1. Introduction
Porous functionalized silica nanoparticles with tailored morphology and structure have attracted increasing attention both for fundamental research and their widespread potential applications. Specifically, researchers have taken advantage of nanotechnology to explore such nanostructured materials for their use in antibacterial applications [[1], [2], [3], [4]]. Three-dimensional (3D) structural materials, especially porous structures, due to their high surface area and pore volume have attracted much interest for their technological significance and potential biomedical applications. Recently, plant and marine biont extracts have been found to have excellent antibacterial effects, such as antibacterial proteins or peptides and capsaicin [5,6]. Capsaicin can be used as an antibacterial agent and anti-inflammatory drug. The controlled-release of those extracts based on mesoporous materials has gradually been used in antibacterial research. Silica particles have been very popular among industrial nanotechnology and scientific fundamental research studies, considering their biocompatibility and porous structure [7,8]. The mesoporous silica-based material MCM-41 was first proposed as a drug delivery system, as were SBA-15 and MCM-48 [[9], [10], [11]]. Therefore, various approaches have been proposed for the synthesis of well-defined mesoporous silica particles due to their potential applications, such as chemistry catalysis [[12], [13], [14], [15], [16]], drug delivery and controlled release [[17], [18], [19], [20]], adsorption of heavy metals and organic pollutants [21], enzyme immobilization, and biomolecule separation [[22], [23], [24], [25]].
Since first raised, several attempts have been made to synthesize mesoporous silica spheres by modifying the Stöber method by the addition of soft or hard templates [[26], [27], [28], [29], [30]]. Various templates have been employed in combination with surfactant templates for the dual-templating fabrication of 3D structure particles, such as emulsions and polymer matrix [31,32], while some other synthetic techniques, including acoustic cavitation aid-tools have also been applied [33]. Wang et al. [34] synthesized a mesoporous shell silica by Water/Oil emulsion method, which could have potential applications as controlled release capsules for drugs, dyes, and cosmetics. Rankin et al. [35] synthesized hollow spherical silica particles with hexagonally ordered mesoporous shells via the dual use of cetyltrimethylammonium bromide (CTAB) and unmodified polystyrene latex microspheres as templates in concentrated aqueous ammonia. Qi et al. [36] has readily synthesized mesoporous silica in mixed water-ethanol solvents at room temperature using CTAB as the template. Zhang et al. [37] has successfully prepared a hollow mesoporous silica nanoparticle by using a carbon nanosphere as a hard template and CTAB as a soft template for drug delivery and protein adsorption. Li et al. [38] has successfully developed amino-functionalized mesoporous silica with an easily tuned mesostructure by a facile self-assembly/solvothermal strategy. Michailidis et al. [39] synthesized quaternary ammonium salts-modified and biocide-loaded mesoporous silica nanoparticles as functional fillers for antifouling/antibacterial coatings with a dual synergetic effect. Nevertheless, it remains a challenge to explore one-step templating routes toward porous functionalized silica particles with designed structures and morphologies.
Herein, we report the one-step synthesis of porous aminated-silica nanoparticles (SiO2-NH2 NPs) through the ammonia-catalyzed hydrolysis of tetraethyl orthosilicate (TEOS) in mixed water-ethanol solutions with a CTAB template. In order to synthesize the desired morphology, the order of regents injected was taken into consideration and we kept the pH value around 9.0 through the whole reaction. After condensation and pore-forming, porous SiO2-NH2 NPs were finally fabricated. Through characterization, porous SiO2-NH2 NPs were found to have a large surface area and the surface was modified by many amino groups. The drug release pattern at different pH values of epirubicin (EPB)-loaded SiO2-NH2 NPs was observed, and the result showed a pH-selective release characteristic. Under immersion in capsaicin-ethyl alcohol, porous SiO2-NH2 NPs were able to absorb capsaicin well. When immersed in water, those porous SiO2-NH2 NPs could release capsaicin gradually. Finally, the porous SiO2-NH2@capsaicin NPs exhibited antibacterial properties against Escherichia coli, as revealed by an antibacterial experiment.
2. Experimental
2.1. Materials
Hexadecyltrimethylammonium bromide (CTAB), (3-aminopropyl) triethoxysilane (APTES), and tetraethylorthosilicate (TEOS) were purchased from Sigma Aldrich. Capsaicin (98%, Energy Chemical), acetic acid (99.5%, analytical reagent (AR), Aladdin), ammonia solution (AR, 28%), epirubicin (AR, EPB), and anhydrous ethanol (AR) were provided by Sinopharm Chemical Reagent Co., Ltd., China. Deionized water was distilled through a Milli-Q water purification system.
2.2. Synthesis of porous aminated-silica (SiO2-NH2) NPs by a one-step approach
For the synthesis of porous aminated-silica nanoparticles, 2.3 mmol of CTAB, and 27 μL of ammonia solution (28%) were added into 100-mL mixture of deionized (DI) water and anhydrous ethanol (ratio of 1:1). Then the solution was stirred at 30 °C and 25 rpm for 30 min until the CTAB had fully dissolved. After that, 4.6 mmol of TEOS and 2.3 mmol of APTES were added into the solution with different orders under vigorous stirring at 30 r/min for 30 min, and the solution was stirred at 25 r/min and 30 °C for another 12 h. In the next step, the temperature was increased from 30 to 80 °C and the stirring decreased to 20 r/min at 80 °C for another 24 h. After cooling to room temperature, the solution in the dialysis tube (Pierce, molecular weight cut-off of 3500) was dialyzed in acid solution (a mixture of DI water, ethanol, and acetic acid with a volume ratio of 1:1:0.007) for 24 h to extract CTAB out of the particles. This process was repeated three times. The solution was then dialyzed in DI water for another 24 h. This process was again repeated three times. The particles were finally filtered through a 200-nm syringe filter (Fisher brand) and then stored after freeze-drying (1 Pa, -13.5 °C for 24 h, LGJ-18, Beijing Songyuanhuaxing Technology Develop Co., Ltd) for further investigation.
2.3. Measurements
Transmission electron microscopy (TEM) images and high-resolution TEM (HRTEM) images of the samples were recorded on a field-emission transmission electron microscope (JEOL JEM-2001 F, accelerating voltage 200 kV) to measure the size, size distribution, and composition percentage of the aminated-silica nanoparticles (SiO2-NH2 NPs) by depositing them on carboncoated copper grids (holey carbon, 200-mesh Cu) and leaving them to dry at room temperature. Thermogravimetric analysis (TGA) was performed using a simultaneous thermal analyzer (STA 499C, Netzsch). Fourier transform infrared (FT-IR) spectra were obtained on an FT-IR spectrometer (Thermo Nicolet Nexus) using KBr pellets. Small-angle X-ray powder diffraction (XRD) patterns were acquired on a D/MaxIIIA (Rigaku) diffractometer. The specific surface area determination and pore volume and size analysis were performed by Brunauer-Emmett-Teller (BET) methods by use of an ASAP 2460 analyzer. Prior to the measurements, the samples were degassed at 80 °C for 4 h under vacuum. Zeta potential measurements were performed on a Brookhaven Zeta-PALS with Bi-Mas particle sizing option. Zeta potentials were recorded for a solution of SiO2-NH2 NPs in neutral deionized water. The pH values of solution were adjusted to 7.0 using 10 mM HCl and 10 mM NaOH with a digital pH meter (PHS-3C, LEICI). The hydrodynamic diameter of the SiO2-NH2 NPs was measured by dynamic light scattering (DLS) using a Malvern Zetasizer nano ZS (Malvern Instruments, Malvern, United Kingdom). The photo-luminescent (PL) intensity was tested by a spectrofluorophotometer (RF-5301 pc, Shimadzu).
2.4. Drug absorption and release abilities of porous SiO2-NH2 NPs and bacterial test against Escherichia coli
First, 10 mg SiO2-NH2 NPs were immersed in 20 mL capsaicin-ethanol solution (0.1 μg mL-1), and the photo-luminescence (PL) spectrum of the residue solution was characterized for capsaicin absorption after different times using a spectrofluorophotometer (RF-5301 pc, Shimadzu). After the absorption test, 10 mg SiO2-NH2@capsaicin NPs were obtained through centrifuging and freeze- drying. Subsequently, the SiO2-NH2@capsaicin NPs were immersed in deionized water, and the PL spectrum of the solution was tested for the characterization of capsaicin release after different times. In order to observe the drug release pattern of porous SiO2-NH2 NPs at different pH values, 10 mg SiO2-NH2 NPs were immersed in 20 mL EPB-water solution (0.5 mg mL-1), and the supernatant was centrifuged to assess the adsorption amount after 24 h using a spectrofluorophotometer (RF-5301 pc, Shimadzu). After that, the EPB-loaded SiO2-NH2 NPs (1 mg/mL, 100 μL) were mixed with phosphate buffer saline (PBS) buffer (5 mL) at pH = 3.6, 4.4, 5.4, 6.6, and 7.4, respectively. After that, the mixture was placed in a shaker (37 °C, 100 rpm) for 24 h. The mixture was centrifuged (5000 rpm, 15 min) to assess the release pattern. Capsaicin showed peaks at 338 nm and EPB showed peaks at 557 nm according to the PL spectrum.
Again, 10 mg SiO2-NH2 NPs were immersed in 20 mL capsaicin-ethanol solution (0.1 μg mL-1) for 24 h. Then, 10 mg SiO2-NH2@capsaicin NPs were obtained through centrifuging and freeze-drying. Finally, different concentrations of SiO2-NH2@capsaicin NPs solution were obtained. As an antibacterial test, all the materials and procedures were treated to kill all bacteria. After an overnight Luria-Bertani (LB) culture, Escherichia coli (E. coli) was established by a dilution of (ca. 107 CFU mL-1) in 200 mL of LB medium and divided into four parts, each with a volume of 50 mL. Then, 10 μl 0.5 μg mL-1 SiO2-NH2 NPs water solution, 0.1 μg mL-1 SiO2-NH2@capsaicin NPs water solution, and 0.5 μg mL-1 SiO2-NH2@capsaicin NPs water solution were added to each 50-mL sample of E. coli in Luria-Bertani (LB) medium. From the numbers of E. coli in each solution, the antibacterial properties of the NPs were tested. The antibacterial activity of SiO2-NH2@capsaicin NPs was tested by the agar diffusion method; the established E. coli was diluted twice before use. Then, 40 μl diluted (1:100) E. coli suspension was spread over the surface of the LB medium uniformly, followed by the addition of a small piece of cellulose paper (with a diameter of 6 mm). Next, the medium was divided into three zones and each zone was added to SiO2-NH2 NPs, 0.1 μg mL-1 SiO2-NH2 @capsaicin NPs and 0.5 μg mL-1 SiO2-NH2 @capsaicin NPs, respectively. The LB medium was placed in a bacterial incubator to cultivate at 37 °C for 24 h. The zone of inhibition was detected by the transparent halos on the surface of the LB medium.
3. Results and discussion
3.1. Characterization of synthesized porous SiO2-NH2 NPs
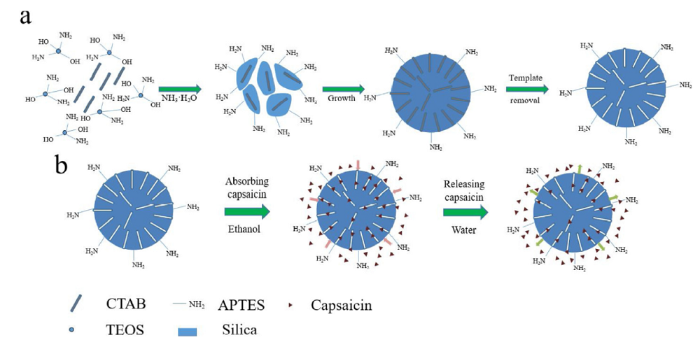
The synthesis of porous aminated-silica (SiO2-NH2) NPs was achieved by a one-step approach, shown in Scheme 1(a). TEOS and APTES were used to fabricate SiO2-NH2 NPs in mixed water-ethanol system. It was desirable that the hydrolysis of TEOS contributed to the SiO2 NPs, with surface modification by APTES. Furthermore, the hydrolysis process was completed before the majority of particles were condensed. SiO2-NH2 NPs were facilitated through hydrolyzation and condensation at appropriate rates and thereby the particles growth could be adjusted over a convenient period of time. And the capsaicin absorption-release experiment was shown in Scheme 1(b).
Scheme 1
Scheme 1
Schematic of the synthesis of porous SiO2-NH2 nanoparticles (NPs) (a) and capsaicin absorption-release abilities (b).
By analyzing the previous research on synthesizing porous SiO2 NPs, TEOS seemed much easier to employ for the design specially structured particles [32,40,41]. On the other hand, APTES could promote hydrolyzation and affected condensation [42]. Both the hydrolysis rate and condensation rate greatly depend on the pH value and the temperature of the aqueous solution mixture [26,32]. Different from reported SiO2 nanoparticles, porous SiO2-NH2 NPs needed more fine mediation. The mixed water-ethanol system was also used to prepare porous amino-functionalized structural SiO2 NPs by the hydrolysis of TEOS. Based on these considerations, we synthesized SiO2-NH2 NPs using both TEOS and APTES as silica sources and ammonia solution as a catalyst. The initial pH of the reaction was around 9.0 and this was maintained through the whole reaction, while the starting temperature stayed at 30 °C. The order of reagents injected also affected the morphology of the final SiO2-NH2 NPs. In order to construct porous SiO2-NH2 NPs, the order of reagents was controlled. Specifically, 4.6 mmol of TEOS and 2.3 mmol of APTES were injected into CTAB and ammonia solution at the same time. After the reaction and extraction of CTAB, SiO2-NH2 NPs with many pores inside and around the surfaces of particles finally formed.
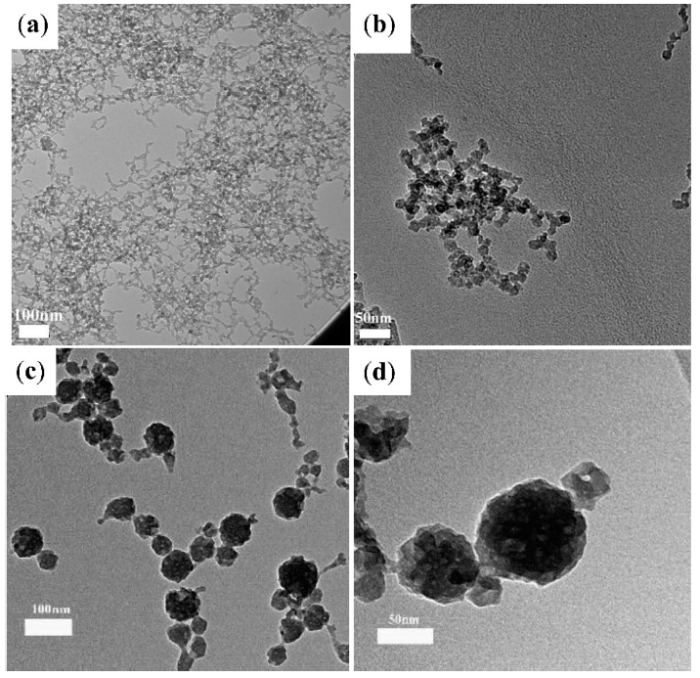
Once the CTAB and ammonia solution was dissolved into the water-ethanol system, then TEOS was injected and allowed to react for 30 min before APTES was added under vigorous stirring. Because of the high concentration of CTAB in the mixture, the hydrolysis of TEOS and APTES formed fiber-like SiO2-NH2 NPs (Fig. 1(a), labeled as “FL-SiO2-NH2 NPs”). Even for the process of the formation of NPs at 30 °C, adding the APTES after the TEOS correspondingly affected the morphology of particles. The diameter of the fibers was about 10 nm while the length was about 100 nm. When the order of the addition of TEOS and APTES was changed, monodispersed SiO2-NH2 NPs (labeled as “MD- SiO2-NH2 NPs”) were fabricated after 4 h, as shown in Fig. 1(b). When TEOS and APTES were added at the same time, SiO2-NH2 NPs with the size of 10 nm finally formed and there were multi-pores observed in the SiO2-NH2 NPs (Fig. 1(b)). During the process, the SiO2-NH2 NPs first formed and grew at 30 °C up to 12 h, and condensation occurred at 80 °C for another 24 h. At the end of process, the final particles were purified by extracting CTAB. At this stage, the pores on the particles were clearly formed, and those pores were visible on the TEM images. The growth process was subject to mediation; if the particles were grown for a long period of time, the size of the particles became larger but the pores still existed. As shown in Fig. 1(c), the reaction began at 30 °C and continued for 12 h. The period of hydrolysis of TEOS and APTES helped the particles to grow gradually larger, resulting in synthesized particles with a mean diameter of 170.3 nm with and a small size distribution. From Fig. 1(d), clear and obvious pores around the surface of the particles were observed on individual SiO2-NH2 NPs (labeled as “SiO2-NH2 NPs”).
Fig. 1.
Fig. 1.
TEM images of FL-SiO2-NH2 NPs obtained by the traditional order (first tetraethylorthosilicate then (3-aminopropyl) triethoxysilane) of reagents addition (a), MD-SiO2-NH2 NPs obtained by the control order (tetraethylorthosilicate and (3-aminopropyl) triethoxysilane at the same time) for 4 h (b), SiO2-NH2 NPs obtained for 12 h (c) and individual SiO2-NH2 NPs (d).
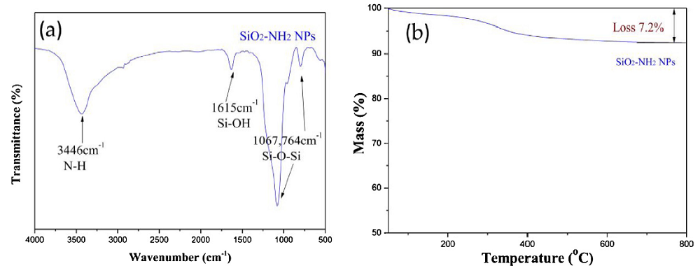
After reaction under the proper ratios of TEOS and APTES injected at the same time, the SiO2-NH2 NPs were freeze-dried before testing. According to the FT-IR spectrum in Fig. 2(a), the SiO2-NH2 NPs exhibited strong peaks at 1615, 1067, and 764 cm-1 attributed to the asymmetric and symmetric stretching vibrations of Si-O-Si. The peaks at 3448 cm-1 in the spectrum of SiO2-NH2 NPs could be assigned to the vibrations of the -NH2 groups. It indicated that the hydrolysis of TEOS occurred as well as APTES modification, resulting in a large of -NH2 groups on the SiO2-NH2 NPs. Hydrolysis, condensation, pore-forming, and purification are all processes that contribute to the final production of SiO2-NH2 NPs, and there were no bands attributed to the groups of CTAB. Although the CTAB concentrated solution acted as a template during the processes, the pores of SiO2-NH2 NPs were finally achieved by the extraction of CTAB. The organic content in SiO2-NH2 NPs was measured by TGA measurement. Based on the weight loss, the organic content of SiO2-NH2 NPs was calculated to be 7.2 wt% (Fig. 2(b)). By comparing to the conventional two-step synthesis of amino-functionalized mesoporous SiO2 NPs, with a number of amino groups of 1-3.8 mmol g-1 (1.6-6.08 wt%) [43]. It indicated that there were a number of -NH2 groups on the surface of the SiO2-NH2 NPs.
Fig. 2.
Fig. 2.
Fourier transforms infrared (FT-IR) spectrum (a) and thermogravimetric analysis (TGA) curve of SiO2-NH2 NPs (b).
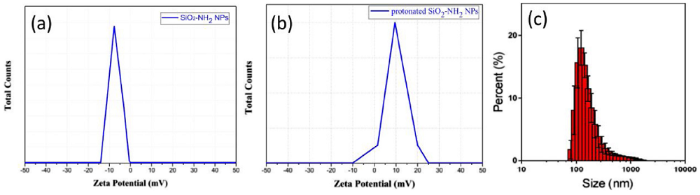
To further study the groups on the surfaces of particles, SiO2-NH2 NPs were handled by a diluted HCl aqueous solution through a protonation process. After protonation, the SiO2-NH2 NPs were then dialyzed in DI water until the pH value of the SiO2-NH2 NPs suspension became neutral. Zeta potentiometer characterization showed that primary SiO2-NH2 NPs (1 mmol L-1) had a negative potential of -7.41 mV (Fig. 3(a)) in a neutral aqueous environment. After protonation, Zeta potentiometer characterization showed that the protonated SiO2-NH2 NPs had a positive potential of +9.8 mV (Fig. 3(b)) due to the -NH3+ groups in a neutral aqueous environment. This confirmed that there were a large number of -NH2 groups on the surfaces of SiO2-NH2 NPs assigned to the FT-IR figures. We have also tested the hydrodynamic particle sizes with DLS, and the result is as below, the average particle size is around 170.3 nm, which meet the TEM result in Fig. 1(c) and (d).
Fig. 3.
Fig. 3.
Zeta potential of SiO2-NH2 NPs (a), protonated SiO2-NH2 NPs (b) at pH = 7 in the aqueous phase and the hydrodynamic particle sizes (c).
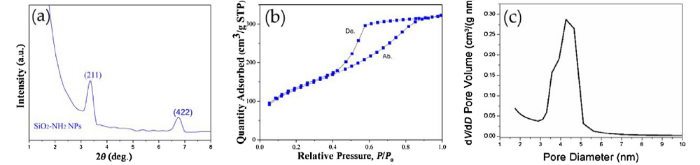
As seen in the small-angle X-ray diffraction (XRD) patterns of the porous SiO2-NH2 NPs in Fig. 4(a), the diffraction peaks at 3.3° and 6.8° also indicated the pores on the surface of the SiO2-NH2 NPs. According to the Bragg equation [44], the approximate diameters of those pores were 4 nm. From the TEM image in Fig. 1(b), those initial fine-size pores were inflected directly. Further, the optimum ethanol-water ratio was a base condition toward the synthesis of mono-dispersed SiO2-NH2 NPs with a porous and spherical structure in TEOS and APTES at 30 °C. At present, we believe that the combination of TEOS-CTAB-ethanol-water-ammonia was the best to obtain structural SiO2-NH2 NPs.
Fig. 4.
Fig. 4.
Small-angle XRD patterns of SiO2-NH2 NPs (a), nitrogen adsorption-desorption isotherm of SiO2-NH2 NPs (b) and pore size distribution of SiO2-NH2 NPs (c).
The nitrogen adsorption and desorption isotherm curves in Fig. 4(b) show the nitrogen adsorption and desorption isotherms of SiO2-NH2 NPs. In Fig. 4c the pore size distribution was showed. From the nitrogen adsorption measurement, the samples of SiO2-NH2 NPs were found to have a high specific surface area with the average pore size of around 4 nm. The specific surface area determination and pore volume and size analysis were performed by Brunauer-Emmett-Teller (BET) methods. From Fig. 4(b), SiO2-NH2 NPs had a BET surface area of 476 m² g-1, while the average (BJH) pore size was 4.3 nm. Compared to conventional amino-functionalized silica nanoparticles, with a BET surface area of 367-1287 m² g-1 and pore size of 2.66-3.64 nm, our one-step synthesis of SiO2-NH2 NPs resulted in nanoparticles with a moderate surface area but a larger pore size [43]. According to the XRD pattern of the SiO2-NH2 NPs, the combination of TEOS-CTAB-ethanol-water-ammonia system could obtain a large surface area and small pore size in SiO2-NH2 NPs.
3.2. Properties of synthesized porous SiO2-NH2 NPs
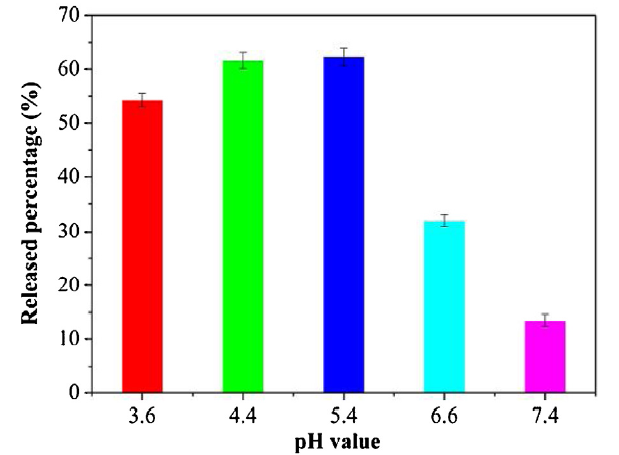
Porous SiO2-NH2 NPs could act as a carrier for drugs because of their porous structure and excellent stability. The SiO2-NH2 NPs could well disperse, and the dispersion exhibited excellent stability for more than three months in the water at room temperature (Fig. 5), because there are -NH2 groups on the surface of the particles, the strong positive surface charge was the main reason to keep this nanoparticle stable. In order to observe the drug release pattern of porous SiO2-NH2 NPs at different pH values, we simulated an in vitro drug release experiment of porous SiO2-NH2@EPB NPs, with a loading efficiency of 73.3 wt%. We selected EPB as a model drug because we want to confirm the absorption-release ability of the porous structure of SiO2-NH2 NPs. As shown in Fig. 6, there was only a 13.5% release of the EPB at pH = 7.4 and a 32.0% release at pH = 6.6. However, an abrupt release of 62.3% occurred at pH = 5.4. Similarly, there was a 61.6% release and a 54.2% release at pH = 4.4 and 3.6, respectively. When the SiO2-NH2 NPs absorb the EPB, the amino groups on the surface of porous SiO2-NH2 NPs can form hydrogen bonds with hydroxyl groups on the surface of EPB, but the amino groups can only be protonated once the SiO2-NH2@EPB NPs is in an acidic condition, at which point the EPB can be released [45,46]. Therefore, pH = 5.4 is a switch point for the release of EPB from porous SiO2-NH2@EPB NPs, which is similar to the acidity of tumor tissue in human bodies. Notably, such pH-dependent release behavior is of great significance for the controlled drug delivery of chemotherapeutics to target tumor sites [45,46].
Fig. 5.
Fig. 5.
Porous SiO2-NH2 NPs dispersed in water before and after three months.
Fig. 6.
Fig. 6.
Released epirubicin (EPB) percentage from SiO2-NH2@EPB NPs at different pH values (3.6, 4.4, 5.4, 6.6, and 7.4). Each independent experiment was tested three times.
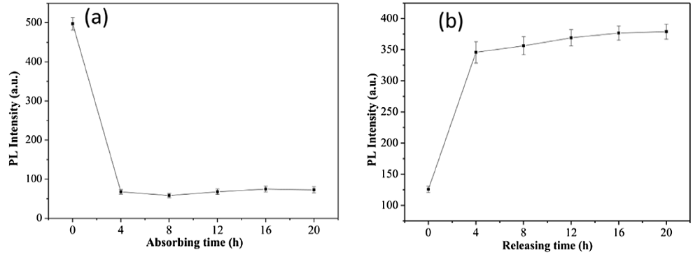
Meanwhile, capsaicin showed peaks at 338 nm according to its photo-luminescent (PL) spectrum. When SiO2-NH2 NPs were immersed capsaicin-ethanol solution, 10 mg SiO2-NH2 NPs exhibited good absorption of capsaicin after testing the PL spectrum of the solution at 4, 8, 12, 16, and 20 h. From the PL intensity peaks at 338 nm of residue capsaicin-ethanol solution with different immersion times of particles, the maximum absorption occurred at 4 h, after which it remained stable (Fig. 7(a)). However, When 10 mg SiO2-NH2@capsaicin NPs were immersed in water, the adsorbed capsaicin was be gradually released into the water, observed by testing the PL intensity at 338 nm of solutions at 4, 8, 12, 16, and 20 h. After SiO2-NH2@capsaicin NPs were immersed in water, more and more capsaicin was released from porous SiO2-NH2 into the water, and the PL spectrum of capsaicin in the water at different releasing times revealed that the maximum release of capsaicin by SiO2-NH2 NPs occurred at 16 h (Fig. 7(b)). According to our analysis, the best absorption (desorption) time of porous SiO2-NH2 NPs for capsaicin in ethanol (water) solutions was 4 (16) h. Because of the abundant amino groups in SiO2-NH2 NPs, formed by hydrogen bonds with the phenolic hydroxyl and amide groups in capsaicin, these nanoparticles were shown to have good absorption/desorption abilities for capsaicin [47].
Fig. 7.
Fig. 7.
Photo-luminescent (PL) intensity of capsaicin residue in ethanol after SiO2-NH2 NPs were immersed in capsaicin-ethanol solution for different absorption times (a) and capsaicin release in water for different releasing times (b). Each independent experiment was tested three times.
3.3. Characterization of antibacterial properties of synthesized porous SiO2-NH2 NPs
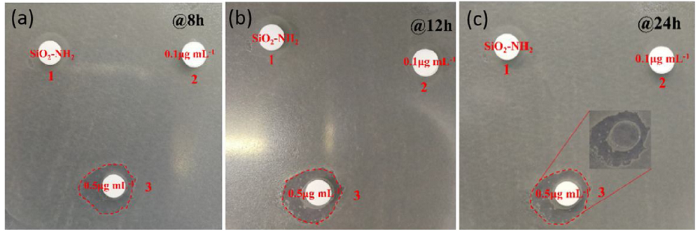
From the numbers of E. coli in Fig. 8, the Luria-Bertani (LB) medium was shown to inhibit the growth of E. coli. From the bacterial growth curves, the antibacterial property of SiO2-NH2@capsaicin NPs was shown gradually with the release of capsaicin, even at a relatively low concentration of 0.1 μg mL-1. Compared to the numbers of only E. coli in LB medium, the SiO2-NH2 NPs exhibited almost no antibacterial property, which can be seen from their similar growth curves. All the bacterial growth curves showed a plateau or decrease after 16 h. Furthermore, the antibacterial property of SiO2-NH2@capsaicin NPs with a concentration of 0.5 μg mL-1 was better than that with a concentration of 0.1 μg mL-1, which can be seen from the lower bacterial growth curve. The antibacterial activity of SiO2-NH2@capsaicin NPs was tested using the agar diffusion method in order to confirm the antibacterial property of SiO2-NH2@capsaicin NPs more intuitively. As shown in Fig. 9, the SiO2-NH2 @capsaicin NPs exhibited antibacterial properties from the zone of inhibition at different times. It can be clearly seen that the bacteria in zone one grew very well, and there was almost no influence in zone two. However, the bacteria in zone three was greatly influenced, as seen clearly from the zone of inhibition. The inhibition zone width in zone three was measured to be 3.7 mm, 4 mm, and 4.2 mm at 8 h, 12 h, and 24 h, respectively. The inhibition zone width can be calculated using the equation:
where H represents the inhibition zone width (mm), D represents the inhibition zone outer diameter (mm), and d represents the sample diameter (mm). Moreover, there was almost no bacteria on the LB medium when the paper was removed (insert of Fig. 9(c)). Therefore, it can be concluded that SiO2-NH2@capsaicin NPs have antibacterial properties against E. coli and the concentration of the SiO2-NH2@capsaicin NPs is not negligible. As a drug delivery carrier, the SiO2-NH2 NPs exhibited good absorption and release properties of capsaicin, while the SiO2-NH2@capsaicin NPs showed antibacterial properties.
Fig. 8.
Fig. 8.
The numbers of E. coli after 0.5, 1, 2, 4, 8, 16, and 20 h of E. coli in Luria-Bertani (LB) medium, with 0.5 μg mL-1 SiO2-NH2 NPs, 0.1 μg mL-1 SiO2-NH2@capsaicin NPs, and 0.5 μg mL-1 SiO2-NH2@capsaicin NPs added to E. coli in Luria-Bertani (LB) medium. Each independent experiment was tested three times.
Fig. 9.
Fig. 9.
Antibacterial activity of SiO2-NH2@capsaicin NPs at (a) 8 h, (b) 12 h and (c) 24 h against E. coli (insert: part 3, after the paper was removed). SiO2-NH2 NPs, 0.1 μg mL-1 SiO2-NH2 @capsaicin NPs, and 0.5 μg mL-1 SiO2-NH2@capsaicin NPs were added to parts 1 to 3, respectively.
4. Conclusion
In summary, we have successfully developed a one-step approach to synthesize porous SiO2-NH2 NPs through the ammonia-catalyzed hydrolysis of TEOS and APTES in a mixed addition system. After condensation, pore-forming, and purification, porous SiO2-NH2 NPs had a large surface area of 476.02 m² g-1 and an average pore width of 4 nm. These SiO2-NH2 NPs exhibited efficient absorption and release of capsaicin, while SiO2-NH2@EPB NPs showed a pH-response release characteristic. SiO2-NH2@capsaicin NPs showed antibacterial properties, but the concentration of NPs was not negligible. The antibacterial properties can be attributed to the unique porous structure and the amine surface for enhanced interactions with cells. So far, we believe that the combination of TEOS-CTAB-ethanol-water-ammonia was the best to obtain structural SiO2-NH2 NPs. These porous SiO2-NH2 NPs can be used in antibacterial applications as drug carriers.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (No. 51706166 and No. 51773163), the Innovation Group of Natural Science Foundation of Hubei Province (No. 2016CFA008) and the Joint Funds of China (No. 20171f0107).
Reference
DOI
URL
PMID
[Cited within: 1]

Drug resistance of bacteria has become a global health problem, as it makes conventional antibiotics less efficient. It is urgently needed to explore novel antibacterial materials and develop effective treatment strategies to overcome the drug resistance of antibiotics. Herein, we successfully synthesized silver decorated sandwich-like mesoporous silica/reduced graphene oxide nanosheets (rGO/MSN/Ag) as a novel antibacterial material through facile method. The rGO and Ag nanoparticles can be reduced in the reaction system without adding any other reductants. In addition, the rGO/MSN/Ag showed higher photothermal conversion capacity due to the modification of silver nanoparticles and exhibited excellent antibacterial activities against Pseudomonas putida, Escherichia coli and Rhodococcus at relatively low dosages, which was confirmed by the minimum inhibitory concentration (MIC) test. Meanwhile, the E. coli with a high concentration was selected for exposure using an 808 nm laser, and the antibacterial effect was obviously enhanced by the near-infrared irradiation induced photothermal effect. Moreover, the hepatocyte LO2 were used for the cytotoxicity evaluation, and the rGO/MSN/Ag showed low toxicity and were without detectable cytotoxicity at the antimicrobial dose. As the prepared rGO/MSN/Ag nanosheets have the advantages of low-cost and high antibacterial activity, they might be of promising and useful antibacterial agents for different applications.
DOI
URL
PMID
[Cited within: 1]

To efficiently deliver anti-cancer drug to tumor site and reduce its toxic side effects on normal tissues, a polyethylene glycol (PEG) shielding and tumor microenvironment triggering cascade pH-responsive hollow mesoporous silica nanoparticles (HMSNs) drug delivery system was fabricated. 3-(3, 4-dihydroxyphenyl) propionic acid (DHPA) functionalized beta-cyclodextrin (beta-CD) was grafted onto the surfaces of HMSNs via boronic acid-catechol ester bonds. Then, PEG conjugated adamantane (Ada) was anchored on HMSNs-beta-CD nanocarrier via host-gust interaction. Various techniques proved the successful fabrication of the system. The in vitro tests confirmed that the system was biocompatible. After the system permeating into tumor via enhanced permeability and retention (EPR) effect, the benzoic-imine bonds between the PEG and Ada were cleaved under weak acid condition in tumor microenvironment (pH 6.8), while the dissociated PEG protective layer facilitating cellular uptake of HMSNs system. Subsequently, the boronic acid-catechol ester bonds linkers further hydrolyzed under even low endosomal pH (4.5-6.5) condition for intracellular drug delivery, leading to efficient cell apoptosis. The in vivo results demonstrated that drug loaded HMSNs significantly inhibited tumor growth while only with minimal toxic side effects. The strategy provides new insight into the development of new generation of drug delivery carriers triggering by tumor microenvironment.
DOI
URL
PMID
[Cited within: 1]

Use of amphiphilic triblock copolymers to direct the organization of polymerizing silica species has resulted in the preparation of well-ordered hexagonal mesoporous silica structures (SBA-15) with uniform pore sizes up to approximately 300 angstroms. The SBA-15 materials are synthesized in acidic media to produce highly ordered, two-dimensional hexagonal (space group p6mm) silica-block copolymer mesophases. Calcination at 500 degrees C gives porous structures with unusually large interlattice d spacings of 74.5 to 320 angstroms between the (100) planes, pore sizes from 46 to 300 angstroms, pore volume fractions up to 0.85, and silica wall thicknesses of 31 to 64 angstroms. SBA-15 can be readily prepared over a wide range of uniform pore sizes and pore wall thicknesses at low temperature (35 degrees to 80 degrees C), using a variety of poly(alkylene oxide) triblock copolymers and by the addition of cosolvent organic molecules. The block copolymer species can be recovered for reuse by solvent extraction with ethanol or removed by heating at 140 degrees C for 3 hours, in both cases, yielding a product that is thermally stable in boiling water.
DOI
URL
PMID
[Cited within: 1]

To improve and extend the SiO2 template method for preparing metal oxide hollow spheres, a general and facile
DOI URL PMID [Cited within: 1]
DOI
URL
PMID
[Cited within: 1]

A new ultrasound-responsive system based on mesoporous silica nanoparticles was developed for biomedical applications, grafting a copolymer on their surface that acts as gatekeeper of the pores. The nanoparticles can be loaded with a cargo at low temperature (4 degrees C), taking advantage of the open conformation that the polymer presents under these conditions. Then, at 37 degrees C the copolymer collapses closing the pore entrances and allowing the nanoparticles to carry the drugs at physiological temperature without premature release, which is of great importance when dealing with cytotoxic drugs in cancer treatments. Upon ultrasound irradiation, the sensitive polymer changes its hydrophobicity and, therefore, its conformation toward coil-like opening the gates and releasing the cargo. These hybrid nanoparticles have been shown to be noncytotoxic and can be internalized into LNCaP cells retaining their ultrasound-responsive capability in the cytoplasm of the cells. Moreover, doxorubicin-loaded hybrid MSNs were incubated with LNCaP cells to show their capacity to induce cell death only when the nanoparticles had been exposed to ultrasound. This work demonstrates that our hybrid-MSNs can be triggered by remote stimuli, which is of capital importance for future applications in drug delivery and cancer therapy.
DOI
URL
PMID
[Cited within: 1]

A novel multifunctional envelope-type mesoporous silica nanoparticle (MEMSN) system combining the merits of pH-responsiveness, non-toxicity and biological specificity, is demonstrated for drug delivery and magnetic resonance imaging (MRI). This system is constructed by immobilizing acetals on the surface of mesoporous silica, and then coupling to ultra small lanthanide doped upconverting nanoparticle, which act as a gate keeper. The anticancer drug DOX is thus locked in the pores, and its burst release can be achieved under acidic environment on account of the hydrolyzation reactions of acetals. The nanogated drug release system is highly efficacious for cancer therapy both in vitro and in vivo. Importantly, the nanocomposite could be harmlessly metabolized and degraded into apparently non-toxic products within a few days. The nanoscale effect of the system allows for passive tumor targeting and increased tumor accumulation of the probes via the enhanced permeation and retention (EPR) effect, which is visualized by MRI in vivo. Therefore, such nanosystem should be of great significance in the future development of highly efficient and tumor targeted drug delivery vehicles for cancer chemotherapy.
DOI
URL
PMID
[Cited within: 1]

Good control of the morphology, particle size, uniformity and dispersity of mesoporous silica nanoparticles (MSNs) is of increasing importance to their use in catalyst, adsorption, polymer filler, optical devices, bio-imaging, drug delivery, and biomedical applications. This review discusses different synthesis methodologies to prepare well-dispersed MSNs and hollow silica nanoparticles (HSNs) with tunable dimensions ranging from a few to hundreds of nanometers of different mesostructures. The methods include fast self-assembly, soft and hard templating, a modified Stober method, dissolving-reconstruction and modified aerogel approaches. In practical applications, the MSNs prepared by these methods demonstrate good potential for use in high-performance catalysis, antireflection coating, transparent polymer-MSNs nanocomposites, drug-release and theranostic systems.
DOI
URL
PMID
[Cited within: 3]

Highly monodisperse, dendritic, and functionalized mesoporous silica nanospheres (MSNs) with sub-200 nm size were synthesized in a one-pot sol-gel reaction, by a dual-templating micelle system consisting of a partially fluorinated short-chain anionic fluorocarbon surfactant and cetyltrimethylammonium bromide. This kind of anionic fluorocarbon surfactant works simultaneously as a swelling agent to enlarge the pore of the MSNs, an ion-pair agent to the structure-directing silane in the preparation of amine-functionalized MSNs, and a surface tension reducing agent to make the system thermodynamically more stable for producing more uniform MSNs. The particle size and the morphology of the resultant MSNs can be fine-tuned by changing the amount of the fluorocarbon surfactant added and the ratio of the functional group containing organosilane to tetraethoxysilane. Subsequently, the as-prepared MSNs were used as base materials for the preparation of drug delivery nanomaterials through the surface grafting of a pH-sensitive drug-conjugated polymer and fluorescent nanomaterials through the embedding of europium(III) complex or the immobilization of large molecule fluorescein isothiocyanate-bovine serum albumin.
DOI
URL
PMID
[Cited within: 1]

Multifunctional integration based on a single nanostructure is emerging as a promising paradigm to future functional materials. In this paper, novel magnetofluorescence nanobowls built with ferroferric mandrel and quantum dots exoderm is reported. Magnetic mandrels are stacked into nanobowls though hydrophobic primary Fe3O4 nanocrystals dragged into anion polyelectrolyte aqueous solution via forced solvent evaporation. Bright luminescence core/shell/shell CdSe/CdS/ZnS quantum dots (QDs) are modified with cationic hyperbranched polyethylenimine (PEI). Through electrostatic interactions, positively charged PEI-coated QDs are anchored on the surface of magnetic mandrel. Under this method, the luminescence of QDs is not quenched by magnetic partners in the resultant magnetoflurescence nanobowls. Such magnetoflurescence nanobowls exhibit high saturation magnetization, superparamagnetic characteristics at room temperature, superior water dispersibility, and excellent photoluminescence properties. The newly developed magnetoflurescence nanobowls open a new dimension in efforts toward multimodal imaging probes combining strong magnetization and efficient fluorescence in tandem for biosensors and clinical diagnostic imaging.
DOI
URL
PMID
[Cited within: 1]

Hollow spherical silica particles with hexagonally ordered mesoporous shells are synthesized with the dual use of cetyltrimethylammonium bromide (CTAB) and unmodified polystyrene latex microspheres as templates in concentrated aqueous ammonia. In most of the hollow mesoporous particles, cylindrical pores run parallel to the hollow core due to interactions of CTAB/silica aggregates with the latices. Effects on the product structure of the CTAB:latex ratio, the amount of aqueous ammonia, and the latex size are studied. Hollow particles with hexagonally patterned mesoporous shells are obtained at moderate CTAB:latex ratios. Too little CTAB causes silica shell growth without surfactant templating, and too much induces nucleation of new mesoporous silica particles without latex cores. The concentration of ammonia must be large to induce co-assembly of CTAB, silica, and latex into dispersed particles. The results are consistent with the formation of particles by addition of CTAB/silica aggregates to the surface of latex microspheres. When the size and number density of the latex microspheres are changed, the size of the hollow core and the shell thickness can be controlled. However, if the microspheres are too small (50 nm in this case), agglomerated particles with many hollow voids are obtained, most likely due to colloidal instability.
DOI
URL
PMID
[Cited within: 1]

Mesoporous silica materials with a variety of morphologies, such as monodisperse microspheres, gigantic hollow structures comprising a thin shell with a hole, and gigantic hollow structures consisting of an outer thin shell and an inner layer composed of many small spheres, have been readily synthesized in mixed water-ethanol solvents at room temperature using cetyltrimethylammonium bromide (CTAB) as the template. The obtained mesoporous silica generally shows a disordered mesostructure with typical average pore sizes ranging from 3.1 to 3.8 nm. The effects of the water-to-ethanol volume ratio (r), the volume content of tetraethyl orthosilicate TEOS (x), and the CTAB concentration in the solution on the final morphology of the mesoporous silica products have been investigated. The growth process of gigantic hollow shells of mesoporous silica through templating emulsion droplets of TEOS in mixed water-ethanol solution has been monitored directly with optical microscopy. Generally, the morphology of mesoporous silica can be regulated from microspheres through gigantic hollow structures composed of small spheres to gigantic hollow structures with a thin shell by increasing the water-to-ethanol volume ratio, increasing the TEOS volume content, or decreasing the CTAB concentration. A plausible mechanism for the morphological regulation of mesoporous silica by adjusting various experimental parameters has been put forward by considering the existing state of the unhydrolyzed and partially hydrolyzed TEOS in the synthesis system.
DOI
URL
PMID
[Cited within: 1]

In this work, a novel type of hollow mesoporous silica nanoparticle (HMSN) with a rough surface has been successfully prepared via a facile soft-hard template route by using a carbon nanosphere as a hard template and cetyltrimethylammonium bromide (CTAB) as a soft template, respectively. This method involves the preparation of a carbon nanosphere, sequential coating of double SiO2 layers, and the removal of the inner carbon core and CTAB to produce HMSNs. The obtained HMSNs possess spherical morphology, a mesoporous shell, and crumpled surfaces. The controlled experiments prove that the addition of 3-ammonia propyl triethoxy silane (APTES) is very crucial for the formation of desired HMSNs. The cell tests indicate that HMSNs show a good biocompatibility. As a result, the potential applications of HMSNs are further explored for drug delivery and protein adsorption, using doxorubicin hydrochloride (DOX) and Cytochrome c (Cyt c) as the model drug and protein, respectively. The HMSNs exhibit high drug loading and protein adsorption capacity, as well as the controlled pH-responsive release behavior for DOX. Therefore, the HMSNs prepared are ideal candidates for various applications such as nanoreactors, drug delivery and protein adsorption.
It can be larger: A facile self-assembly/solvothermal approach to synthesize monodispersed, large-pore (>12 nm) silica nanospheres (LPSNs) with ordered, accessible, and interconnected pore channels has been successfully developed by utilizing an amphiphilic block copolymer (polystyrene-b-poly (acrylic acid), PS-b-PAA) as pore template and cetyltrimethylammonium bromide (CTAB) as structure-stabilizing agent.
Multifunctional dual-compartment Janus mesoporous silica nanocomposites of UCNP@SiO2@mSiO(2)&PMO (UCNP = upconversion nanoparticle, PMO = periodic mesoporous organosilica) containing core@shell@shell structured UCNP@SiO2@mSiO(2) nanospheres and PMO single-crystal nanocubes have been successfully synthesized via a novel anisotropic island nucleation and growth approach with the ordered mesostructure. The asymmetric Janus nanocomposites show a very uniform size of similar to 300 nm and high surface area of similar to 1290 m(2)/g. Most importantly, the Janus nanocomposites possess the unique dual independent mesopores with different pore sizes (2.1 nm and 3.55.5 nm) and hydrophobicity/hydrophilicity for loading of multiple guests. The distinct chemical properties of the silica sources and the different mesostructures of the dual-compartments are the necessary prerequisites for the formation of the Janus nanostructure. With the assistance of the near-infrared (NIR) to ultraviolet/visible (UVvis) optical properties of UCNPs and heat-sensitive phase change materials, the dual-compartment Janus mesoporous silica nanocomposites can be further applied into nanobiomedicine for heat and NIR light bimodal-triggered dual-drugs controllable release. It realizes significantly higher efficiency for cancer cell killing (more than 50%) compared to that of the single-triggered drugs delivery system (similar to 25%).
DOI
URL
PMID
[Cited within: 2]

Recent studies with inorganic nanoparticles modified with functional groups have demonstrated improvement in drug delivery into cancer cells. In the present study, we prepared, characterized, and evaluated mesoporous silica nanoparticles (MSNs) as carriers for epirubicin hydrochloride (EPI) in order to improve the antitumor efficacy of this drug. MSNs were prepared and functionalized with phosphonate, polyethylene glycol (PEG) and polyethylenimine-polyethylene glycol (PEI-PEG) groups. Different nanoparticulate formulations were loaded with EPI. The in vitro cytotoxicity and the in vivo antitumor efficacy of MSNs containing EPI were evaluated versus free EPI. The EPI release from nanoparticles was shown to be pH-dependent. The size of MSNs functionalized with polyethyleneimine-polyethylene glycol (MSN-PEI-PEG) was 123.8 +/- 4.8 nm. This formulation showed the best antitumor effects at an EPI dose of 9 mg/kg in C-26 colon carcinoma model. The biodistribution results proved that MSN-PEI-PEG-EPI had a higher tumor accumulation compared to free EPI, 3h after drug administration. The results indicated that this formulation could be effective nanocarriers for anti-tumor therapies.






 WeChat
WeChat