1. Introduction
Soft magnetic amorphous and nano-crystalline materials have attracted considerable interest for their energy-saving potential due to their high magnetic performance [1,2]. Today, silicon steels are predominately used for various magnetic applications, but have the drawback of high core losses (∼ hysteresis losses + eddy current losses). Research has shifted from improving the magnetic properties of conventional materials to ferromagnetic amorphous alloys, and today, towards nano-crystalline materials [3,4]. Nano-crystalline soft ferromagnetic alloys were introduced 30 years ago and show excellent soft magnetic properties through precisely controlled nano-grains embedded in an amorphous matrix [[5], [6], [7], [8], [9]]. These particular materials are expected to substitute Fe-Si alloys or ferrites for high performance power transformers and electric motors [10]. Soft ferromagnetic glass-forming alloys produced with high purity exhibit a high glass-forming ability, a high degree of magnetic softness (low coercivity, high effective permeability, and low core losses), as well as a high saturation magnetization, mechanical strength, and corrosion resistance [11].
The quality of amorphous materials depends on alloy composition and cooling conditions during solidification. The melt must be cooled quickly to circumvent crystallization. The required cooling rates limit the sample geometry and size, as cooling or quenching is often governed by time-dependent heat conduction that causes crystallization. Melt spinning is the most commonly used technique to produce amorphous ribbons. Amorphous ribbons are subsequently heat-treated to adjust their soft magnetic properties. These ribbons are usually wound around transformer rings, but cannot be manufactured in complex shapes while having a low packing density, leading to reduced soft magnetic properties. These limitations can be overcome by alternative techniques such as the combined use of powder synthesis and consolidation to introduce soft ferromagnetic glass-forming alloys to a broad commercial market [12]. Despite rapid development of soft ferromagnetic glass-forming alloys in a few industrial applications, they are still limited to only a few sectors, as they depend on expensive high purity materials and clean laboratory conditions to avoid crystallization. From an economic and ecological point of view, compositions with commercial purity such as ferrous metals like iron, nickel, and chromium are preferred [13]. However, present impurities or oxygen in commercial purity materials promote heterogeneous crystal nucleation, causing a shift in the crystallization time [14]. Crystallization for soft ferromagnetic glass-forming alloys is orders of magnitudes faster than in many common polymers, silicate glass-forming liquids, and even in robust metallic glasses [15]. Therefore, a high cooling rate is inevitable during solidification in molten metal gas atomization to avoid kinetically related crystal growth.
The droplet solidification time depends on the droplet size, the temperature gradient between the melt droplet and the surrounding cooling gas, as well as the convective heat transfer coefficient. Increasing the heat transfer coefficient in conventional gas atomization is very challenging [16], though it is desired to increase the amorphous powder fraction. The atomization of soft ferromagnetic glass-forming alloys with commercial purity on the commercial scale has been rarely studied, whereas the production of soft ferromagnetic glass-forming alloys with high purity has been successfully demonstrated [2,17,18].
The aim of this paper is therefore the production of soft ferromagnetic glass-forming powders with commercial purity through the development of novel cooling strategies. These cooling strategies increase the cooling rate, creating new process windows that are inaccessible in conventional gas atomization. However, these cooling strategies require water as a cooling medium, promoting the formation of an oxide layer around the resulting particles. The oxide layer may decrease material properties as well as alter powder consolidation. In order to investigate the powder consolidation ability, Spark plasma sintering experiments were performed. The subsequent powder consolidation enables the determination of soft magnetic properties on the final bulk samples.
Another critical aspect is the formation of hydrogen during liquid quenching. The positive effect of hydrogen on metallic glasses is rarely discussed with respect to transport processes, structural changes, glass formation, and material properties [[19], [20], [21], [22], [23], [24], [25]]. Thus, hydrogen and oxygen content in the powders were measured in the powders and correlated with the GFA and final soft magnetic properties.
2. Cooling strategies during molten metal gas atomization
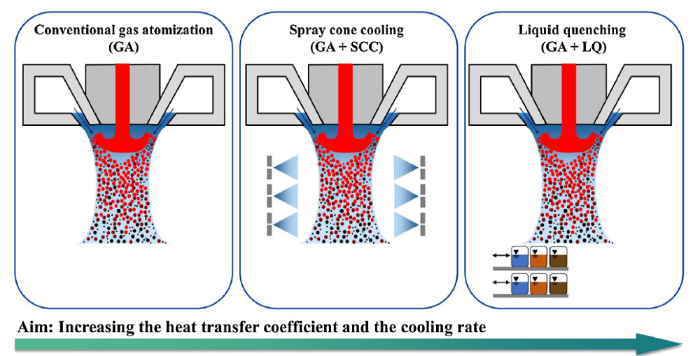
Besides gas atomization (GA) with conventional cooling, different cooling strategies were developed to increase the heat transfer coefficient and thus the cooling rate by combining conventional gas atomization with spray cone cooling (GA + SCC) and liquid quenching (GA + LQ). The developed cooling strategies have the potential to synthesize soft ferromagnetic glass-forming alloys with commercial purity and fully amorphous particles larger than 100 μm that normally tend to crystallize during solidification. Fig. 1 schematically summarizes the conventional gas atomization process and the two developed cooling strategies.
Fig. 1.
Fig. 1.
The goal of this work was to increase the heat transfer coefficient and thus the cooling rate by developing novel cooling strategies during molten metal gas atomization. Gas atomization (GA) with conventional cooling, spray cone cooling (GA + SCC), and liquid quenching (GA + LQ) were used in this study. Both cooling techniques used distilled water as a cooling medium. Spray cone cooling used distilled water to cool the spray cone from all angles, whereas liquid quenching was performed to quench the molten droplets. Experimental details are listed in Supplementary Material.
2.1. Materials
Two soft ferromagnetic glass-forming alloys ({(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 [26] and Fe76B10Si9P5 [27]) with commercial purity were selected to challenge the validity of our novel cooling strategies. The abbreviations FeCoBSiNb and FeBSiP for the two alloys are used from now on. These two soft ferromagnetic glass-forming alloys are difficult to atomize into a glassy state with commercial purity due to cooling rate and kinetically related growth limitations. For instance, the critical cooling rate for the FeCoBSiNb alloy during droplet solidification varies from 700 to 900 K s-1 using POEM (pulsated orifice ejection method) and high purity [17]. When impulse atomization and commercial purity are used, the critical cooling rate increases to 5000 K s-1 [12]. Atomization of the FeBSiP glass-forming alloy with commercial purity feedstock during gas atomization is a new field in literature. Melt spinning samples of the same composition were also produced for comparison. The nominal composition including purity information is given in Table 1.
Table 1 Selected soft ferromagnetic glass-forming alloys including purity information. The alloys have been processed with commercial purity. Melt spinning samples were produced for comparison.
| Alloy (at.%) | Nomenclature | Elements used (wt%) | Comments |
|---|---|---|---|
| {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 [26] (Melt atomization) | FeCoBSiNb | Fe80.51B18.26 Fe33Nb65.5 Fe (99.97), Si (99.99) Co (99.8) | Binary alloys and raw elements were melted in the atomization tower (no master alloy was used). |
| Fe76B10Si9P5 [27] (Melt atomization) | FeBSiP | Fe80.51B18.26 Fe75.92P23.23 Fe (99.97), Si (99.99) | Binary alloys and raw elements were melted in the atomization tower (no master alloy was used). |
| {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 [26] (Melt spinning) | FeCoBSiNb | Fe80.51B18.26 Fe33Nb65.5 Fe (99.97), Si (99.99) Co (99.8) | Master alloy and melt-spun samples were made using an induction furnace and melt spinning, respectively. Binary alloys and raw elements were used. |
| Fe76B10Si9P5 [27] (Melt Spinning) | FeBSiP | Fe80.51B18.26 Fe33Nb65.5 Fe (99.97), Si (99.99) Co (99.8) | Master alloy and melt-spun samples were made using an induction furnace and melt spinning, respectively. Binary alloys and raw elements were used. |
2.2. Atomization and melt spinning sample production
Soft ferromagnetic glass-forming alloys were synthesized into powders with a novel close-coupled gas atomizer. Atomizer details can be found in Refs. [28,29]. Binary alloys and raw elements with commercial purity were heated in a commercial Al2O3 crucible to the temperature of interest under an argon atmosphere. The melt temperature was maintained for 20 min to ensure homogenization. The vacuum level inside the vessel was reduced to 1 kPa. After homogenization, a stopper rod was pulled to start the atomization process. All experiments were performed with a nozzle outlet diameter of 2 mm, leading to melt mass flow rates ranging from 140 to 150 kg h-1. After running through the nozzle outlet, the molten metal stream subsequently disintegrates into micrometer-sized droplets due to the interactions with a high-energy gas jet. The molten droplets solidify in the presence of an inert gas during their flight inside the spray chamber. All atomization experiments were run with Ar ≥ 99.996 and an oxygen content of 4 ppm as the atomization gas. Table 2 summarizes all process parameters as well as atomization characteristics, including the gas-to-melt mass flow ratio (GMR).
Table 2 Process parameters including cooling strategies and alloy selection as well as gas atomization characteristics.
| Cooling strategy | Alloy (at.%) | Atomizer | Inert gas | Vacuum (kPa) | D (mm) | Tliq (K) | TM (K) | ΔTM (K) | p (MPa) | m˙L (kg h-1) | m˙G (kg h-1) | GMR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional gas atomization (GA) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 149 | 780 | 5.2 |
| Conventional gas atomization (GA) | Fe76B10Si9P5 | CD-CCA-0.8 | Argon | 1 | 2 | 1338 | 1688 | 350 | 1.6 | 142 | 780 | 5.5 |
| Spray cone cooling (GA + SCC) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 146 | 780 | 5.3 |
| Spray cone cooling (GA + SCC) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 151 | 780 | 5.2 |
| Spray cone cooling (GA + SCC) | Fe76B10Si9P5 | CD-CCA-0.8 | Argon | 1 | 2 | 1338 | 1688 | 350 | 1.6 | 142 | 780 | 5.5 |
| Spray cone cooling (GA + SCC | Fe76B10Si9P5 | CD-CCA-0.8 | Argon | 1 | 2 | 1338 | 1688 | 350 | 1.6 | 149 | 780 | 5.2 |
| Liquid quenching in H2O (GA + LQ) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 175 | 780 | 4.5 |
| Liquid quenching in H2O (GA + LQ) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 168 | 780 | 4.7 |
| Liquid quenching in PEG (GA + LQ) | {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 | CD-CCA-0.8 | Argon | 1 | 2 | 1484 | 1834 | 350 | 1.6 | 141 | 780 | 5.5 |
| Liquid quenching in H2O (GA + LQ) | Fe76B10Si9P5 | CD-CCA-0.8 | Argon | 1 | 2 | 1338 | 1688 | 350 | 1.6 | 144 | 780 | 5.4 |
| Liquid quenching in H2O (GA + LQ) | Fe76B10Si9P5 | CD-CCA-0.8 | Argon | 1 | 2 | 1338 | 1688 | 350 | 1.6 | 148 | 780 | 5.3 |
D = Nozzle outlet diameter, Tliq = Liquidus temperature, TM = Melt temperature, ΔTM = Melt superheat temperature, p = Atomization pressure, ${{\dot{m}}_{\text{L}}}$= Melt mass flow rate, ${{\dot{m}}_{\text{G}}}$= Gas mass flow rate, GMR = Gas-to-melt mass flow ratio, H2O = Distilled H2O, PEG = Poly(ethylene glycol).
Spray cone cooling and liquid quenching experiments were performed with distilled water. An additional liquid quenching experiment was performed with a water-soluble polymer (poly(ethylene glycol)) provided by Sigma Aldrich (PEG, BioUltra, CAS: 25322-68-3, (C2H4O)n·H2O) for comparison, as the cooling effect of poly(ethylene glycol) is assumed to be between water and mineral oils [30,31]. Since water can promote undesirable residual stresses, cracking, and embrittlement during quenching, poly(ethylene glycol) was introduced. PEG has a significantly higher boiling point (> 423 K) than water.
Spray cone cooling experiments were carried out with four dual argon/water nozzles (KL1, Diva Sprühtechnik), arranged at an incident angle of 45° and operated with a water flow of∼400 mL min-1 and an argon pressure of 0.55 MPa. The four nozzles were placed 0.2 m away from the melt outlet and arranged at 90° relative to each other, ensuring cooling from all angles. Furthermore, liquid quenching experiments were performed with eleven collectors at each falling distance (0.5 and 0.7 m). Experimental setups for both cooling strategies are shown in supplementary material Figs. S1 and S2.
Initial melt spinning samples were synthesized for reference from a master alloy by melting the same commercial purity binary alloys and raw elements in a high frequency induction furnace (VMF 1-11, DIAVAC) under a vacuum level of 10-2 Pa. 10 g of the master alloy were subsequently placed in a quartz crucible with an outlet diameter of 0.5 mm. The vacuum level inside the melt spinning device (NEV-A04, NISSIN GIKEN) was reduced to 2 × 10-2 Pa. The Cu wheel rotation was 4000 rpm. The produced melt-spun samples were ∼ 20 μm thick and ∼ 1.2 mm wide.
The soft ferromagnetic powders were consolidated using spark plasma sintering (Dr. Sinter, Sumitomo Coal Mining, SPS-1050). The particle size classes of < 25 μm and 25-45 μm were consolidated to toroidal rings with an outer and inner diameter of 13 and 8 mm as well as a height of 2.6±0.15 mm, representing a typical geometry to determine magnetic properties. The powders were pressed without a binder under a vacuum level of ∼ 6 Pa. The samples were heated in a graphite die to a temperature around the glass transition temperature and simultaneously compressed at 1 GPa at a holding time of ∼ 30-45 s, followed by a cooling step of ∼ 0.42 K s-1 to reduce residual stresses and thus micro-cracks. The relative density of the bulk samples was measured by Archimedes’ method with n-Tridecane (Cat. No. 40265-00) as the working fluid to avoid corrosion.
2.3. Powder and bulk sample characterization
The synthesized powders were sieved into eight size fractions: < 25, 25-45, 45-63, 63-90, 90-125, 125-150, 150-180, 180-200 μm. Particles in contact with distilled water were initially cleaned with ethanol and then dried. Secondary electron microscopy (SUPRA 40, Carl Zeiss) was used to qualitatively analyze particle shape and surface quality.
A theta-theta X-ray powder diffractometer (D8 Discover, Bruker) equipped with a CuKα source and a fixed divergence slit was used. Fe-fluorescence was electronically suppressed through the detector. The powder samples were run from 10° to 90° with a total measurement time of 3 h.
Continuous heating experiments on as-atomized powders and melt spinning samples were performed using a Mettler Toledo Differential Scanning Calorimetry instrument (TGA/DSA3+) with a heating rate of 0.5 K s-1. The DSC equipped with a DTA sensor was initially calibrated with In, Zn, Al, Au, and Pd elements.
The chemical powder composition was determined using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) provided by Thermo Fisher Scientific Inc. (IRIS Advantage DUO).
The oxygen and hydrogen powder content was measured with an ELTRA ONH-2000 device. The powders were placed in Sn capsules (90252, LECO Instruments) and dropped into an inner graphite crucible. The powders were then heated with 5 kW and 4 kW to determine oxygen and hydrogen content, respectively. The oxygen and hydrogen channels were initially calibrated with steel standards (91400-1003, Eltra for hydrogen and 91100-1006, Eltra for oxygen). After calibration, five blank measurements with empty crucibles were run and finally subtracted from the actual measurements (typically 3-5 measurements).
The saturation magnetization Bs of as-atomized powders and ribbons were measured with a vibrating sample magnetometer (VSM, VSM-5, Toei Industry). The VSM device was initially calibrated with a Ni standard (purity of 99.99 wt%, 7 mm × 7 mm, 230.49 mg, and a magnetic moment of 12.54 emu). A magnetic field of ± 1600 A m-1 was used. In addition, magnetic properties of sintered bulk samples with respect to saturation magnetization Bs, coercivity Hc, and permeability μ were measured with a B-H curve tracer (BHH-50, Riken Denshi) and a magnetic field of ± 19 kA m-1 was used.
3. Results and discussion
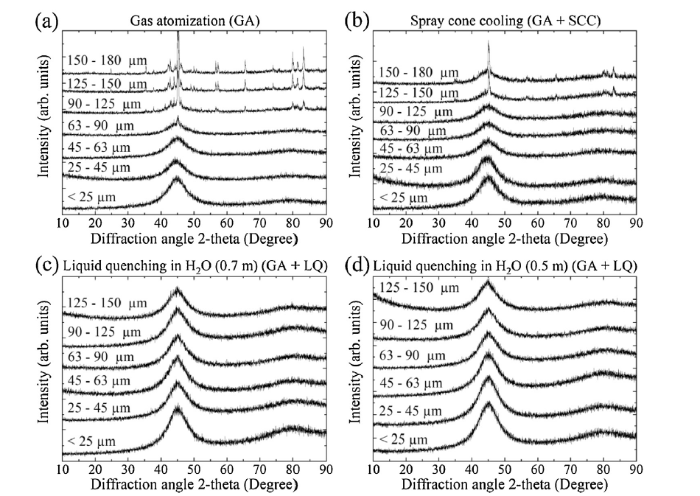
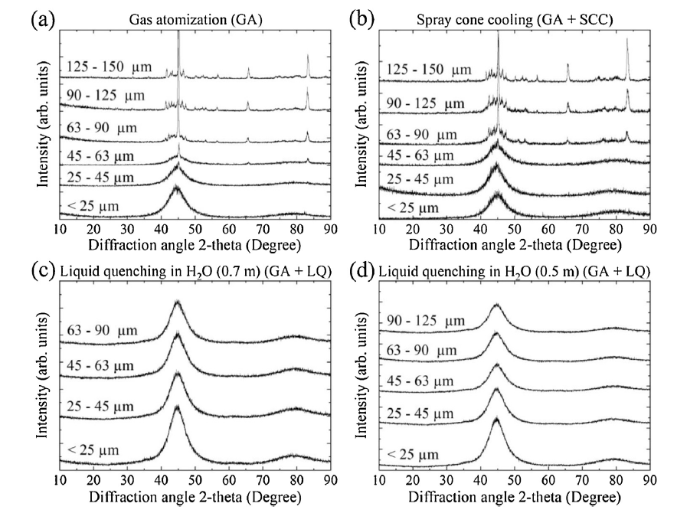
Fig. 2, Fig. 3 shows XRD traces for as-atomized powders which were synthesized by gas atomization with conventional cooling and the two cooling strategies (spray cone cooling and liquid quenching). FeCoBSiNb particles atomized with conventional cooling were fully amorphous up to 63 μm (Fig. 2(a)), whereas FeBSiP particles atomized with conventional cooling exhibited small crystalline peaks even for the smallest particle size class (Fig. 3(a)). FeBSiP particles were partially-amorphous using conventional cooling. Note that the intensity and the number of crystalline peaks increased with particle size due to a lower droplet surface-to-volume ratio (lower cooling rate). To further overcome cooling rate limitations, spray cone cooling and liquid quenching cooling strategies during molten metal gas atomization were used. FeCoBSiNb and FeBSiP quenched particles, collected at distances of 0.5 m and 0.7 m, resulted in the highest cooling rates and in amorphous powders. However, in the particle size class of 125-150 μm for the FeCoBSiNb alloy, the main amorphous halo is slightly sharper, suggesting that a few crystals may be present. Out of the presented strategies, spray cone cooling exhibited the second highest cooling rate.
Fig. 2.
Fig. 2.
XRD analyses for {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 powders using different cooling strategies.
Fig. 3.
Fig. 3.
XRD analyses for Fe76B10Si9P5 powders using different cooling strategies.
DSC traces are shown for the two soft ferromagnetic glass-forming alloys in Fig. 4 (particle size classes of 25-45 μm and 125-150 μm). The DSC traces were consistent with the presented XRD analyses in Fig. 2, Fig. 3. The DSC traces were less pronounced with increasing particle size (lower cooling rate), resulting in a lower amorphous fraction. DSC traces for the melt spinning reference samples were also plotted for comparison. The shape of the DSC curves of the melt spinning sample is similar to that of the DSC curves of the powders. DSC traces of the liquid quenching cooling strategy were unchanged regardless of the particle size, demonstrating that the particles were fully amorphous. DSC measurements for FeBSiP particles showed the same behavior as seen for the FeCoBSiNb alloy. However, two crystallization events were observed for the liquid quenching cooling strategy, whereas only one crystallization peak has been previously reported in literature. Yamaura et al. [32] also reported a second DSC peak for a Ti50Ni25Cu25 melt-spun ribbon. They detected a broad endothermic peak due to hydrogen desorption, a secondary exothermic peak corresponding to the crystallization of hydride, and a final sharp endothermic peak due to hydrogen desorption. Kováč et al. [33] argued that hydrogen absorption is determined by the sign of the enthalpy related to the process. An exothermic peak is typically detected for hydride-forming systems (e.g. Zr, Ti, V, and rare-earth metals) and endothermic peaks are generally observed for metals and alloys, which do form stable hydrides at room temperature. However, DSC measurements provided limited insights into the formation of the second exothermic peak for the FeBSiP glass-forming alloy.
Fig. 4.
Fig. 4.
DSC analyses on (a) {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and (b) Fe76B10Si9P5 powders for two different particle size classes (25-45 μm and 125-150 μm). The abbreviations GA, GA + SCC, and GA + LQ stand for conventional gas atomization, conventional gas atomization and spray cone cooling, and conventional gas atomization and liquid quenching, respectively (see
Fig. 5 shows measured enthalpy of crystallization values from DSC measurements as a function of particle diameter for conventional cooling and the different cooling strategies. For up to 63 μm, FeCoBSiNb particles atomized under conventional cooling conditions showed the same enthalpy of crystallization values as the melt spinning reference. Particles > 63 μm distinctly decreased as particle size increased. This trend was also observed in the spray cone cooling strategy. However, the decrease in enthalpy of crystallization is less pronounced, concluding that spray cone cooling is an effective cooling strategy, as the cooling rate and the amorphous fraction was significantly increased. The highest cooling rate is obtained for liquid quenching experiments, as the entire powder produced is fully amorphous regardless of particle size. The same observations were made for the FeBSiP glass-forming alloy. It can be summarized that liquid quenching provided the highest cooling rate, followed by spray cone cooling. These results are a progressive development for the possible commercialization of metallic glasses with commercial purity considering synthesis and consolidation. Many manufacturers suffer under the limitation that only a small amount of amorphous powder in a certain particle size range is typically available.
Fig. 5.
Fig. 5.
Enthalpy of crystallization describing the amorphous fraction as a function of particle diameter. (a) {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and (b) Fe76B10Si9P5 soft ferromagnetic glass-forming alloys were considered for gas atomization with conventional cooling and the different cooling strategies. The {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 alloy was additionally quenched in poly(ethylene glycol) (PEG). The horizontal error bars represent the d16,3 and d84,3 percentiles measured by particle laser diffraction. Measurements on melt spinning (MS) samples were also plotted for comparison. The dashed line refers to the average enthalpy of crystallization and the upper and lower edges of the orange area defines the upper and lower standard deviation. The abbreviations GA, GA + SCC, and GA + LQ stand for conventional gas atomization, conventional gas atomization and spray cone cooling, and conventional gas atomization and liquid quenching, respectively (see
Particles atomized under the liquid quenching cooling strategy showed higher enthalpy of crystallization values than the melt spinning reference sample. Reported values such as characteristic temperatures or enthalpy of crystallization were also consistent with previous literature studies on monolithic FeCoBSiNb alloys (though a slightly different alloy composition was used) [34], demonstrating that the particles and the melt spinning samples were fully amorphous. The question then arises: what causes the increase in enthalpy of crystallization, as for instance, the saturation magnetization of as-atomized FeCoBSiNb powders also significantly increased. The results of the magnetic properties are presented and discussed later.
The same results were obtained after carefully repeating the liquid quenching experiments with distilled water and poly(ethylene glycol) (see Table 2). Compositional errors in feedstock preparation can be neglected, since the compositional analysis was validated by ICP-OES measurements (Supplementary Material, Tables S1 and S2). Hydrogen enhances structural changes [[22], [23], [24]] caused by internal stresses when hot melt droplets impinge on the quench medium. In addition, particles atomized under liquid quenching showed a significant increase in a number of non-spherical particles, demonstrating that the droplets were either partially-solidified or completely molten before impinging the liquid medium (Supplementary Material, Fig. S3). Thus, partially-solidified or molten droplets may be affected by hydrogen.
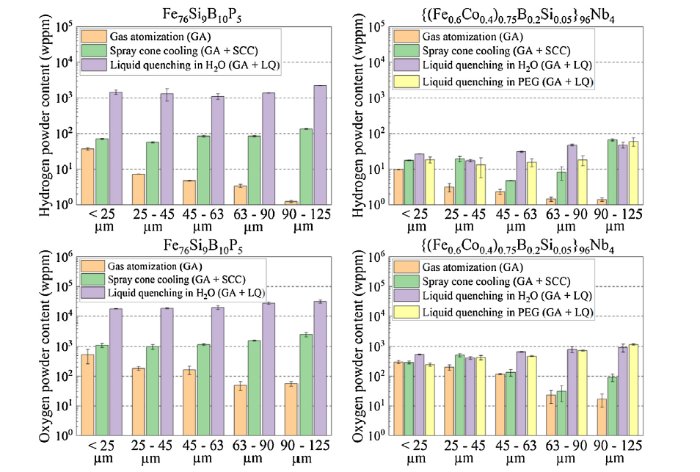
Oxygen and hydrogen content in the powder was determined (Fig. 6). The results for both soft ferromagnetic powders indicated that the measured hydrogen and oxygen level increased up to one order of magnitude, depending on the investigated particle size class. The oxygen content is one order of magnitude higher than the hydrogen level. The hydrogen and oxygen levels for FeCoBSiNb and FeBSiP particles atomized with conventional cooling significantly decreased with increasing particle size. For both cooling strategies, the hydrogen and oxygen levels were almost constant. This is because particles atomized under conventional cooling are governed by the surface-to-volume effect, where the larger the particles, the less the surface area available for interaction with hydrogen and oxygen. Particles atomized with spray cone cooling and liquid quenching are alternatively dominated by mass diffusion, resulting in constant hydrogen and oxygen values. The FeBSiP alloy has a high iron content, resulting in low corrosion resistance. The particles were strongly corroded after being quenched in distilled water. The hydrogen and oxygen levels were typically an order of magnitude higher when compared with the FeCoBSiNb alloy.
Fig. 6.
Fig. 6.
Hydrogen and oxygen measurements for {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and Fe76B10Si9P5 powders. Different particle size classes were considered.
The water-quenched FeBSiP particles exhibited a second exothermic peak as seen in the DSC measurements in Fig. 4(b). In order to determine the cause of the second exothermic peak, two particle size classes (25-45 μm and 125-150 μm) were heat-treated to two temperatures before (1023 K) and after the peak (1353 K). The oxygen content remained the same at both temperatures. The hydrogen level decreased from around 2230 ± 15 ppm to 5 ± 1 ppm at the higher temperature (1353 K), whereas the heat-treated hydrogen level measured before the peak (1023 K) remained almost constant at 2099 ± 47 ppm. Therefore, the second peak can be attributed to hydrogen inducement. For the FeCoBSiNb alloy, particle size classes of 25-45 μm and 125-150 μm were heated to 1273 K. Analogous to the FeBSiP particles, the oxygen level remained constant regardless of the heat-treatment procedure used, whereas the hydrogen level slightly decreased from 60 ± 5 ppm to 40 ± 4 ppm in contrast to the FeBSiP particles where the hydrogen level strongly decreased.
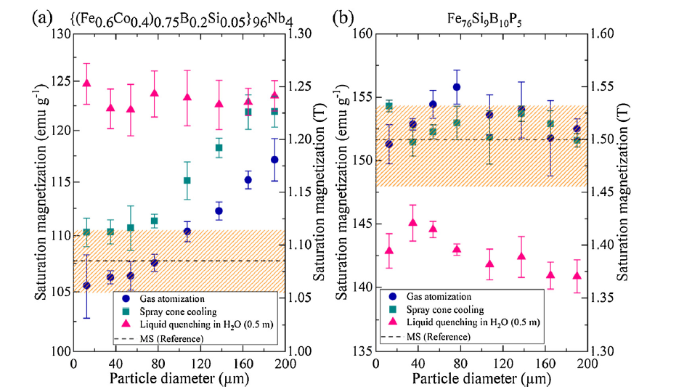
The soft magnetic properties of the two soft ferromagnetic powders with respect to saturation magnetization were determined from vibrating sample magnetometer measurements (Fig. 7). The saturation magnetization was considered as an indicator in this study, since it is affected by local short range order, which in turn may be altered due to high cooling rates and hydrogen [35]. The saturation magnetization for FeCoBSiNb powders increased with particle size for conventional gas atomization and spray cone cooling, likely caused by hydrogen inducement. However, a significant increase in saturation magnetization was observed for quenched particles in distilled water. The saturation magnetization value of the melt spinning reference sample was plotted. The measured saturation magnetization values for the melt spinning reference sample are consistent with high purity monolithic bulk samples [36], showing that commercial purity particles quenched in water can be synthesized to a similar or even greater saturation magnetization value. However, the quenched FeBSiP powders showed a distinctly lower saturation magnetization when compared to the melt spinning reference sample and particles synthesized with conventional or spray cone cooling due to a lower corrosion resistance.
Fig. 7.
Fig. 7.
Saturation magnetization determined from vibrating sample magnetometer measurements as a function of particle size for (a) {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and (b) Fe76B10Si9P5 powders. Conventional gas atomization and novel cooling strategies were considered for this study. Measurements on melt spinning samples (MS) were also plotted for comparison (the dashed line refers to the average saturation magnetization and the upper and lower edges of the orange area defines the upper and lower standard deviation).
An important aspect for the commercialization of soft ferromagnetic glass-forming alloys is the subsequent powder consolidation step, since densification primarily depends on the particle size distribution and an oxide layer around the particles [37]. The spray cone cooling and liquid quenching technique used distilled water that increased the oxygen and hydrogen content in the powder, as well as on the surface of the quenched particles (Fig. 6). A large oxide layer around the particles typically hinders consolidation, since it restricts the particle bonding ability [38]. For this purpose, binder-free consolidation was carried out using spark plasma sintering on two different particle size classes (< 25 μm and 25-45 μm). The particles were pressed into toroidal rings with a relative density greater than 98 %. The pressed samples were fully amorphous according to XRD analyses (shown in Supplementary Material Fig. S4). The reproducibility of particles larger than 63 μm was low (for spark plasma sintering parameters, see supplementary material Table S3). The cracking and fracturing of the sintered rings during handling prevented mechanical testing. Quantifying the mechanical properties was beyond the scope of this study. The densification and the final soft magnetic properties were of primary interest. Recent research has shown that the mechanical properties of soft ferromagnetic glass-forming alloys can be significantly increased by introducing a second metallic glass powder as a binder [39]. A binder typically promotes larger core losses, but increases the mechanical strength. For example, the authors could reach a yield stress of ∼ 500 MPa for nano-crystalline soft ferromagnetic glass-forming alloys, which is sufficient for most commercial magnetic applications and thus is a promising approach for the industrialization of commercial purity soft ferromagnetic glass-forming alloys [39].
Fig. 8 compares saturation magnetization values between as-atomized powders and sintered toroidal rings for two different particle size classes (< 25 μm and 25-45 μm) considering gas atomization with conventional cooling, spray cone cooling, and liquid quenching. The blue data points correspond to the same points seen in Fig. 7 and the green points represent the sintered rings. The saturation magnetization for the water-quenched FeCoBSiNb particles slightly decreased after being pressed. The saturation magnetization values for these pressed particles were slightly below the average melt spinning reference, demonstrating that consolidation has a small effect on the final saturation magnetization. However, when considering spray cone cooling and liquid quenching cooling strategies, a strong decrease in saturation magnetization is observed for the sintered FeBSiP rings, likely caused by oxides. The relative densities were slightly lower when compared to the same sintered FeCoBSiNb rings (for relative density details, see Table S3 in Supplementary Material).
Fig. 8.
Fig. 8.
Saturation magnetization determined from B-H curve tracer measurements as a function of two particle size classes (< 25 μm and 25-45 μm) for (a) {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and (b) Fe76B10Si9P5 as-atomized powders, as well as sintered toroidal rings. The toroidal rings had an outer and inner diameter of 13 and 8 mm as well as a height of 2.6 ± 0.15 mm. Conventional gas atomization and novel cooling strategies were considered for this study. Measurements on melt spinning samples (MS) were also plotted for comparison (the dashed line refers to the average saturation magnetization and the upper and lower edge of the orange area defines the upper and lower standard deviation). The abbreviations GA, GA + SCC, and GA + LQ stand for conventional gas atomization, conventional gas atomization and spray cone cooling, and conventional gas atomization and liquid quenching, respectively (see
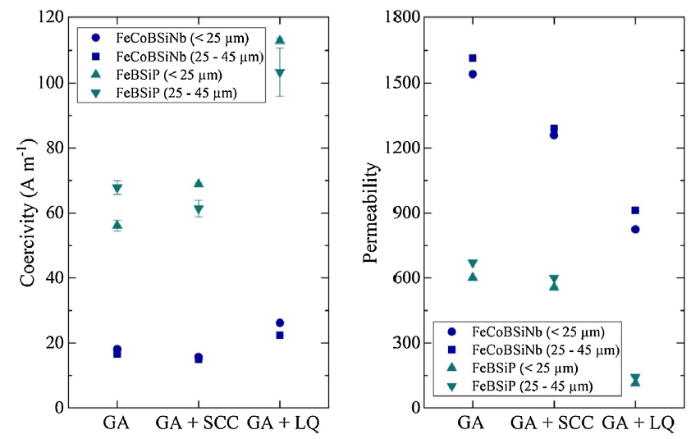
The magnetic properties with respect to coercivity and permeability were determined from hysteresis curves as a measure of consolidation ability when considering novel cooling strategies (Fig. 9). Literature reports coercivity values of 0.8 A m-1 for a FeBSiP bulk sample [40], whereas a coercivity of 1.5 A m-1 as well as a permeability value of 32.000 for the FeCoBSiNb bulk sample is measured [41]. While the saturation magnetization of the sintered FeCoBSiNb rings were nearly unaffected by consolidation, the coercivity increased slightly, regardless of the cooling strategies used. Sintered FeBSiP rings showed an even stronger increase in coercivity. The permeability decreased significantly for both considered soft ferromagnetic glass-forming alloys when considering spray cone and liquid quenching cooling strategies.
Fig. 9.
Fig. 9.
Coercivity (a) and permeability (b) of sintered toroidal rings for two soft ferromagnetic glass-forming alloys ({(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and Fe76B10Si9P5). The rings had an outer and inner diameter of 13 and 8 mm as well as a height of 2.6 ± 0.15 mm. Two particle size classes were used to consolidate the powders (< 25 μm and 25-45 μm). The abbreviations GA, GA + SCC, and GA + LQ stand for conventional gas atomization, conventional gas atomization and spray cone cooling, and conventional gas atomization and liquid quenching, respectively (see
4. Conclusion
Two soft ferromagnetic glass-forming alloys ({(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 and Fe76B10Si9P5) with commercial purity were atomized by using conventional close-coupled gas atomization and novel cooling strategies. The novel cooling strategies included spray cone cooling and liquid quenching. The implementation of these strategies extended the common process window in molten metal gas atomization, and resulted in an increased heat transfer and thus in higher cooling rates. These strategies enabled the production of fully amorphous particles with commercial purity for the entire particle size range, which is generally restricted in conventional gas atomization. Distilled water is used as the cooling medium in most strategies, promoting higher hydrogen and oxygen content in the powder. The glass-forming ability and soft magnetic properties with respect to saturation magnetization for the {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 alloy were significantly improved by the presence of hydrogen. Hydrogen-inducement caused a second crystallization event for the Fe76B10Si9P5 alloy. The consolidation ability of the water-quenched particles (particle size class of < 25 μm and 25-45 μm) were investigated with spark plasma sintering. Amorphous toroidal rings were consolidated with a relative density > 98 % regardless of the cooling strategy used. The saturation magnetization was nearly unchanged after consolidation, the coercivity decreased slightly and permeability decreased significantly.
Acknowledgements
This work was financially supported by the Industrielle Gemeinschaftsforschung IGF (Grant No. 19219 N/1) and the Japan Society for the Promotion of Science (Grant No. 18K04767). V. Uhlenwinkel and L. Mädler also greatly acknowledge funding from the Deutsche Forschungsgemeinschaft (DFG)-Project (No. 276397488-SFB 1232) for partly supporting this research. This work was partly conducted at Tohoku University, Sendai, Japan and N. Ciftci appreciates the financial support through the following scholarships: MAPEX Center for Materials and Processes at the University of Bremen and the Cooperative Research and Development Center for Advanced Materials at Tohoku University (No. GIMRT-18GK0015). Dr. M. Wendschuh, Dr. S. Pokhrel, Dr. N. Ellendt, Prof. R. Busch, Dr. B. Bochtler, J. Eitzen, and L. Ludwig are acknowledged for their fruitful contributions. We are also grateful for the experimental and scientific support from E. Matthaei-Schulz, S. Evers, F. Peschel, K. Harata, and Dr. Z. Yan.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jmst.2020.03.077.
Reference
This article surveys amorphous and nanocrystalline alloys for soft magnetic applications. Both materials have much in common, starting from the technique of production and including the key factors that determine their properties. Thus the magneto-crystalline anisotropy randomly fluctuates on a scale much smaller than the domain wall width and, as a consequence, is averaged out by exchange interactions so that there is no net anisotropy effect on the magnetization process, the prerequisite for good soft magnetic behaviour. Superior soft magnetic properties additionally require low magnetostriction, which is true of amorphous Co-based alloys and, more recently, nanocrystalline Fe-based alloys, but at a significantly higher saturation induction and with better thermal stability. Both materials reveal low losses of up to several hundred kilohertz and their B–H loop can be tailored by magnetic field annealing according to the demands of the application.
The development of metal alloys that form glasses at modest cooling rates has stimulated broad scientific and technological interest. However, intervening crystallization of the liquid in even the most robust bulk metallic glass-formers is orders of magnitude faster than in many common polymers and silicate glass-forming liquids. Crystallization limits experimental studies of the undercooled liquid and hampers efforts to plastically process metallic glasses. We have developed a method to rapidly and uniformly heat a metallic glass at rates of 10(6) kelvin per second to temperatures spanning the undercooled liquid region. Liquid properties are subsequently measured on millisecond time scales at previously inaccessible temperatures under near-adiabatic conditions. Rapid thermoplastic forming of the undercooled liquid into complex net shapes is implemented under rheological conditions typically used in molding of plastics. By operating in the millisecond regime, we are able to "beat" the intervening crystallization and successfully process even marginal glass-forming alloys with very limited stability against crystallization that are not processable by conventional heating.
Zr55Cu30Ni5Al10 alloys with different amounts of hydrogen have been prepared by arc melting under the gaseous mixture of hydrogen and argon. Proper additions of hydrogen have been proved to effectively increase the glass-forming ability (GFA) of this alloy. Positive effect of hydrogen on GFA has been interpreted from the thermodynamic and structural points of view. Proper additions of hydrogen can decrease the liquidus temperature, which leads to more stable glass-forming liquid. Structure analysis by positron annihilation lifetime spectroscopy shows that proper additions of hydrogen can increase the concentration of shortest open volume and decrease the concentration of intermediate and largest open volumes. This leads to formation of a denser random packed structure, and thus increases the GFA of Zr55Cu30Ni5Al10 alloys. (C) 2012 Elsevier B.V.
AbstractNew Fe-based bulk glassy alloys with diameters up to 5 mm were formed in [(Fe1−xCox)0.75B0.2Si0.05]96Nb4 system by the copper mold casting method. The substitution of Co for Fe causes an increase in the glass-forming ability (GFA). As the Co content increases, the liquidus temperature (Tl) decreases, resulting in an increase of reduced glass transition temperature (Tg/Tl) from 0.566 to 0.587. The bulk glassy alloys exhibit super-high fracture strength of 3900–4250 MPa, Young's modulus (E) of 190–210 GPa, elastic strain (εe) of 0.02, plastic strain (εp) of 0.0025 and Vickers hardness (Hv) of 1150–1220. The σf/E and Hv/3E of these glassy alloys are 0.02, respectively, in agreement with the previous data for a number of bulk glassy alloys. The agreement indicates that these Fe-based bulk glassy alloys have an elastic–plastic deformation mode. The syntheses of high-strength Fe-based bulk glassy alloys with super-high fracture strength of over 4000 MPa and high glass-forming ability are encouraging for future development of Fe-based bulk glassy alloys as structural materials.]]>








 WeChat
WeChat