1. Introduction
With the increase of environmental pollution and the depletion of traditional fossil energy reserves, it is obligatory imperative to explore more renewable energy sources to promote sustainable social and technological development [1]. For this reason, researchers have been working hard to find alternative clean energy. To collect the energy of sunlight and covert it into other forms of energy is an efficient way of using the solar energy. Photocatalytic splitting water to produce H2 is a widely developed method for investgating a new green energy [2]. To search for effective photocatalysts is the key to the method, but it still faces some challenges. The ideal photocatalyst should meet stringent requirements, such as (Ⅰ) appropriate band gap for collecting a wide range of sunlight; (Ⅱ) suitable bandwidth level for energy conversion; (Ⅲ) outstanding charge separation and transfer performance under visible light illumination [3].
So far, many semiconductors have been used in the research of photocatalytic hydrogen production, such as TiO2 [4], CdS [5], g-C3N4 [6], ZnS [7], ZnIn2S4 [8], and Zn0.5Cd0.5S [9]. Wherein, graphitic carbonitride (g-C3N4) is one of the excellent candidates for this kind of photocatalyst, and has been widely studied due to its excellent optical, thermal and electrical properties and low cost [[10], [11], [12], [13]]. After intensive researchs, it is found that the catalytic activity of g-C3N4 is significantly depend on its morphology, size, surface area and defects andenergy states, including its electronic properties [14,15]. Regrettably, the photocatalytic activity of pure g-C3N4 (CN) is confined because of weak light absorption capacity, tardy charge mobility and fast photo-induced electron-hole pairs recombination rate [16,17]. Aiming at solving the problem that the absorption capacity of g-C3N4 to visible light is very weak, at present, the mainstream has considered introducing a photosensitizer into the reaction system [10]. EY, as a typical organic photosensitizer, which is often used in g-C3N4 reaction system, can strengthen the visible light absorption capacity of photocatalyst and provide a rich electron source to the system [11]. In the current study, much work remains to be done in the photocatalysis domain of g-C3N4 to further improve its photocatalytic performance. In order to effectively realize the efficient production of solar photon-driven hydrogen, immobilized promoter on CN surface is one of the valid strategies to improve the performance of photocatalytic H2 evolution [18,19]. As is known to all that precious metals (such as Au [20], Ag [21,22], and Pt [23,24], Pd [25],) are efficient cocatalysts that can improve the corresponding photocatalytic properties of CN to some extent. Nevertheless, precious metals are not suitable for future industrialization of photocatalysis due to rare and expensive properties. Hence, develop a new kind of abundant non-noble metal cocatalysts with a low cost to replace precious metals is necessary [26,27].
Due to the synergy effect between different active sites, bimetallicnanoparticles (composed of two different metal elements) generally exhibit distinguished electronic and optical performances [[28], [29], [30]]. In this work, graphite carbon nitride (g-C3N4) was modified by Ni-Cu bimetallic nanoparticles, according to a simple calcination process and ultimately formed a highiy efficient visible light-driven photocatalyst. Nickel acetate and copper acetate are used as precursors to obtain nickel and copper elemental materials in a nitrogen atmosphere at 300 °C. A composite catalyst can be obtained after secondary calcination. Assuredly, the test results showed that hydrogen evolution reaction can be promoted by the strategy of bimetal modified. The doping effect of single metal Ni and bimetal Ni-Cu was studied by experimental and theoretical methods. Based on a series of characterization analysis results, a reasonable photocatalytic mechanism for enhancing hydrogen evolution activity is proposed. This work provides a new method for the development of non-noble metal promoters.
2. Experimental
The used chemical reagents were analytical reagent, and the water used for the experiment was not further purified as ultra-pure water
2.1. Preparation of g-C3N4
A certain quality of melamine was weighed and placed in a covered crucible. Putting the crucible in a muffle furnace and it was calcined at 550 °C for 2 h. Cooling it to room temperature and obtaining yellow solid product, which was ground into powder and collected.
2.2. Preparation of composite photocatalyst
A certain amount of nickel acetate and copper acetate were kept at a heating rate of 2 °C min-1 in a nitrogen atmosphere at 300 °C for 2 h. After calcination, washing the obtained catalysts with a small amount of diluted hydrochloric acid, nickel and copper element could be obtained. Nickel and copper element were identified as N-300 and C-300, respectively. The CNN-n (; ‘n’ refers to the mass percentage of metal to g-C3N4, and n = 5, 10, 15, 20.) composite was obtained by calcining 0.20 g of the g-C3N4 sample and the Ni element with different mass percentage to g-C3N4 together in a nitrogen atmosphere at 300 °C for 1 h. CNNC-x (x = 5, 10, 15, 20) composite catalyst was obtained by adjusting the proportion of copper elemental on the basis of CNN-15 catalyst. The specific preparation process was shown in Fig. 1.
Fig. 1.
Fig. 1.
Synthesis procedure for Ni-Cu/g-C3N4.
2.3. Characterization
To investigate the corresponding information about the phase composition of the samples, XRD measurement was used to characterize the results (HORIBA Scientific, France; 40 kV and 30 mA, with a scan ranging from 5° to 80°, scan a rate of 10°/min). SEM, TEM and HRTEM were used to obtain the microstructures of the samples (JSM-6701 F. JEOL, FEI Tecnai TF20 high-resolution transmission electron microscope). XPS results were represented on an energy dispersive X-ray instrument (ESCALAB 250Xi). Heat stability of the samples was also be explored by the Thermogravimetric experiments (German NETZSCH STA449 Synchronous Thermal Analyzer). Employing BaSO4 as the reference for baseline correction, UV-vis diffuse reflectance spectra (DRS) of the catalyst was characterized by a UV-2550 (Shimadzu) spectrometer for investigating the corresponding optical performance information. According to the BET method, specific surface areas of the samples were determined by the N2 adsorption-desorption isotherms (ASAP2020 M). The results of photoluminescence spectroscopy were acquired by a FLUOROMAX-4 spectrophotometer at indoor temperature.
2.4. Photoelectrochemical measurement
The photoelectrochemical measurement was performed with a standard three-electrode cell and the 0.2 M Na2SO4 as electrolyte on an electrochemical workstation (VersaStat4-400, Advanced measurement Technology, Inc). The prepared photoanode was used as the working electrode, a Pt plate was used as the counter electrode, and a saturated calomel electrode (SCE) was used as the reference electrode. A 300 W xenon lamp was used as the light source, and a 420 nm cut filter removed the ultraviolet light. Transient photocurrent measurements at open circuit potential were recorded under visible light illumination. LSV curves were measured at room temperature and a scan rate of 5 mV s-1. An electrochemical impedance spectroscopy (EIS) pattern was acquired at an open circuit potential with a frequency ranging from 1 MHz to 1 Hz
2.5. Hydrogen production experiments
The tests of photocatalytic H2 evolution performance were carried out in a 62 mL quartz reaction flask with a flat window. Specific steps were as follows: using TEOA as the electron donor, adding 10 mg photocatalyst powder and 10 mg EY into 30 mL of TEOA aqueous solution (15 %). Before light source irradiation, using ultrasonic dispersion lasted for 5 min to form homogeneous solution, and using nitrogen to replace the system air for 10 min to form anaerobic environment. The photocatalyst in suspension was evenly dispersed with a magnetic stirrer. A 5 W LED white-light multi-channel was used as simulated solar light source in the photocatalytic reaction system. Each time, 0.5 mL of evolved gas was extracted from the reaction flask to analyze the produced hydrogen amount by taking gas chromatography (Tianmei GC7900, TCD, 13Xcolumn, N2 as carrier).
The apparent quantum efficiency (QE) was measured under a 300 W xenon lamp. The light source was transformed by using 420, 450, 475, 500, 550, and 600 nm band-pass filters. And the output intensity was measured by a PL-MW2000 optical radiometer. The distance between the reaction bottle and the lamp source was 15 cm, and the light receiving area was as the same as the optical radiometer. The QE value can be calculated according to Eq. (1):
3. Results and discussion
3.1. XRD and FT-IR analysis
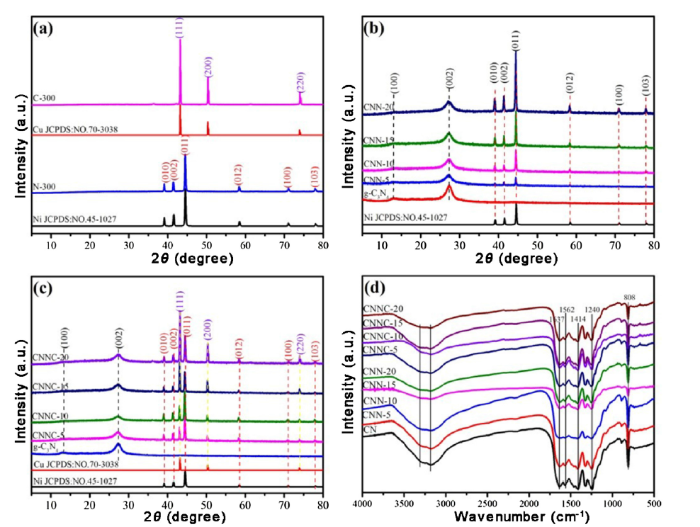
The phase structure of the sample was investigated via taking XRD spectrometer. As displayed in Fig. 2(a), N-300 and C-300 obtained by a calcination method under nitrogen atmosphere can correspond very well to nickel element and copper element standard card, respectively. The diffraction peaks of N-300 at about 39.15°, 41.57°, 44.54°, 58.42°, 71.03° and 78.02°correspond to (010), (002), (011), (012), (110) and (103) crystal planes of Ni (JCPDS-45-1027), respectively. Similarly, the diffraction peaks at 43.17°, 50.32° and 73.89° in the C-300 samples are corresponded to (111), (200) and (220) crystal planes of Cu (JCPDS-70-3038), respectively. These results show that the purest nickel and copper elements can be obtained by the above method. Fig. 2(b) shows a comparison of XRD patterns of obtained samples that with different nickel contents. The g-C3N4 photocatalyst exhibited two major diffraction peaks at 13.1° and 27.4°, which related to the crystal planes of (100) and (002). The diffraction peak appeared at 13.1° corresponds to the repeating structural unit of the heptazine ring (C6N7), and the interplanar spacing was 0.765 nm. Another strong diffraction peak is located at 27.4°, and the interlayer spacing is 0.336 nm, which is attributed to the interlayer accumulation of the aromatic system [[31], [32], [33]]. In CNN-n (n = 5, 10, 15, 20), except (100) and (002) crystal planes which belong to bare g-C3N4, the intensity of all diffraction peaks on Ni elemental are enhanced with the increasing of n. And no other new diffraction peaks appeare. By contrst, the (111), (200) and (220) crystal planes of Cu can be clearly observed in CNNC-x (x = 5, 10, 15, 20) after the introduction of copper element (Fig.2(c)), and the diffraction peak intensity enhanced with the increasing Cu content too. This phenomenon showed that compounding g-C3N4 with element nickel and/or element copper was successful.
Fig. 2.
Fig. 2.
XRD patterns (a, b, c) and FT-IR (d) of bare g-C3N4 (CN), CNN-n (n = 5, 10, 15, 20), CNC and CNNC-x (x = 5, 10, 15, 20) samples.
As shown in Fig. 2(d), infrared spectra of g-C3N4, CNN-n (n = 5, 10, 15, 20), CNC and CNNC-x (x = 5, 10, 15, 20) are displayed. The broad peak between 3000 and 3600 cm-1 is derived because of the extendible vibration of N—H band and the water species physical adsorption on the surface of g-C3N4 [34]. The peak at 1637 cm-1 indicates the stretching of C (sp2) = N units which belongs to the aromatic heptazine heterocycle. And the peak appeares at about 1562 cm-1 belongs to the typical CN-heterocyclic stretching mode [35]. The peak appeares at approximately 1246 cm-1 corresponded to the unit of C—NH—C [35,36]. The last absorption peak which appeares at about 1406 cm-1 could attributable to the mode of pyridazine unit stretching vibration, while the other strong infrared band at 808 cm-1 can be attributed to the out-of-plane bending oscillation of the pyridazine unit [37].
3.2. XPS analysis
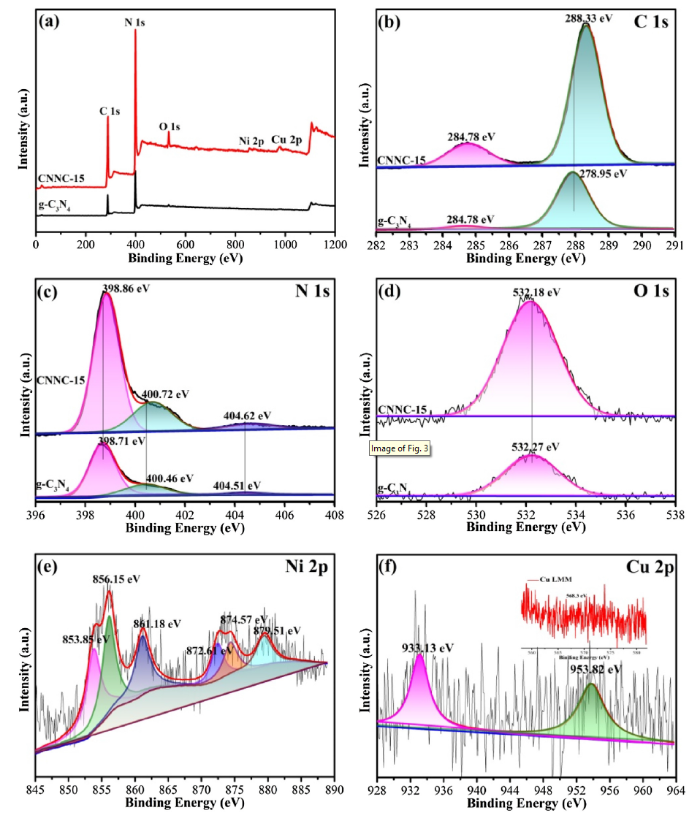
To investigate the chemical state of all elements in CNNC-15 photocatalyst, we performed XPS test. As shown in Fig. 3(a), the measured spectra of CNNC-15 and pure g-C3N4 indicates that the sample contained the signal peaks of C, N, O elements. Moreover, CNN-15 composite sample also had signal peaks of Ni and Cu compared with pure g-C3N4. Fig. 3(b) is C element fine spectrum. And the binding energy which appeares at 284.78 eV is caused by contaminated during the test. The peak at 288.33 eV could be attributed to the attachment between one carbon atom and three N atoms in the g-C3N4 lattice [38]. Compared with the fine spectrum of C element in pure g-C3N4, the binding energy of C element in CNNC-15 composite sample increases, indicating the existence of charge transfer. Fig. 3(c) shows three peaks in the XPS results of N 1s. The peak appeares at 398.86 eV corresponded with aromatic nitrogen (C=N-C) bondes to a carbon atom by the sp2 hybrid [39]. And the weaker peak with the higher binding energy at 400.72 eV can be attributed to three carbon atoms bonded with quaternary nitrogen in the aromatic ring [40]. The peak centered at 404.62 eV belongs to π excitation [41]. The corresponding binding energy of different elements in CNNC-15 composite is higher than that in the bare g-C3N4, which demonstrates that after the secondary calcination to modify the element of Ni-Cu to bare g-C3N4, the charge of the bulk g-C3N4 phase would move to the metal, that resulting the increase of the binding energy of C and N elements. Fig. 3(d) shows that the binding energy of O1 s peak is 532.18 eV, which is related to the surface of the sample oxidized during the test. In the high resolution XPS spectrum of Ni 2p (Fig. 3(e)), 853.85 eV and 872.61 eV correspond to Ni 2p3/2 and Ni 2p1/2 of nickel element (Ni°), respectively [42]. The other two peaks which located 856.15 eV and 874.57 eV relate to Ni 2p3/2 and Ni 2p1/2 of NiO(Ni2+), respectively, and there are two accompanied satellite peaks at 861.18 eV and 879.51 eV [43,44]. Fig. 3(f) showes the characteristic peaks of Cu 2p which binding energy appeares at about 933.13 eV and 953.82 eV belong to the Cu 2p3/2 and Cu 2p1/2, respectively [45,46]. Further analysis of Cu 2p spectra reveals unaccompanied satellite peaks, and the peak of Cu LMM in the illustration is 568.3ev, indicating the presence of Cu element (Cu°) in the composite material, which can also be proved by the XRD pattern.
Fig. 3.
Fig. 3.
XPS analysis of CNNC-15 composite: (a) XPS survey spectrum of CNNC-15 and pure g-C3N4; High-resolution XPS spectrum of C 1s (b), N 1s (c), O 1s (d), Ni 2p (e) and Cu 2p (f) that belongs to CNNC-15 and pure g-C3N4, respectively.
3.3. SEM
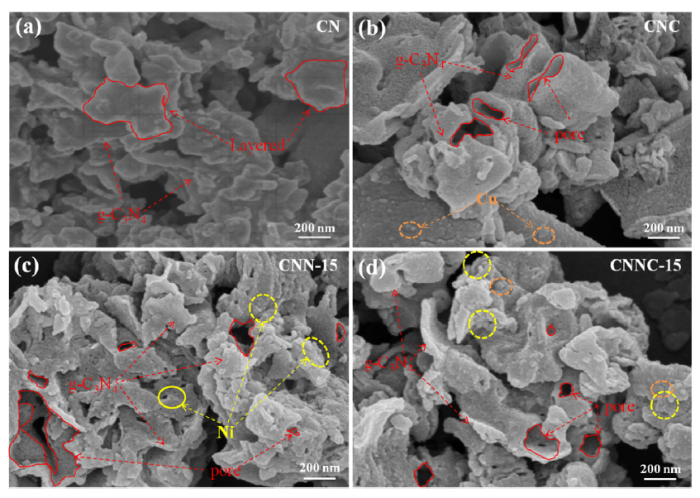
Morphology is one of the important factors that affect photoactivity. Fig. 4(a-d) are scanning electron microscope (SEM) images of g-C3N4(CN), CNC, CNN-15 and CNNC-15, respectively. As shown in Fig. 4(a), agglomerated irregular flakes and particles composed the morphology of pure g-C3N4, which is a representative structural feature of g-C3N4 that synthesized by the method of thermal polymerization [47,48]. Fig. 4(b) and (c) are the SEM images of CNC and CNN-15 samples calcined with Cu and Ni. It is noticeable that the size of composite sample is smaller than that of the bare bulk g-C3N4, and the surface has distinct small particles. After the secondary calcination, the g-C3N4 shows a sheet-like structure at before but in the composite, it shows obvious bending and appeares some voids. These voidage results from the stacking and secondary heat treatment of g-C3N4 sheets. Fig. 4(d) of the bimetallic loaded on CNNC-15 sample is like that of the single metal. In addition, the porous structure is conducive to heterogeneous catalytic reactions, promoting the diffusion and transport of reactant molecules and it may help to improve the photocatalytic H2 evolution activity [49,50].
Fig. 4.
Fig. 4.
SEM pictures of g-C3N4 (a), CNC (b), CNN-15 (c) and CNNC-15 (d).
3.4. TEM
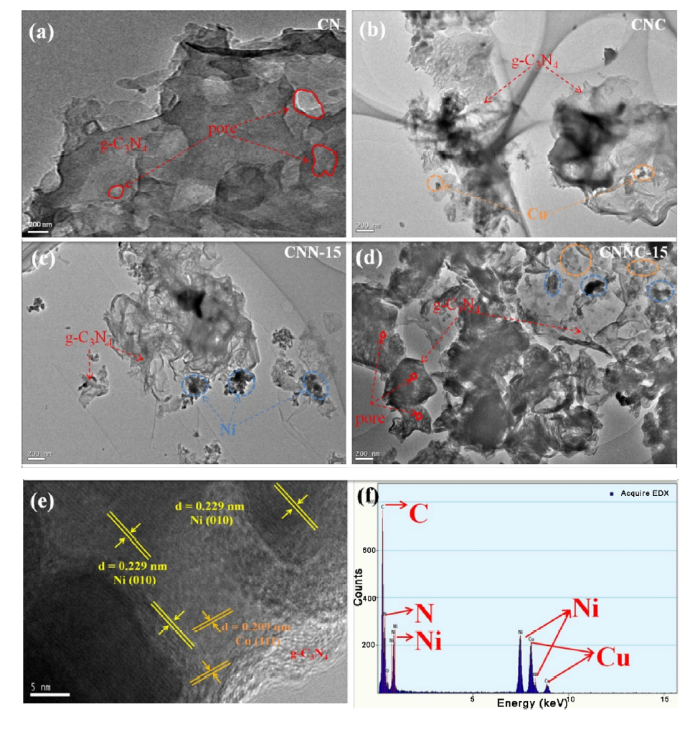
A typical 2D nanosheet morphology with a porous structure is displayed in the g-C3N4 TEM image (Fig. 5(a)) due to gas bubbling of ammonia during the melamine pyrolysis process [51,52]. Besides that, the 2D nanosheet structure of g-C3N4 was not as the same as the large smooth 2D laminate, which representes that it formed by many 2D nanosheets overlapping layers. After the secondary calcination, the dispersion degree of the nanosheets is somewhat stronger. The nanosheets of CNC, CNN-15 and CNNC-15 exhibit more transparent nature than bare g-C3N4 nanosheets. As shown in Fig. 5(b), the Cu/g-C3N4 (CNC) composite still maintained the nanosheet structure of g-C3N4, and there are many smaller Cu particles on the surface. The result for Ni/g-C3N4 (Fig. 5(c)) is like that shown in Fig. 5(b). The fine particles appearing on the surface of the nanosheet are Ni simple substance. This indicats that the planning of samples that had been successfully anchored to 2D g-C3N4 by heat treatment of nickel or/and copper elemental materials is successful. Further analysis of the TEM image (Fig. 5(d)) of CNNC-15 shows that this extensive metal-to-semiconductor contact is the key to form a tight Schottky interface. The loading of the nickel-copper elements contribut to the increase of surface area and provid more enough sites for active proton reduction. It is generally believed that it is advantageous to weaken the probability of photo-induced electrons and holes recombination by providing a rich surface-active site, thereby enhancing the separation of photoexcited charge carriers [[53], [54], [55]]. HRTEM image of the CNNC-15 sample is shown in Fig. 5(e). according to the analysis, one can find two types of lattice fringes with lattice spacing of d =0.229 nm and 0.209 nm, which can be attributed to the (002) crystal plane of Ni and the (010) crystal plane of Cu. Besides that, the test results of EDX of CNNC-15 samples also indicates that C, N, Ni and Cu elements exist in the sample (Fig.5(f)). This shows that the strategy of modifying nickel-copper metal element on g-C3N4 by secondary calcination is successful.
Fig. 5.
Fig. 5.
TEM image of g-C3N4 (a), CNC (b), CNN-15 (c) and CNNC-15 (d); HRTEM images of CNNC-15(e). EDX of CNNC-15 (f).
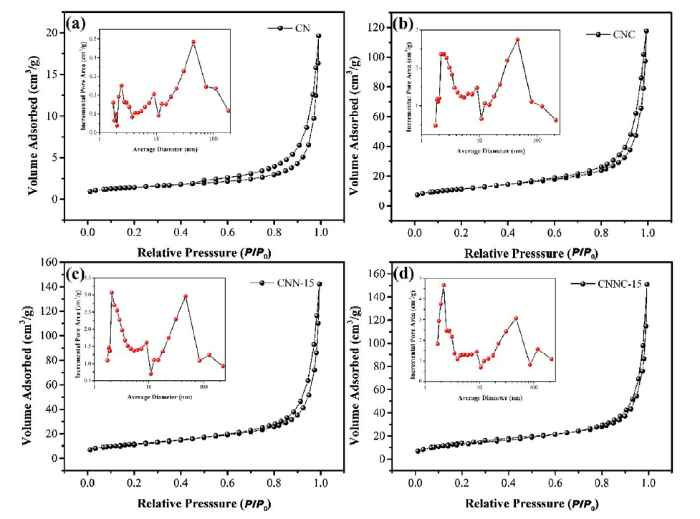
3.5. BET characterization
The specific surface area (SSA) is an important factor to influence the hydrogen evolution performance. To investigate the transformation of SSA, the samples were tested by brunauer-emmett-teller (BET) method to obtain the corresponding information. The adsorption equation of multi-molecular layer was as follows [43]:
where, Va is the adsorption amount under pressure p; $V_{\text{m}}^{\text{a}}$ is the saturated adsorption amount of the monolayer; c is the adsorption constant related to the adsorption heat; p* is the saturated vapor pressure of the adsorbed liquid at the adsorption temperature.
The presented Table 1 was the information about pore volume, pore size and porosity results of different composites. Fig. 6 shows the nitrogen adsorption-desorption isotherms of g-C3N4(CN), CNC, CNN-15 and CNNC-15. The pore distribution curves are shown in the inset. According to the classification of BDDT, the shape of the isotherm of g-C3N4 is the type IV isotherm. CNC, CNN-15 and CNNC-15 composites all show as type II isothermal lines. At the higher relative pressures, Type IV isotherms shows a hysteresis loops, which demonstrates the presence of mesoporous structures in the synthesized nanomaterials. Type II isotherms means multi-layer adsorption isotherms, which is consistent with the morphology structure [56]. This indicats that the adsorption heat of the first layer of the CNC, CNN-15 and CNNC-15 samples is greater than their heat of condensation [57]. The surface areas of CN, CNC, CNN-15 and CNNC-15 nanomaterials calculated by Brunauer-Emmett-Teller (SBET) are 5, 40, 42 and 51 m2/g, respectively. This indicates that the SSA of the nanomaterials greatly increases according to heat treatment of nickel or/and copper elements to modify bare g-C3N4. Increased specific surface areas plays an important role in photocatalysis. Larger specific surface areas generally meant that the system can provide more active sites to absorb more reactant molecules and enhance the hydrogen evolution activity eventually. Average pore diameters of CN, CNC, CNN-15 and CNNC-15 were 21, 18, 19 and 19 nm, respectively, which indicates that the proportion of macropores in the composite catalyst after loading Ni and Cu elenents decrease. The pore volume was measured by the Barrett-Joyner-Halenda (BJH) method and the results shows that the pore volumes of CN, CNC, CNN-15 and CNNC-15 ARE 0.025, 0.002, 0.219 and 0.223 cm3/g, respectively.
Table 1 SBET, pore volume and average pore size comparisons results for different catalysts.
| Samples | SBET (m2/g) | Pore volume (cm3/g) | Average pore size (nm) |
|---|---|---|---|
| g-C3N4 | 5 | 0.025 | 21 |
| CNC | 40 | 0.002 | 18 |
| CNN-15 | 42 | 0.219 | 19 |
| CNNC-15 | 51 | 0.233 | 19 |
Fig. 6.
Fig. 6.
N2 adsorption-desorption isotherms and corresponding pore-size distribution curves (inset) of the CN (a), CNC (b), CNN-15 (c) and CNNC-15 (d) samples.
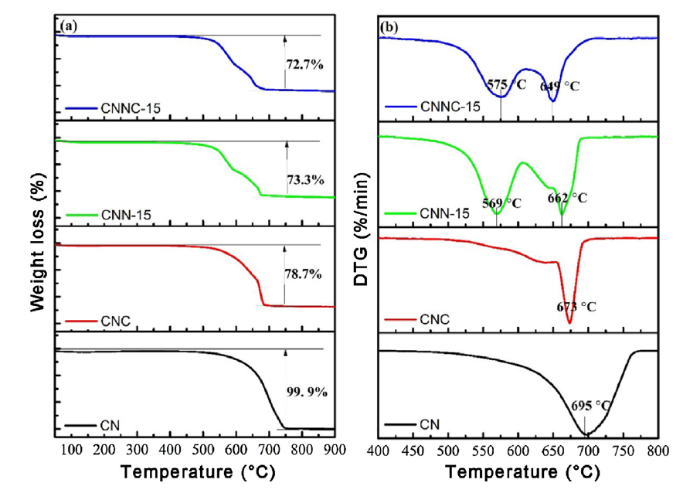
3.6. TG analysis
The TG curves of g-C3N4 (CN), CNC, CNN-15 and CNNC-15 are shown in Fig. 7(a). The thermogravimetric analysis was carried out under the atmosphere of N2 with the temperature rise of 10 °C/min. Pure g-C3N4 has only one thermogravimetric platform in the pyrolysis process. It can be seen from Fig. 7(b) that there is only one obvious peak on the differential thermogravimetric curve, indicating that there is only one decomposition process for the decomposition of g-C3N4. The bare g-C3N4 is released with the form of ammonia gas during the decomposition process [58], and the decomposion is completed at 750 °C. After the introduction of Cu element, the thermal decomposition process of the CNC composite is like CN. The difference between these two processes was that the CNC is completely decomposed at about 700 °C, and the release peak temperature appeares at 673 °C. The CNC composite was not pyrolyzed and still had some residual, indicating that the sample contains a certain amount of Cu. With the introduction of Ni element, the thermal decomposition process of CNN-15 and CNNC-15 composite samples significantly changed, and two distinct peaks appeares on the differential thermogravimetric curve obviously. The two exothermic peaks at 569 °C and 662 °C are attributable to the sublimation and thermal heat separation of CNN-15 samples, as well as CNNC-15. The results show that the thermal stability of CN, CNC, CNN-15 and CNNC-15 samples have changed after the introduction of Cu and Ni elements. It may be due to the change of stacking structure of the composite catalyst, which affectes the heatstability [59,60].
Fig. 7.
Fig. 7.
TG curves (a) and DTG curves (b) of CN, CNC, CNN-15, and CNNC-15.
3.7. Photocatalytic H2 production activity of catalyst
Using TEOA as the electron donor, the photocatalytic reaction of different catalysts was tested under the condition of visible light irradiation. The hydrogen evolution activity of bare g-C3N4 was almost negligible without EY and cocatalyst, and there were no H2 produced in all samples under the dark state. Under the condition of EY sensitiztion, the photocatalytic hydrogen evolution activity of the composite was constructed by introducing a certain amount of Ni/Cu metal elements on bare g-C3N4 surface by in-situ calcination got improvement at some extend (Fig.8(a)). As displayed in Fig. 8(b), the H2 production activity of the composites with different content of Ni metal is investigated at the condition of pH = 9. The hydrogen production activity of CNN-15 reaches to 62.6 μmol after 5 h, which reaches to 14.5 times higher than that of bare g-C3N4. Next, CNNC-x (x = 5, 10, 15, 20.) composites is constructed by further modifying the CNN-15 composite catalyst with Cu element too. The hydrogen production activity of CNNC-15 composite reaches to 104.4 μmol after putting into the double promoter strategy, which attained 24.3 times higher than bare g-C3N4 (Fig. 8(c)). Which demonstrates that simultaneous introduction of Ni and Cu elemental substances have a remarkable improvement in the photocatalytic activity for g-C3N4 nanosheet. Because of the synergistic interaction among different active sites, CNNC-15 has more active sites and faster electron motion rate, which helps to enhance photocatalytic activity. On the contrary, extreme introduction of the metal elemental promoter in the composite photocatalyst could inhibit its activity, thereby inhibiting the photocatalytic production of H2. For example, the visible light catalytic activity of CNNC-20 photocatalyst is significantly reduced. The effect of pH values of the system on the photocatalytic activity of CNNC-15 photocatalysts could also be further investigated. The results that under the different pH solution of the photocatalytic reaction could be seen from Fig. 8(d). And this effect shows obviously at pH = 11 compared with pH = 9 and pH = 10, the degree of improvement is not obvious. Under the condition of pH = 12, the hydrogen evolution performance decreases greatly compared with the the best hydrogen evolution activity. It can be seen from Fig. 8(d) that all conditions including high alkaline conditions (pH = 12) and neutral conditions (pH = 7) are not conducive to the formation of hydrogen. When the value of pH is relatively low, the solution may be protonated because the existence of too many H+ and resulting the serious effectiveness decrease of the electron donor, thereby reducing the dye inducing efficiency [61]. In the strong alkaline reaction conditions, the thermodynamic driving force for hydrogen production is significantly insufficient due to the decrease of hydrion concentration. Furthermore, different solution pH in the reaction system would also affect the adsorbability of EY molecules on the surface of CNNC-15 composite. The photocatalytic hydrogen production performance of different catalysts are shown in Table 2. To investigate the stability of CNNC-15 photocatalyst, a cycle test was conducted under the same conditions. As shown in Fig. 8(e), the stability of CNNC-15 photocatalyst could be investigated after three cycles. It performed better in photocatalytic H2 production property during the first 5 h cycle. Because the slight photo-corrosion effect, the hydrogen production activity of CNNC-15 composite decreased in the second and third runs. Besides that, due to the EY molecule easily desorbed and decomposed under long-term light irradiation, which also could result in the decrease of H2 production activity under visible light radiation. Fig. 8(f) showed the wavelength-dependent apparent quantum efficiency (QE) of hydrogen evolution, the QE of CNNC-15 reaches to 6.83 % when the wavelength is 475 nm.
Fig. 8.
Fig. 8.
Comparison of different catalyst H2 production activity of CN, CNC, CNN-15, and CNNC-15 (a). Comparison of hydrogen production performance of composite with different nickel content (b) and copper content (c). Effect of different pH triethanolamine aqueous to the photocatalytic property of CNNC-15 (d). Stability testing of CNNC-15 composite in this system (e). Apparent quantum efficiency (QE) of the CNNC-15 (f).
Table 2 Comparison of photocatalytic activity.
| Photocatalyst | Co-catalyst | Light source | Sacrificial reagent | Activity (μmol/ (h g)) | Ref. |
|---|---|---|---|---|---|
| g-C3N4 | Ni-Cu | 5 W LED (≥ 420 nm) | 15 vol.% TEOA | 2088.28 | This work |
| g-C3N4 | S-Ni | 300 W Xe-lamp (> 420 nm) | 20 vol.% TEOA | 2021.3 | [12] |
| g-C3N4 | Pt-Pd | 300 W Xe-lamp (≥ 400 nm) | 10 vol.% TEOA | 1600.8 | [28] |
| g-C3N4 | NiS | 350 W Xe-lamp, (≥ 420 nm) | 15 vol.% TEOA | 593.6 | [35] |
| g-C3N4 | Pt-C | 350 W Xe-lamp, (≥ 420 nm) | 15 vol.% TEOA | 212.8 | [52] |
| g-C3N4 | C | Xe-lamp, (> 420 nm) | 15 vol.% TEOA | 410.1 | [55] |
| g-C3N4 | MoP | 300 W Xe-lamp (> 400 nm) | 10 vol.% TEOA | 327.5 | [65] |
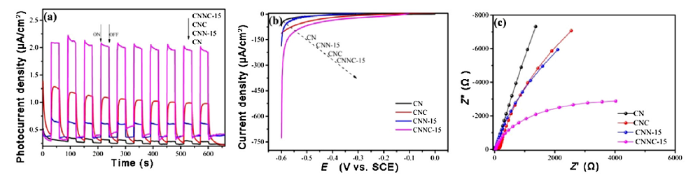
3.8. Photoelectric performance tests
Bimetallic nanoparticles generally exhibit superior electronic and optical properties due to synergistic effects among different active sites. In order to study the photoelectrochemical properties of CN, CNC, CNN-15 and CNNC-15, all tests were carried out in a 0.2 M Na2SO4 electrolyte solution. As shown in Fig. 9(a), all catalysts exhibit different photocurrent responses under discontinuous intermittent illumination. The photocurrent density of all photocatalysts sharply increases under irradiation. It is worth mentioning that the transient photocurrent response of CNNC-15 is the highest among all tested samples. This directly proves that CNNC-15 catalyst has the highest electron transport efficiency under the illumination condition, so the hydrogen production performance is higher compared to other composites [61]. The polarization curves further determine the kinetics of hydrogen evolution at high current densities on the surface of catalyst. Fig. 9(b) shows the polarization curves of CN, CNC, CNN-15 and CNNC-15 photocatalysts. Obviously, CNNC-15 exhibits the highest current density and lower hydrogen evolution overpotential. It shows that CNNC-15 electrode is a promising photocatalyst. This is mainly due to the modification of the surface of g-C3N4 by the bimetallic nanoparticles. The radius of curvature of the Nyquist curve reflectes the mass transfer rate of the charge in the interfacial reaction. The smaller the curvature radius of the curve, the faster charge transfer can be realized, thus exhibiting excellent optoelectronic performance [43,57]. Fig. 9(c) shows the Nyquist curves of the original measurements for CN, CNC, CNN-15 and CNNC-15 materials. It is obvious that CNNC-15 composites exhibit the smallest radius of curvature. The synergistic effect of the bimetallic nanoparticles promots the surface charge transfer rate and greatly improves the catalytic performance of the composite. Because nickel-copper bimetal nanoparticles provide abundant active sites, the synergy among different active sites is the main reason for the excellent photoelectric performance of CNNC-15 composite photocatalyst.
Fig. 9.
Fig. 9.
i-t curve (a), LSV curve (b) and Nyquist curve (c) curve for CN, CNC, CNN-15, and CNNC-15 photocomposites.
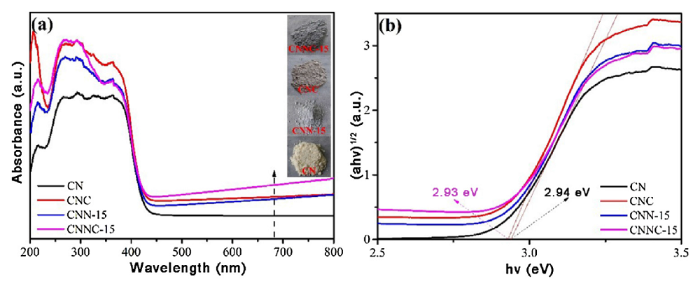
3.9. Optical performance test of photocatalysts
Fig. 10(a) depicts the absorption spectra of the g-C3N4 nanocomposite catalyst in the UV-vis-NIR region. All the samples have sharp absorption edges in the visible region, indicating that their absorption is related to the intrinsic band gap. The energy band gap of the catalyst is evaluated by the Kubelka-Munk method (Fig. 10(b)) [42,62]. The introduction of Ni-Cu elements can improve the absorbility of optical radiation, and reduce the band gap of the photocatalyst. From the function of Kubelka-Munk, the band gaps of CN and CNNC-15 could be approximately calculated as 2.94 eV and 2.93 eV, respectively.
Fig. 10.
Fig. 10.
UV-vis absorption spectra of CN, CNC, CNN-15, and CNNC-15 photocomposites.
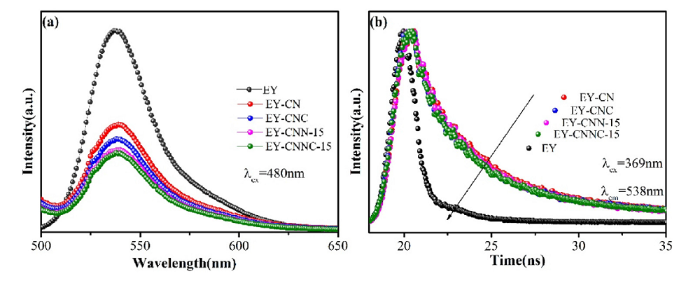
In EY solution, steady-state fluorescence spectrum was used to characterize the fluorescence properties of different catalysts, the electron transport and excited state interaction between EY and different composite are discussed. The catalysts in EY solution were denoted as EY-CN, EY-CNC, EY-CNN-15 and EY-CNNC-15 respectively. As shown in Fig. 11(a), the fluorescence quenching degree is different after addition different photocatalyst in EY solution. The catalysts in EY solution were excited by light at 480 nm, and emission peak value is about 538 nm after excitation. Under the irradiation condition, the compound was triggered, ground state electrons will absorbe the enough energy and transfer the electrons from the LUMO to HUMO level. Because of that, the photo-induced electrons and holes are generated [35]. And due to the excited electrons are unstable, they are decay because of radiation and immediately return to the ground state. And the decay of the radiative transition is accompanied with the emission of fluorescent photons. In this process, many EY which in the heavy excited state are returned to the ground state, and then generate fluorescence emission peaks. As displayed in Fig. 11(a), it can be easily observed that the strongest fluorescence is generated by the bare EY solution under the excitation of 480 nm light which meant that its electron-hole pairs recombination rate is the highest. When other catalyst is sensitized by EY, it could be found that the fluorescence intensity drops sharply, especially CNNC-15, which reaches the lowest level. The weakening of the fluorescence emission intensity indicates that the electron-hole pairs recombination rate of catalyst was inhibited. Photo-generated electrons would transfered to the conduction band of the catalyst [36]. The modified g-C3N4 greatly promotes the transport electrons due to the presence of nickel-copper element, and vastly improves charge separation efficiency and hydrogen production activity.
Fig. 11.
Fig. 11.
PL spectra of CN, CNC, CNN-15, and CNNC-15 (a) and transient fluorescence spectra (b).
where τ is emission lifetimes; A is the corresponding amplitude.
where ket is electron transfer rate constants; τF,s is short lifetimes; τF,l is long lifetimes.
The corresponding information of fluorescence lifetimes of the charge carriers, the corresponding amplitude, weighted average lifetime and electron transfer rate of EY, EY-CN, EY-CNC, EY-CNN-15 and EY-CNNC-15 are shown in Table 3. It can be easily observed from the Table 3 that the average lifetime of bare g-C3N4 is 4.0702 ns, after introduction of Ni and/or Cu metal, the average lifetime is reduced, and the CNNC-15 composite under the action of bimetal is 2.9948 ns. In addition, as a indicators of charge carriers, the ket of CN, CNC, CNN-15 and CNNC-15 samples is calculated, and the corresponding results are ket = 5.920 × 108, 6.575 × 108, 5.900 × 108 and 9.218 × 108, respectively. These results demonstrate that the loading of nickel and copper elements provides a new path for charges transfer, thereby enhancing the separation efficiency of photogenerated charges. Moreover, it is further explained that the promoting effect of Ni-Cu bimetal is better than that of metal alone. Therefore, it means that the CNNC-15 catalyst has the fastest transfer rate of photogenerated charges and the highest separation efficiency, thus inducing excellent hydrogen production activity. This is consistent with the results of hydrogen evolution kinetics and photoelectrochemical measurements results.
Table 3 Attenuation parameters o photocatalyst.
| Samples | Pre-exponential factors, A | Lifetime, <τ> (ns) | Average lifetime, <τ> (ns) | ket (s-1) | χ2 |
|---|---|---|---|---|---|
| EY | A = 100 | τ=0.1979 | 0.1979 | --- | 1.07 |
| EY-CN | A1 = 36.61 A2 = 33.00 A3 = 30.39 | τ1=8.357 τ2 = 1.660 τ3=96.99 | 4.0702 | 5.920 × 108 | 1.02 |
| EY-CNC | A1 = 35.79 A2 = 31.39 A3 = 32.39 | τ1=8.274 τ2 = 99.52 τ3 = 1.498 | 3.7674 | 6.575 × 108 | 1.09 |
| EY-CNN-15 | A1 = 36.20 A2 = 26.43 A3 = 37.37 | τ1=8.469 τ2 = 91.23 τ3 = 1.664 | 3.7009 | 5.900 × 108 | 1.08 |
| EY-CNNC-15 | A1 = 41.98 A2 = 26.68 A3 = 31.35 | τ1=5.519 τ2 = 1.061 τ3=48.44 | 2.9948 | 9.218 × 108 | 1.09 |
3.10. Reasonable photocatalytic mechanism
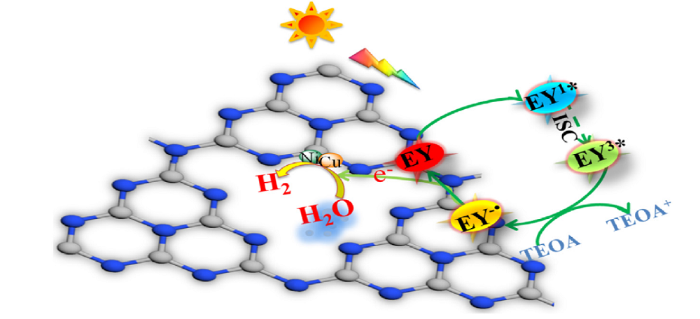
Through the above characterization analyses, the reasonable mechanism of photocatalytic hydrogen evolution was proposed (Fig. 12). g-C3N4 absorbed enough energy from the irradiation of visible light to produce electron-hole pairs. The EY molecule as a sensitizer was adsorbed on the photocatalyst surface under the radiation of visible light to form a single excited state EY1*, after that, more stable triple excited state EY3* was formed via undergoes band gap conversion. TEOA was used as the electron donor, due to the reducing and quenching of EY3*, EY-̇ was formed with strong reducing ability which played a key role in the hydrogen evolution performance [61]. The electron of EY-˙ moved to the surface of the composite, where H2 was generated in the reduction reaction. At the same time, the dye molecules returned to the ground state. Highly conductive Ni-Cu bimetallic nanoparticles were implanted between g-C3N4 layers, and electrons located at the CB of g-C3N4 could be quickly transferred to the metal layer. Hydrogen evolution reaction occurred on the surface of Ni-Cu and holes remain in the VB of g-C3N4 to react with the sacrificial reagent. A close Schottky junction between the Ni-Cu bimetallic nanoparticles and g-C3N4 layers caused fast and efficient electron-hole separation, therefore, the photocatalytic activity was significantly improved [[63], [64], [65], [66]]. The synergy effect between Ni-Cu bimetals increased the charges transfer rate and the specific surface area. And Ni-Cu bimetals were given the ability to enhance H2-evolution and TEOA oxidation kinetics just because of the tight interfaces which existed in Ni-Cu/g-C3N4 composites.
Fig. 12.
Fig. 12.
Possible photocatalytic hydrogen evolution mechanism of CNNC-15 under visible light radiation.
4. Conclusion
In summary, it can demonstrate the high efficiency of the g-C3N4 nanosheets modfied with bimetallic Ni-Cu nanoparticles according to in-situ chemical reduction based on heat treatment. The in-situ supported single metal Ni, bimetallic Ni-Cu significantly improves the performance of photocatalytic hydrogen evolution compared with pure g-C3N4 through this route. As the increase of the precursor at same heat treatment time, and the content of Ni or Ni-Cu metals increases too. The CNNC-15 sample can be obtained by adjusting the metal content, which has the highest H2 production activity and excellent stability. The H2 evolution properties of Ni-Cu/g-C3N4 composites are significantly improved, it is caused by the enhanced light absorption intensity, more efficient charge separation rate and faster interface charge migration. The tight Schottky interface of Ni-Cu/g-C3N4 composite enhances the performance of H2 production and optimizes TEOA oxidation kinetics. It can be believed that a new inspiration for rational design and exploration g-C3N4 and other semiconductor nanocomposites is sanguine according to this sample in-situ deposition method, enabling efficient carrier separation on the surface.
Acknowledgments
This work was financially supported by the Open Project of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University (No. 2019-KF-36), the Chinese National Natural Science Foundation (Nos. 21862002 and 41663012), the new technology and system for clean energy catalytic production, Major scientific project of North Minzu University (No. ZDZX201803) and The Ningxia low-grade resource high value utilization and environmental chemical integration technology innovation team project, North Minzu University.
Reference
DOI
URL
PMID
[Cited within: 1]

Developing effective and simple one-pot synthetic strategies regarding the formation of heterojunction photocatalytic semiconductors remains an intense challenge in research pursuits. Further scheming of the p-n heterojunction has sustained renewed interest in catalysis, photocatalysis, energy storage, and conversion because they easily accelerate the bulk charge separation efficiency. Thus we have successfully designed a Au-MoS2/ZnIn2S4 heterojunction photocatalyst for the first time by adopting a simple one-pot hydrothermal technique, followed by a deposition-precipitation method. By adjusting the mole ratio of Mo with that of Zn and In precursors, we have fabricated a MoS2/ZnIn2S4 p-n heterojunction photocatalyst, and the established p-n heterojunction between MoS2 and ZnIn2S4 is demonstrated by various physicochemical and morphological characterizations. An interfacial junction is created between MoS2 and ZnIn2S4 at the depletion region via an in situ formation mechanism, leading to the enhancement of the charge separation through the p-n heterojunction and thus improving the photocatalytic activity. Moreover, the photocatalytic activity is projected to further improve by the incorporation of Au nanodots on the surface of MoS2/ZnIn2S4 photocatalysts. The increase in activity is due to the generation and participation of a large number of direct-electron-transfer-induced hot electrons in the photochemical reaction. From the experimental results, Au-MoS2/ZnIn2S4 heterojunction photocatalysts with only 1% MoS2 and 1% Au loading content displayed a 561.25 mumol/h H2 evolution rate and 84% degradation of phenol, which are nearly 15 and 6 times higher than those neat ZnIn2S4. In addition Au-MoS2/ZnIn2S4 photocatalysts exhibit a photocurrent density of approximately 2.56 mAcm(-2), which is nearly 2.4 times higher than that of the MoS2/ZnIn2S4 heterojunction photocatalyst. This exertion represents the synergetic enhancement of photocatalytic activity through the p-n heterojunction as well as the hot-electron participation by the metal nanocatalyst, which is an inspiration for developing efficient photocatalysts.
DOI
URL
PMID
[Cited within: 1]

A straightforward approach is developed for fabrication of a visible-light-driven Ag/g-C3N4 catalyst. Morphological observation shows that the g-C3N4 sheets are decorated with highly dispersed Ag nanoparticles having an average size of 5.6 nm. The photocatalytic activity measurements demonstrate that the photocatalytic degradation rates of methyl orange (MO), methylene blue (MB), and neutral dark yellow GL (NDY-GL) over Ag/g-C3N4-4 can reach up to 98.2, 99.3 and 99.6% in the presence of borohydride ions (BH4(-)) only with 8, 45, and 16 min visible light irradiation, respectively. The significant enhancement in photoactivity of the catalyst is mainly attributed to the high dispersity and smaller size of Ag nanoparticles, the strong surface plasmon resonance (SPR) effect of metallic Ag nanoparticles, the efficient separation of photogenerated charge carriers, the additional superoxide radicals (O) generated from the reduction of dissolved oxygen in the presence of BH4(-) and the synergistic effect of Ag nanoparticles and g-C3N4.
DOI
URL
PMID
[Cited within: 2]

Splitting of alcohols into hydrogen and corresponding carbonyl compounds has potential applications in hydrogen production and chemical industry. Herein, we report that a heterogeneous photocatalyst (Ni-modified CdS nanoparticles) could efficiently split alcohols into hydrogen and corresponding aldehydes or ketones in a stoichiometric manner under visible light irradiation. Optimized apparent quantum yields of 38%, 46%, and 48% were obtained at 447 nm for dehydrogenation of methanol, ethanol, and 2-propanol, respectively. In the case of dehydrogenation of 2-propanol, a turnover number of greater than 44000 was achieved. To our knowledge, these are unprecedented values for photocatalytic splitting of liquid alcohols under visible light to date. Besides, the current catalyst system functions well with other aliphatic and aromatic alcohols, affording the corresponding carbonyl compounds with good to excellent conversion and outstanding selectivity. Moreover, mechanistic investigations suggest that an interface between Ni nanocrystal and CdS plays a key role in the reaction mechanism of the photocatalytic splitting of alcohol.
DOI
URL
PMID
[Cited within: 1]

Semiconductor-based photocatalysis is considered to be an attractive way for solving the worldwide energy shortage and environmental pollution issues. Since the pioneering work in 2009 on graphitic carbon nitride (g-C3N4) for visible-light photocatalytic water splitting, g-C3N4 -based photocatalysis has become a very hot research topic. This review summarizes the recent progress regarding the design and preparation of g-C3N4 -based photocatalysts, including the fabrication and nanostructure design of pristine g-C3N4 , bandgap engineering through atomic-level doping and molecular-level modification, and the preparation of g-C3N4 -based semiconductor composites. Also, the photo-catalytic applications of g-C3N4 -based photocatalysts in the fields of water splitting, CO2 reduction, pollutant degradation, organic syntheses, and bacterial disinfection are reviewed, with emphasis on photocatalysis promoted by carbon materials, non-noble-metal cocatalysts, and Z-scheme heterojunctions. Finally, the concluding remarks are presented and some perspectives regarding the future development of g-C3N4 -based photocatalysts are highlighted.
DOI
URL
PMID
[Cited within: 1]

Energy captured directly from sunlight provides an attractive approach towards fulfilling the need for green energy resources on the terawatt scale with minimal environmental impact. Collecting and storing solar energy into fuel through photocatalyzed water splitting to generate hydrogen in a cost-effective way is desirable. To achieve this goal, low cost and environmentally benign urea was used to synthesize the metal-free photocatalyst graphitic carbon nitride (g-C(3)N(4)). A porous structure is achieved via one-step polymerization of the single precursor. The porous structure with increased BET surface area and pore volume shows a much higher hydrogen production rate under simulated sunlight irradiation than thiourea-derived and dicyanamide-derived g-C(3)N(4). The presence of an oxygen atom is presumed to play a key role in adjusting the textural properties. Further improvement of the photocatalytic function can be expected with after-treatment due to its rich chemistry in functionalization.






 WeChat
WeChat