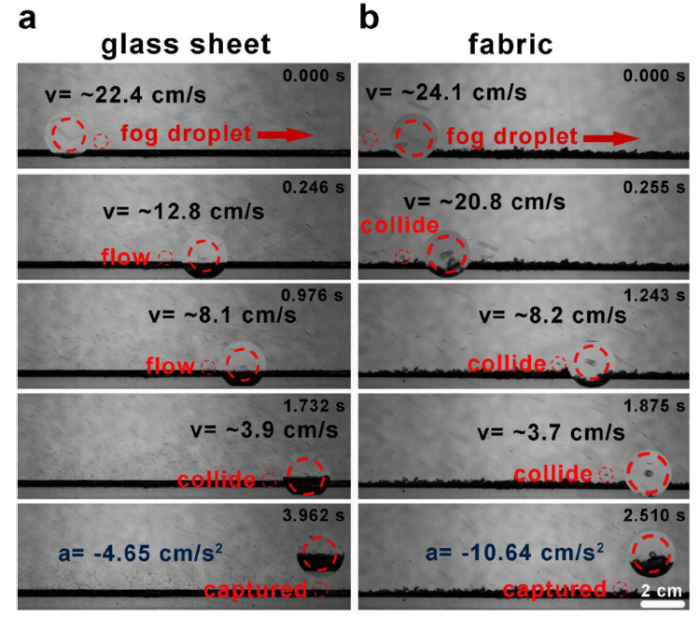
where δ is the thickness of boundary layer, x is the distance between the fog collector and the fog flow (ranges from 0 to 30 mm). From Eq. (5), the calculated δ is less than 67 μm, which indicates that the droplets decelerate sharply as they flow through the area. With the same thickness of the boundary layer, the humps of rough fabric can increase the collision probability between the fog droplets and the substrate, and consume the kinetic energy of the droplet. Therefore, the fabric with humps is easier to capture the fog droplets than the smooth glass sheet. We have carried out a laboratory experiment to verify the validity of the above discussion. Under the effect of boundary layer, the droplets near the surface of the smooth plate have a slow speed (~3.9 cm/s). However, it takes a long time to run out of the fog droplet’s kinetic energy, which is not conducive to the collection device to its capturing (Fig. 1(a) and Movie S1). On the contrary, the humps on the fabric can significantly reduce the effect of boundary layer. The fog droplets decelerate sharply as they approach the surface of the soft fabric (from 20.8 cm/s to 3.7 cm/s), which indicates that the fog droplets can consume its kinetic energy in a short time (Fig. 1(b) and Movie S2). Moreover, a simple calculated of the acceleration (Eq. 6) further proves the importance of humps:
where a, d and t is the acceleration, distance and time of fog droplets during the flow process. Accordingly, the acceleration of the fog droplet is -4.65 cm/s2 on the smooth glass sheet and -10.65 cm/s2 on the fabric.
Fig. 1.
Fig. 1.
The behavior of the fog droplets when it colloids, decelerates and spreads on a smooth glass sheet (a) and fabric with rough surface (b). One typical droglet was magnified for clear view. The scale bar represents 2 cm and all images have the same scale.
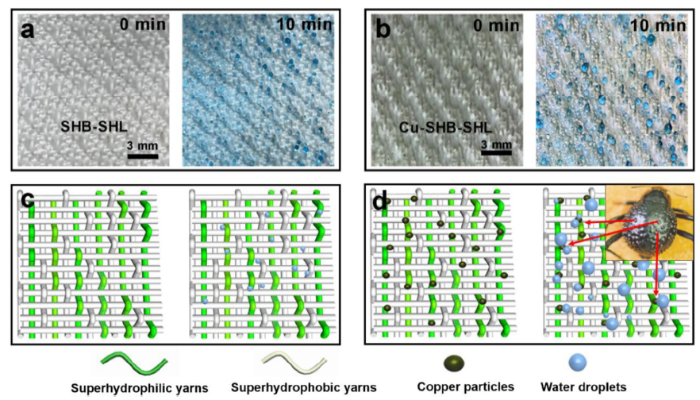
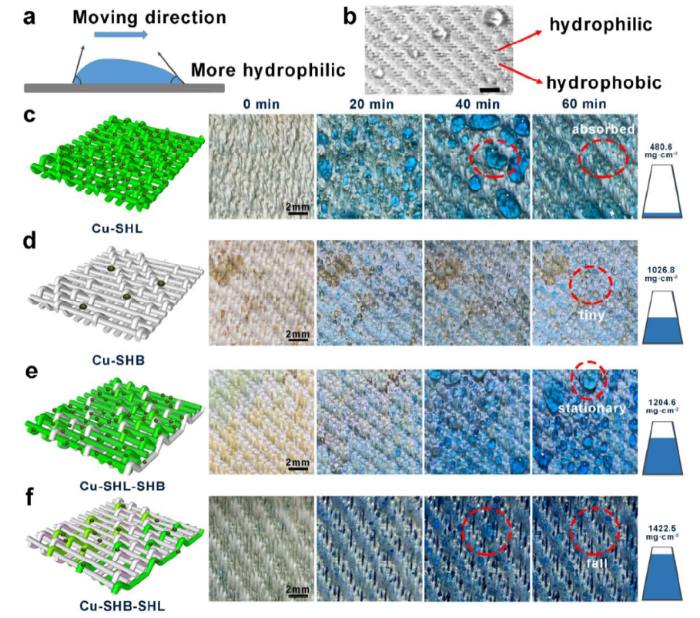
The thermal conductance of the substrate material is also an important factor for the efficiency of fog harvesting. When the fog is in contact with the substrate surface, it transfers the latent heat to the substrate before it condenses. The thermal conductivity of substrate material is therefore an important factor in the process of fog condensation. Unfortunately, the thermal conductivity of polyester is low at 0.084 W/m/K). To improve the thermal conductivity of the substrate, we deposited copper (thermal conductivity 377 W/m/K) particles on the polyester yarns. Fig. 2(a) shows the condensation dynamics of the SHB-SHL patterned fabric from 0 min to 10 min. From the optical images, some tiny droplets condensed on the SHB-SHL patterned fabric surface. In comparison, the Cu-SHB-SHL patterned fabric collected more droplets within 10 min (Fig. 2(b)). Moreover, the size of collected fog droplets on the SHB-SHL patterned fabric were smaller than that of Cu-SHB-SHL patterned fabric. Fig. 2(c), (d) further illustrate the mechanism when the fog droplets condensed on the fabrics. As shown in Fig. 2(c), only a small number of droplets have condensed on the surface of the pristine fabric. While after copper coating, the volume and number of condensed fog droplets are larger than in the case without copper coating (Fig. 2(d)). At the same time, it can be seen that a large number of droplets are condensed on the surface of the copper coating, which indicate the copper coating is the key factor for fog condensation (as same as the humps of Namib desert beetle). Based on the higher thermal conductivity of copper particles, Cu-SHB-SHL patterned fabric showed a higher fog harvesting efficiency.
Fig. 2.
Fig. 2.
The effects of copper coating on fog harvesting. (a) The fog harvesting behavior on a SHB-SHL patterned fabric from 0 min to 10 min. After 10 min, the SHB-SHL patterned fabric captured some tiny fog droplets. (b) The fog harvesting behavior on a Cu-SHB-SHL patterned fabric from 0 min to 10 min. After 10 min, the Cu-SHB-SHL patterned fabric captured more tiny fog droplets and some fog droplets grow bigger. (c) and (d) are schematic diagrams of the dynamic collection process of SHB-SHL and Cu-SHB-SHL, respectively. Inserted image is a digital photo of Namib desert beetle (copyright 2001, Nature) [5]. The water in the sprayer was dyed with methyl blue for better viewing. The scale bars are 3 mm.
The effect of the area ratio between the superhydrophilic and superhydrophobic fiber yarns on the water harvesting rate was also investigated. Three fabrics with different superhydrophilic/ superhydrophobic area ratios were weaved by changing the weave organization structure. Fig. S2 showed the effect of the proportion of superhydrophilic and superhydrophobic regions on the fog harvesting. As shown in Fig. S2(a), when area ratio of SHB: SHL = 3: 1 (superhydrophobic area is larger than the superhydrophilic area), there were less water droplets captured and it took a longer time for water droplets to fall down to the surface. Similarly, when area ratio of SHB: SHL = 1: 3, too much the superhydrophilic area caused the droplets to be absorbed into the fabric yarns instead of rolling down into the collection point (Fig. S2(c)). Finally, when the area ratio of SHB: SHL = 2: 2, the fog collector has the best water harvesting performance (Fig. S2(b)). In addition, WHR (water harvesting rate) of 3 samples showed in Fig. S3, further confirmed that the sample under the area ratio of SHB: SHL = 2: 2 is the best one for fog harvesting.
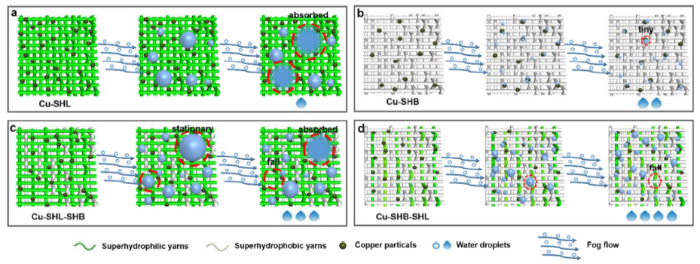
Based on the excellent fog harvesting property of the as-prepared Cu-SHB-SHL patterned fabric, a low-cost and high-efficiency fog collection system was built in the laboratory. The fog collecting process and collection efficiency of this Cu-SHB-SHL patterned fabric were bench marked with the seven other substrates. Fig. 3(a) illustrates the mechanism of droplets movement on superwettable patterned fabric. Driven by the surface wettability gradient, droplets tend to move toward the more wettable area. Fig. 3(b) and Movie S3 display a simple simulation of the fog flow towards the substrate. To further clarify the impact of different wettability on fog collection, we tracked the fog collection process by capturing a series of optical images on four different fabrics (Cu-SHL, Cu-SHB, Cu-SHL-SHB and Cu-SHB-SHL) from 0 min to 60 min. Fig. 3(c) and d respectively show the surface of Cu-SHL and Cu-SHB after collection. The Cu-SHL patterned fabric collected many fog droplets, but it lost the ability to transport the collected droplets because of the superhydrophilic property, the captured droplets are absorbed by the sample. On the contrary, although the Cu-SHB fabric can transport the captured droplets, it can’t capture too many droplets and the captured droplets are very small. As shown in Fig. 3(e), under the interaction of both superhydrophilic and superhydrophobic yarns, the Cu-SHL-SHB fabric was able to capture droplets and transport them to the collection device. However, since the peak points on the fabric surface is superhydrophilic, the fog droplets captured on the surface cannot be transported away quickly and exposed the active condensation sites, which has cause a decrease in the droplet capturing efficiency. The best performance was offered by the Cu-SHB-SHL surface as shown in Fig. 3(f)f, most of the tiny droplets from the fog flow were condensed on the separated superhydrophilic area and aggregated before the droplets overcame the capillary force to roll into the collection beaker through the superhydrophobic channels. Fig. 4 further summarized the detailed fog condensation and droplet transportation mechanism of the four fabrics during the continued fog harvesting processes.
Fig. 3.
Fig. 3.
(a) Illustration of the moving behavior when the droplet on a surface with two different wettabilities. (b) The distribution of water droplets when water droplets fall on the surface of a fabric with two different wettabilities. (c)-(f) Scheme and condensation dynamics of Cu-SHL, Cu-SHB, Cu-SHL-SHB and Cu-SHB-SHL patterned fabric from 0 min to 60 min. The water in the sprayer was dyed with methyl blue. The scale bars are 2 mm.
Fig. 4.
Fig. 4.
The effects of distribution of different wettabilities on fog harvesting. (a), (b), (c) and (d) are schematic diagrams of the dynamic fog harvesting process of Cu-SHL, Cu-SHB, Cu-SHL-SHB and Cu-SHB-SHL, respectively.
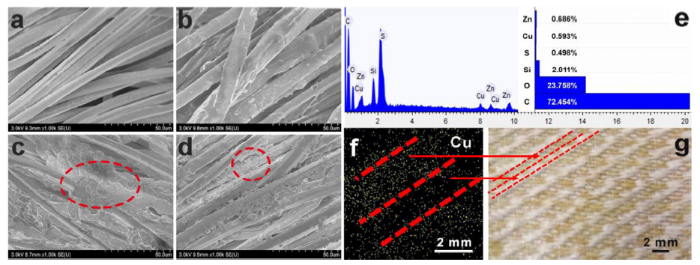
Since the fog collection process is carried out outdoor, the device will be exposed to sunlight for a long time. To improve the UV resistance of the polyester, we added ZnO nanoparticles to PDMS[43]. Meanwhile, the addition of ZnO nanoparticles also increased surface roughness of the fabric. Fig. S4 shows only a slight decrease in the WHR and water contact angle (WCA) of Cu-SHB-SHL at different time intervals, confirming that the Cu-SHB-SHL indeed has improved the UV resistance. Fig. 5(a), (b) show SEM images of polyester yarns before and after being treated with PDMS and ZnO nanoparticles. Fig. S5(a), (b) illustrate that the superhydrophobic area on as-prepared patterned fabric has a high WCA of 155.7° and a rolling angle of 11.3°. After the copper deposition, the wettability of the Cu-SHB-SHL surface remained unchanged, because the superhydrophobic areas basically do not participate in the reaction.
Fig. 5.
Fig. 5.
SEM of the fibers with different treatment processes. (a) Untreated fibers. (b) After PDMS and ZnO coating. (c) The superhydrophilic fibers with in-situ copper deposition. (d) The superhydrophobic fibers after in-situ copper deposition. (e) EDS spectrum and atomic ratio of the Cu-SHL-SHB patterned fabric. (f) Cu distribution on the Cu-SHL-SHB patterned fabric. (g) Digital photo of the Cu-SHB-SHL patterned fabric. The scale bar is 2 mm.
SEM images confirm the presence of copper particles on the surface of the superhydrophilic fibers (Fig. 5(c)) and superhydrophobic fibers (Fig. 5(d)). Energy dispersive spectroscopy (EDS) results of the Cu-SHB-SHL, Cu-SHL and Cu-SHB fabrics are displayed in Fig. 5(e), (f), (g), Fig. S6(a)-(c) and Fig. S7(a)-(c). As expected, new peaks belonging to Cu element on Cu-SHB-SHL appeared after the deposition (Fig. 5(e)). Fig. 5f shows elemental mapping of Cu-SHB-SHL surface. Fig. 5g is the digital photo of the Cu-SHB-SHL fabric, which clearly shows the existence of copper particles uniformly distributed on the Cu-SHB-SHL fabric.
The efficiency of the fog collector is characterized through WHR following Eq. (7):
where wt, w0 represent the weight of the collected water with the sample and without, respectively. S represents the area of the samples and t is the collecting time.
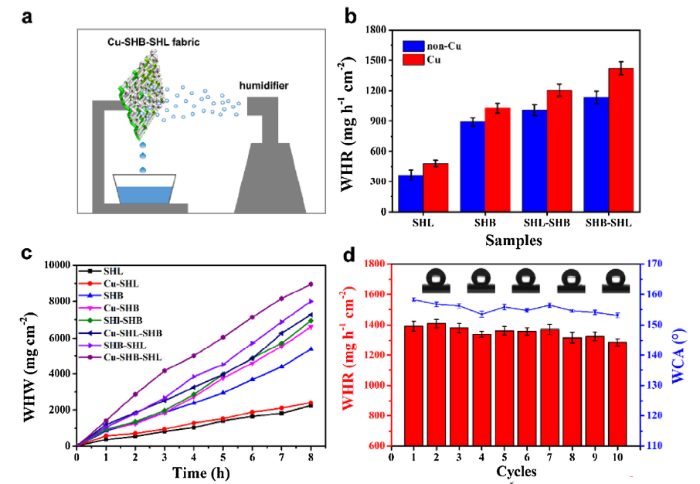
Fig. 6(a) displays a lab-made fog harvesting device, including a humidifier, an iron support stand and a beaker. The water harvesting capacities on multifarious wetting samples and as-prepared Cu-SHB-SHL are illustrated in Fig. 6(b). Fabrics coated with copper are more capable of collecting fog droplets than those without. Meanwhile, fabrics with the SHB-SHL wettability contrast showed an especially high WHR than other three types of samples. Fig. 6(c) shows the water-harvesting capacity of all the 8 samples with increasing time. From the results, the Cu-SHB-SHL sample has the highest WHR value at 1432.7 mg/h/cm2, and the water contact angle exceeds 150°. In addition, the WHR values on SHB and SHL are lower than SHL-SHB and SHB-SHL. This is because the water droplets captured by the superhydrophilic area are difficult to be detached. Similarly, water droplets in the fog flow are difficult to be captured by single superhydrophobic regions. The best solution is with the synergistic effect between SHB and SHL regions as demonstrated by the Namib desert beetle. The better fog collection ability of the SHB-SHL surface than SHL-SHB can be explained by boundary layer theory. The superhydrophilic humps cannot deliver the captured fog droplets in a short period of time, but absorbed some captured fog droplets,therefore hampered the subsequent collection process and the WHR will decrease. While superhydrophobic humps can avoid the adhesion of water droplets, and continuous transport of droplets for fog collection. Fig. 6(d) exhibits that after 10 cycles of fog collection, the superhydrophobic area is able to sustainably maintain its antiwetting ability and the WHR still remained at a high level. Moreover, by compared the WHR of Cu-SHB-SHL with the samples of other literatures (Fig. S8), further proving that Cu-SHB-SHL is one of the most efficient fog collector.
Fig. 6.
Fig. 6.
(a) The lab-made fog harvest device: a fog flow was produced by the humidifier to the prepared samples to harvest water and transport water into the container below. (b) WHR value of all samples. (c) WHW (water harvesting weight) tests of 8 samples during a period of 8 h. (d) WHR on the sample of Cu-SHB-SHL fabric for 10 cycles of fog-harvesting processes. Insets in d are optical WCAs images on the superhydrophobic areas. The size of all samples are 3 cm × 3 cm.
Furthermore, the cost of Cu-SHB-SHL was estimated. The plain polyester with mass per unit of 70 g m-2 is about \$ 0.5, together with CuSO4, Tin chloride dehydrate, potassium sodium tartrate tetrahydrate, sodium hydrosulfite, ammonium hydroxide solution, ZnO particles, N-hexane and polydimethylsiloxane, the total cost is less than \$1.5 per m2. (the price is from local market and the exchange rate is based on the exchange rate on May 16, 2020). Such low costclearly favors practical applications.
4. Conclusion
By learning the fog collection strategy from Namib Desert beetle, a fabric with two different wettability regions with copper coating was prepared via a simple weaving method. The WHR of the Cu-SHB-SHL patterned fabric reached 1432.7 mg/h/cm2, higher than the other 7 reference surface patterns. During the collecting process, the Cu-SHB-SHL fabric performed a rapid, timely, spontaneous, and unidirectional transportation of collected droplets. Through a series of studies, the excellent performance of the Cu-SHB-SHL sample is attributed to the increased thermal conductivity though Cu deposition, the properly designed hump structures, and the synergistic interaction of yarns with distinct wettability. This technology is facile, inexpensive, and highly efficient in water collection from fog. It is potentially suitable for large-scale water harvesting for the arid region to tackle the freshwater scarcity problem.
Acknowledgements
Z.H.Y and H.M.Z contribute equally to this work. The authors thank the National Natural Science Foundation of China (51972063; 21501127; 51502185), Natural Science Foundation of Fujian Province (2019J01256), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1916). We also acknowledge the funds from China postdoctoral science foundation grant (2019TQ0061).
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jmst.2020.05.054.
Reference
DOI
URL
PMID
[Cited within: 1]

Freshwater scarcity is increasingly perceived as a global systemic risk. Previous global water scarcity assessments, measuring water scarcity annually, have underestimated experienced water scarcity by failing to capture the seasonal fluctuations in water consumption and availability. We assess blue water scarcity globally at a high spatial resolution on a monthly basis. We find that two-thirds of the global population (4.0 billion people) live under conditions of severe water scarcity at least 1 month of the year. Nearly half of those people live in India and China. Half a billion people in the world face severe water scarcity all year round. Putting caps to water consumption by river basin, increasing water-use efficiencies, and better sharing of the limited freshwater resources will be key in reducing the threat posed by water scarcity on biodiversity and human welfare.
DOI
URL
PMID
[Cited within: 2]

Some beetles in the Namib Desert collect drinking water from fog-laden wind on their backs. We show here that these large droplets form by virtue of the insect's bumpy surface, which consists of alternating hydrophobic, wax-coated and hydrophilic, non-waxy regions. The design of this fog-collecting structure can be reproduced cheaply on a commercial scale and may find application in water-trapping tent and building coverings, for example, or in water condensers and engines.
DOI
URL
PMID
[Cited within: 1]

Many biological surfaces in both the plant and animal kingdom possess unusual structural features at the micro- and nanometre-scale that control their interaction with water and hence wettability. An intriguing example is provided by desert beetles, which use micrometre-sized patterns of hydrophobic and hydrophilic regions on their backs to capture water from humid air. As anyone who has admired spider webs adorned with dew drops will appreciate, spider silk is also capable of efficiently collecting water from air. Here we show that the water-collecting ability of the capture silk of the cribellate spider Uloborus walckenaerius is the result of a unique fibre structure that forms after wetting, with the 'wet-rebuilt' fibres characterized by periodic spindle-knots made of random nanofibrils and separated by joints made of aligned nanofibrils. These structural features result in a surface energy gradient between the spindle-knots and the joints and also in a difference in Laplace pressure, with both factors acting together to achieve continuous condensation and directional collection of water drops around spindle-knots. Submillimetre-sized liquid drops have been driven by surface energy gradients or a difference in Laplace pressure, but until now neither force on its own has been used to overcome the larger hysteresis effects that make the movement of micrometre-sized drops more difficult. By tapping into both driving forces, spider silk achieves this task. Inspired by this finding, we designed artificial fibres that mimic the structural features of silk and exhibit its directional water-collecting ability.
DOI
URL
PMID
[Cited within: 1]

Titanium dioxide nanotubes (TNTs) have drawn wide attention and been extensively applied in the field of biomedicine, due to their large specific surface area, good corrosion resistance, excellent biocompatibility, and enhanced bioactivity. This review describes the preparation of TNTs and the surface modification that entrust the nanotubes with better antibacterial property and enhanced osteoblast adhesion, proliferation, and differentiation. Considering the contact between TNTs' surface and surrounding tissues after implantation, the interactions between TNTs (with properties including their diameter, length, wettability, and crystalline phase) and proteins, platelets, bacteria, and cells are illustrated. The state of the art in the applications of TNTs in dentistry, orthopedic implants, and cardiovascular stents are introduced. In particular, the application of TNTs in biosensing has attracted much attention due to its ability for the rapid diagnosis of diseases. Finally, the difficulties and challenges in the practical application of TNTs are also discussed.
DOI
URL
PMID
[Cited within: 3]

Harvesting micro-droplets from fog is a promising method for solving global freshwater crisis. Different types of fog collectors have been extensively reported during the last decade. The improvement of fog collection can be attributed to the immediate transportation of harvested water, the effective regeneration of the fog gathering surface, etc. Through learning from the nature's strategy for water preservation, the hydrophobic/hydrophilic cooperative Janus system that achieved reinforced fog collection ability is reported here. Directional delivery of the surface water, decreased re-evaporation rate of the harvested water, and thinner boundary layer of the collecting surface contribute to the enhancement of collection efficiency. Further designed cylinder Janus collector can facilely achieve a continuous process of efficient collection, directional transportation, and spontaneous preservation of fog water. This Janus fog harvesting system should improve the understanding of micro-droplet collection system and offer ideas to solve water resource crisis.
Inspired by the water-collecting strategies of desert beetles and spider silk, a novel kind of surface with star-shaped wettablity patterns has been developed. By combining both wettability and shape gradients, the as-prepared surface has gained higher efficiency in water collection compared to circle-shaped wettability patterns and uniformly superhydrophilic or superhydrophobic surfaces.
DOI
URL
PMID
[Cited within: 1]

One-dimensional materials (1D) capable of transporting liquid droplets directionally, such as spider silks and cactus spines, have recently been gathering scientists' attention due to their potential applications in microfluidics, textile dyeing, filtration, and smog removal. This remarkable property comes from the arrangement of the micro- and nanostructures on these organisms' surfaces, which have inspired chemists to develop methods to prepare surfaces with similar directional liquid transport ability. In this Account, we report our recent progress in understanding how this directional transport works, as well our advances in the design and fabrication of bioinspired 1D materials capable of transporting liquid droplets directionally. To begin, we first discuss some basic theories on droplet directional movement. Then, we discuss the mechanism of directional transport of water droplets on natural spider silks. Upon contact with water droplets, the spider silk undergoes what is known as a wet-rebuilt, which forms periodic spindle-knots and joints. We found that the resulting gradient of Laplace pressure and surface free energy between the spindle-knots and joints account for the cooperative driving forces to transport water droplets directionally. Next, we discuss the directional transport of water droplets on desert cactus. The integration of multilevel structures of the cactus and the resulting integration of multiple functions together allow the cactus spine to transport water droplets continuously from tip to base. Based on our studies of natural spider silks and cactus spines, we have prepared a series of artificial spider silks (A-SSs) and artificial cactus spines (A-CSs) with various methods. By changing the surface roughness and chemical compositions of the artificial spider silks' spindle-knots, or by introducing stimulus-responsive molecules, such as thermal-responsive and photoresponsive molecules, onto the spindle-knots, we can reversibly manipulate the direction of water droplet's movement on the prepared A-SSs. In addition, the A-SSs with nonuniform spindle-knots, such as multilevel sized spindle-knots and gradient spindle-knots, further demonstrate integrated directional transport ability for water droplets. Through mimicking the main principle of cactus spines in transporting water droplets, we were able to fabricate both single and array A-CSs, which are able to transport liquid droplets directionally both in air and under water. Lastly, we demonstrated some applications of this directional liquid transport, from aspects of efficient fog collection to oil/water separation. In addition, we showed some potential applications in smart catalysis, tracer substance enrichment, smog removal, and drug delivery.
DOI
URL
PMID
[Cited within: 1]

Dehydration (10 days at 27 degrees C) of the Namib tenebrionid Stenocara gracilipes resulted in a rapid weight loss (17.5%), and a substantial decline in haemolymph volume (72%). Although the lipid content decreased significantly, metabolic water production was insufficient to maintain total body water (TBW). Rehydration (no food) resulted in increases in haemolymph volume, body weight (sub-normal), and TBW to normality. Haemolymph osmolality, sodium, potassium, chloride, amino acids, and sugars (trehalose and glucose), were all subject to osmoregulatory control during both dehydration and rehydration. Major osmolar effectors in this species are sodium, chloride, and amino acids, with most of the contribution to regulation of haemolymph osmolality coming from changes in the levels of these constituents. Changes in amino acid levels are not the result of interchange with soluble protein during dehydration (the possibility exists during extended rehydration, however). Despite faecal losses of sodium being low (8.2% of that removed from the haemolymph during dehydration), sodium concentrations do not return to normal during rehydration. Chloride concentrations increase supra-normally when access to water is allowed, and remain elevated throughout the rehydration period. Although faecal loss of potassium greatly exceeded the amount removed from the haemolymph (by approximately 1.8 times), haemolymph potassium levels were strongly regulated during rehydration. S. gracilipes demonstrates an exquisite capacity to regulate haemolymph osmolality under conditions of both acute water-shortage and -abundance. Together with an efficient water economy (drinking when fog-water is available, and a superb water conservation mechanism in the form of wax-bloom production), this must serve to contribute to long-term survival of this species in an otherwise harsh abode.
DOI
URL
PMID
[Cited within: 1]

The microdroplets in fog flow have been considered as an important resource for supplying fresh drinking water. Most of the reported works of fog collection focus on the water-collecting ability rather than the environmental reliability of selected materials. In this work, a beetle-inspired hierarchical fog-collecting interface based on the antibacterial needle-array (ABN) and hydrophilic/hydrophobic cooperative structure is displayed. The hydrophilic ABN is coated with zwitterionic carboxybetaine (CB) brushes that endow the fog collector with a long-term cleaning in harsh environment. Due to its strong affinity to water molecules, the tilted needles with a CB coating can facilitate the capture of fog and the rapid delivery of condensed water driven by gravity. After being transported to the connected hydrophobic sheet, the collected droplets can be rapidly detached and stored in the container, achieving a high fog-harvesting rate. Furthermore, CB-patterned channels are integrated on the hydrophobic sheet for the pathway-controlled water delivery. The CB coating is able to efficiently resist bacterial adhesion and contamination during fog harvesting, protecting the device from microbiological corrosion. The current design provides a promising method to incorporate antibacterial ability into fog collectors, which offer great opportunity to develop water harvesters for real-world applications.




 WeChat
WeChat