1. Introduction
Supercapacitor as an energy storage device between the battery and the conventional capacitor has attracted great research interest in recent years [[1], [2], [3]]. Supercapacitors which combine the advantages of batteries and conventional capacitors can not only provide much higher energy density than conventional capacitors but also provide high power density and excellent cycle stability [[4], [5], [6]]. In order to meet the practical application, it is necessary to develop high energy density supercapacitor. Different positive and negative electrode materials are used to form asymmetric supercapacitors (ASCs) so as to improve energy density. Energy density could be increased greatly by increasing operating potential according to the equation E = 1/2CV2. Although a variety of ASC systems have been studied, most studies have focused on the preparation of positive electrode materials, while negative electrode materials have been less studied [7]. Metal oxides as negative electrodes such as Molybdenum oxide [[8], [9], [10]], Iron oxide [[11], [12], [13]], and Bismuth oxide [[14], [15], [16]] have been investigated because of their much higher energy density than carbon materials. Among them, Bismuth oxide has attracted extensive attention owing to its high theoretical capacitance (1370 F g-1), suitable potential window, low cost, non-toxic to environment, and easy preparation [14]. For example, Y. Qiu et al. reported the fabrication of Bi2O3 nanowires with the specific capacitance of 693 F g-1 (1A g-1) [15], and N. M. Shinde et al. had prepared Bi2O3 nanosheets with a specific capacitance of 447 F g-1 [16]. In alkaline solution, Bi2O3 is charged and discharged by the redox reaction between Bi2O3 and Bi, and it can provide high energy density since the valence state of Bi element is converted between trivalence and zero valence, which indicates the feasibility of Bismuth metal as electrode material.
However, Bi2O3 similar to other metal oxides has very poor electrical conductivity, which limits its electrochemical performance severely. In order to overcome the poor conductivity of Bi2O3, researchers have designed and prepared composites of Bi2O3 and carbon materials to improve electrochemical performance [[17], [18], [19]]. Bi2O3 and carbon materials such as GO or CNF had been adopted to form a composite electrode so that the conductivity of the electrode can be improved greatly, and the specific surface area of the Bi2O3 electrode can be also increased to improve the contact area between electrode and electrolyte. For instance, H. Xu et al. had prepared Bi2O3 nanoflower/CNF by a solvothermal method, and the specific capacitance was 545 m F cm-2 - 312 m F cm-2 (3 mA cm-2 - 15 mA cm-2) [17]. R. Liu et al. used a solvothermal method to prepare Bi2O3/GO/BC and the specific capacitance was 6675 m F cm-2 or 681 F g-1 (1 mA cm-2) [18]. X. Li et al. had prepared Bi2O3/CNF/CC by a solvothermal method and the capacity is 110 mA h g-1 (2 A g-1) [19].
Carbon nanotube (CNT) as a kind of quasi-one-dimensional material possesses excellent electrical conductivity, mechanical property, chemical stability, and extremely high specific surface area [20] so that it is suitable to prepare metal oxide and CNT composite electrode to improve electrochemical performance [[21], [22], [23], [24], [25], [26]]. In addition, the high reducibility of carbon nanotubes can be used to prepare metal nanoparticle [[27], [28], [29]]. W. Chen et al. prepared Fe/CNT by annealing treatment of Fe2O3/CNT [27,28]. J. LÜ et al. prepared Co/CNT by annealing Co3O4/CNT [29]. Therefore, it is an excellent method to obtain metal Bi by autoreduction of Bi2O3/CNT.
In this work, we have prepared Bi2O3/CNT composite by facile solvothermal method, and core-shell Bi-Bi2O3/CNT with 3-dimensional neural network structure by annealing treatment of Bi2O3/CNT. The 3-dimensional neural network and the core-shell structure of Bi-Bi2O3 nanosphere provide an efficient electronic transfer path for the electrochemical redox reaction. The Bi-Bi2O3/CNT electrode reveals a high specific capacitance of 850 F g-1 (1 A g-1). The specific capacitance of Bi-Bi2O3/CNT could be 714 F g-1 when the current density is 30 A g-1 indicating excellent rate performance. Asymmetric supercapacitor is fabricated with Bi-Bi2O3/CNT as negative electrode and Ni(OH)2/CNT as positive electrode revealing a high energy density of 36.7 Wh kg-1 and a maximum power density 8000 W kg-1. The asymmetric supercapacitor shows acceptable cycle performance of 72.9% after 1000 cycle.
2. Experimental
Multi-walled carbon nanotubes (CNTs) was purchased from the TIME NANO (purity >98%, length 0.5-2 μm), All chemicals were purchased from Kmart Chemical Co. Ltd and were A.R. grade without further purification. Fabrication of Bi-Bi2O3/CNT: 0.97 g Bi(NO3)3·5H2O was dissolved in a mixed solution consisting of 26 ml ethanol and 13 ml ethylene glycol. Subsequently, 100 mg CNTs was added into the solution above with 30 min magnetic stirring and 30 min ultrasonication. And then the solution was transferred into 50 ml Teflon-lined stainless steel autoclave at 160 °C for 5 h. After cooling to room temperature, the sample was washed several times with distilled water and ethanol and dried in a vacuum oven at 80 °C for 3 h. The dried powder was put in a tube furnace by annealing treatment at 300 °C for 2 h with heating rate of 1 °C min-1 in N2 atmosphere. For comparison, pure Bi2O3 and Bi2O3/CNT samples were prepared. Pure Bi2O3 was prepared by same solvothermal method above without CNTs added. Bi2O3/CNT was prepared by same solvothermal method without annealing treatment. In order to explore the formation mechanism of Bi-Bi2O3/CNT, the sample named as Bi2O3/CNT200 was prepared by 200 °C annealing of Bi2O3/CNT. The positive electrode of Ni(OH)2/CNT is prepared by one-step solvothermal method. Briefly, 0.29 g Ni(NO3)2·5H2O, 0.6 g urea and 50 mg CNTs were added into 40 ml deionized water with 30 min magnetic stirring and 30 min ultrasonication. Then, the solution was transferred into a 50 ml Teflon-lined stainless steel autoclave at 110 °C for 3 h. The sample was washed several times with distilled water and ethanol and dried in a vacuum oven at 80 °C for 3 h after cooling to room temperature.
The phases of samples were characterized by X-ray powder diffraction (XRD, D8 advance, CuKα = 0.1542 nm, 40 kV, 40 mA). The morphology, microstructure, and elemental mapping were characterized by field emission scanning electron microscopy (SEM, Hitachi S-4800, 5 kV), transmission electron microscopy (TEM, JEM-2100 F, 200 kV) and energy dispersive spectrometer (EDS, Oxford instrument). The surface analysis was obtained by X-ray photoelectron spectroscopy (XPS, Thermo SCIENTIFIC ESCALAB 250Xi). The nitrogen adsorption-desorption isotherm and pore distribution isotherm were measured by using Quantachrome NOVA 2200e.
Electrode fabrication and electrochemical measurements: 20 mg sample powders, 2.5 mg acetylene black, 4.2 mg PTFE (60% aqueous solution) were added to 2 ml ethanol for 30 min ultrasonication and then were rolled into films. The films were weighed and pressed on the foamed nickel on 20 MPa for 10 s. The Bi-Bi2O3/CNT negative electrode and the Ni(OH)2/CNT positive electrode were assembled to an asymmetric supercapacitor device with 6 M KOH as the electrolyte and cellulose acetate membrane as the separator. Electrochemical tests including cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) were performed with a CHI660E electrochemical workstation. The three-electrode test was based on 2 cm × 3 cm platinum plate as a counter electrode and calomel electrode (saturated KCl) as a reference electrode in 6 M KOH.
The specific capacitance of electrodes and asymmetric supercapacitor were calculated from the GCD curves according to Eq. (1).
Where Cs is specific capacitance (F g-1), m is the mass of the active material, Δt is the discharge time, and ΔV is the operating voltage window.
The energy density and power density of the asymmetric supercapacitors were calculated according to Eqs. (3) and (4).
Where C is the specific capacitance of the asymmetric supercapacitor devices, V is the voltage change of the discharge, and Δt is the discharge time.
3. Results and discussion
3.1. Morphology and structure
Fig. 1 shows the schematic diagram of the synthesis process of Bi-Bi2O3/CNT. Bi2O3/CNT was prepared by simple solvothermal method where Bi2O3 nanosheets grow on the surface of CNT, and then Bi-Bi2O3/CNT was obtained by annealing treatment. Amorphous Bi2O3 nanospheres begin to precipitate on the surface of Bi2O3 nanosheets during heating, and adhere to the surface of CNT with Bi2O3 nanosheets decompose gradually. Subsequently, amorphous Bi2O3 nanospheres on the surface of CNT convert to core-shell Bi-Bi2O3 by autoreduction between amorphous Bi2O3 and CNT. The amorphous Bi2O3 nanospheres on CNT surface were partially reduced to metal Bi as core to form the core-shell structure of Bi-Bi2O3. The core-shell Bi-Bi2O3 and CNT are assembled into core-shell Bi-Bi2O3/CNT with 3-dimensional neural network structure.
Fig. 1.
Fig. 1.
The schematic diagram of synthesis process.
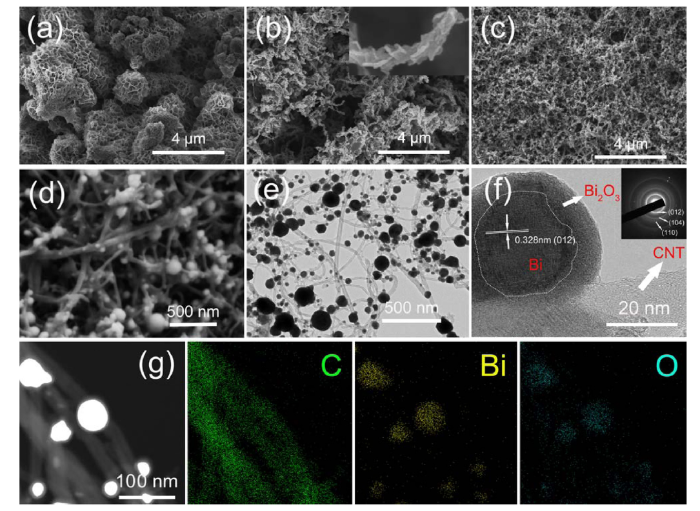
The morphology and nanostructure of pure Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT are analyzed by using SEM. The morphology of pure Bi2O3 prepared by using solvothermal method is upright-standing and intersecting nanosheets in Fig. 2(a). This intersecting nanosheets network can provide a high surface area, which can increase the contact area with electrolyte and facilitate the continuous electron transfer, but the conductivity is still poor due to the long electron transfer path inside nanosheets network. Fig. 2(b) is the morphology of Bi2O3/CNT that Bi2O3 nanosheets grow and wrap on the surface of CNT, and this structure can facilitate the continuous electron transfer inside nanosheets, but the cross grown Bi2O3 nanosheets on the surface of CNT may have no great contact with CNT, and the CNT coated by Bi2O3 nanosheets have no good contact with the collector. Fig. S1 shows the TEM and HRTEM images of Bi2O3/CNT200. It can be observed that nanospheres appear on the surface of CNT and Bi2O3 nanosheets in Fig. S1(a). Amorphous Bi2O3 nanospheres inside dotted circles precipitated from Bi2O3 nanosheets in Fig. S1(b). Amorphous Bi2O3 nanosphere adhered on the surface the CNT with the gradual decomposition of Bi2O3 nanosheets in Fig. S1(c). Fig. 2(c) and (d) displays the structure and morphology of Bi-Bi2O3/CNT, in which Bi-Bi2O3 nanospheres grow on the surface of carbon nanotubes. The Bi-Bi2O3/CNT with 3-dimensional neural network structure composed of Bi-Bi2O3 nanospheres and CNT network is also confirmed by TEM in Fig. 2(e). It displays the Bi-Bi2O3 nanospheres growing on the surface of CNT and Bi-Bi2O3 with core-shell structure in Fig. 2(f). Notably, there is a great contact between the Bi core, Bi2O3 shell and CNT for more efficient electron transfer. Core-shell Bi-Bi2O3 nanospheres like cell bodies of neuronal cells can store energy, and exposed CNTs like synapses are more conducive to electron transfer with collector compared with Bi2O3/CNT. It can be observed that the core of the nanosphere is metal Bi that the lattice distance is 0.328 nm corresponding to (012) plane of metal Bi, and the shell of nanosphere is amorphous Bi2O3 without lattice. It can be also confirmed that the core of nanosphere with lattice is metal Bi from SEAD image inserted in Fig. 2(f), in which three obvious diffraction rings correspond to (012), (104) and (110), respectively. The presence of Bi2O3 is also confirmed from a high-angle annular dark field (HAADF) image of scanning transmission electron microscopy (STEM) and X-ray element mapping images in Fig. 2(g). The distribution of Bi element is approximately the same as O element corresponding to the nanospheres in the STEM image, and the distribution of C element corresponds to the CNTs in the STEM image, so it can be speculated that the shells of the nanospheres growing on the surface of CNTs are amorphous Bi2O3. Moreover, Fig. S2 shows the EDS and element proportion of Bi2O3/CNT200 and Bi-Bi2O3/CNT revealing the presence of C, Bi, and O element. The ratio of Bi and O element of Bi2O3/CNT200 is 2: 2.92 close to 2: 3 of Bi2O3 which indicates Bi2O3 has not been reduced by CNT at 200 °C in Fig. S2(a). The ratio of Bi and O element of Bi-Bi2O3/CNT is 2 : 1.65 due to Bi2O3 reduced to metal Bi partially in Fig. S2(b).
Fig. 2.
Fig. 2.
SEM images of (a) pure Bi2O3, (b) Bi2O3/CNT and (c, d) Bi-Bi2O3/CNT. (e) TEM images of Bi-Bi2O3/CNT. (f) HRTEM images (inset: SAED pattern) of Bi-Bi2O3/CNT. (g) HAADF-STEM image and corresponding elemental mapping images of Bi, C, and O of Bi-Bi2O3/CNT.
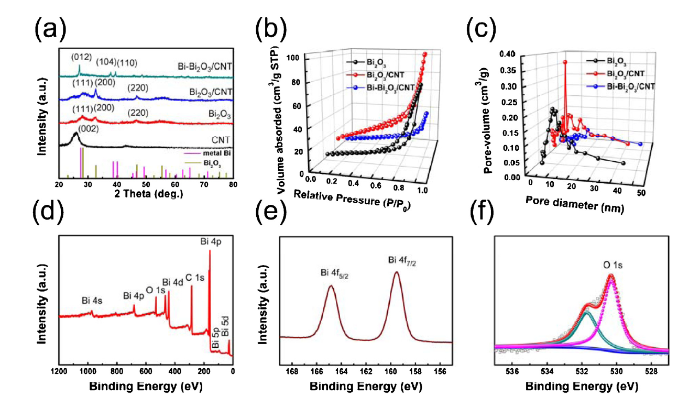
Fig. 3(a) represents the XRD patterns of CNT, pure Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT. Typical (002) plane of the CNT is shown [30]. The fraction peaks of Bi2O3 and Bi2O3/CNT in Fig. 3(a) can be indexed to the cubic phase of δ-Bi2O3 (PDF#02-0542) with (111), (200) and (220) diffraction plane. The diffraction peaks of Bi-Bi2O3/CNT can be indexed to the hexagonal phase of metal Bi (PDF#01-0688) with (012), (104) and (110) diffraction plane. It indicated that Bi crystal will appear by annealing treatment of Bi2O3/CNT. According to the Debye-Scherrer formula, the average grain size of Bi2O3, Bi2O3/CNT, and Bi-Bi2O3/CNT are calculated to be about 5 nm, 8 nm and 32 nm indicating that Bi2O3 is not well crystallized. Fig. S3 shows the XRD comparison of Bi2O3/CNT, Bi2O3/CNT200 and Bi-Bi2O3/CNT. Bi2O3/CNT is indexed to cubic phase of δ-Bi2O3 same as Bi2O3/CNT, but the diffraction peak intensity of Bi2O3/CNT200 is lower than Bi2O3/CNT indicating that crystallinity of Bi2O3 can be reduced during heating. Fig. 3(b, c) show the nitrogen adsorption-desorption isotherms and pore-size distribution plots of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT. H3-type hysteresis in the range of 0.6-1.0 P/P0 confirms the involvement of the mesoporous type character in pure Bi2O3 and Bi2O3/CNT [16]. The specific surface area of pure Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT are calculated to be 28.64, 65.98 and 20.79 m2 g-1 according to the Brunauer-Emmett-Teller (BET) method. The average pore-size distribution maximum and average pore volumes of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT are 7.86 nm (0.21 cm3 g-1), 9.60 nm (0.37 cm3 g-1) and 19.88 nm (0.05 cm3 g-1), respectively. It suggests higher specific surface area, pore size and pore volume of Bi2O3 by adding CNT, but the specific surface area and pore volume are reduced by annealing treatment of Bi2O3/CNT to form Bi-Bi2O3/CNT suggesting the energy of δ-Bi2O3 reduced by decreasing its specific surface area and pore volume due to the instability of δ-Bi2O3 at high temperature. The typical survey XPS spectrum in Fig. 3(d) reveals the presence of C, Bi and O elements in the Bi-Bi2O3/CNT. The Bi 4f spectrum of Bi-Bi2O3/CNT is shown in Fig. 3(e), which exhibits the binding energy at 159.5 eV and 164.8 eV corresponding to Bi 4f7/2 and Bi 4f5/2 suggesting the presence of Bi2O3 [18]. The O 1s of Bi-Bi2O3/CNT at 530.3 eV and 532.0 eV confirms the presence of oxygen species in Fig. 3(f). Therefore, it suggests that the surface of Bi-Bi2O3 nanosphere is Bi2O3 shell.
Fig. 3.
Fig. 3.
(a) XRD patterns of CNT, Bi2O3, Bi2O3/CNT, and Bi-Bi2O3/CNT. (b) Nitrogen adsorption-desorption isotherms, (c) Pore-size distribution plots of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT. (d) XPS Survey spectrum of Bi-Bi2O3/CNT. (e) Bi 4f and (f) O 1s spectrum of Bi-Bi2O3/CNT.
3.2. Growth mechanism
It is interesting and worth discussing to obtain core-shell Bi-Bi2O3/CNT with 3-dimensional neural network structure. The δ-Bi2O3 synthesized by the solvothermal method is not well crystallized because complete δ-Bi2O3 crystal exists only above 730 °C indicating the instability of Bi2O3 nanosheets [31]. The metastable state of δ-Bi2O3 causes the structural transformation of δ-Bi2O3 during heating. However, the annealing temperature is not high enough to transform δ-Bi2O3 into α-Bi2O3 but enough into amorphous nanosphere to reduce its energy at high temperature. M. J. Chen et al prepared the Bi@amorphousBi2O3 nanospheres by prolonging solvothermal time and using glucose as reducing agent, which indicated the possibility of preparing Bi-Bi2O3 with core-shell structure by reduction method and forming Bi2O3 amorphous shell [32]. When the electrochemical reaction occurs in Bi2O3 electrode, one of the steps is 2H2O+3BiO22-→2BiO2-+4OH-+Bi(0), where Bi (II) exists and disproportionates into Bi (0) and Bi (III) [33]. In fact, Bi (II) is an unstable valence state and is highly prone to disproportionation: 3Bi2+→2Bi3++Bi(0) [34]. In addition, CNT has strong reducibility to metal oxides. For instance, Fe2O3 growing on the surface of CNT could be reduced into Fe with CO production from 600 °C to 800 °C [27,28]. Therefore it can be inferred that Bi2O3/CNT reacts as Eqs. (4) and (5) at 300 °C in N2 atmosphere.
3.3. Electrochemical performance
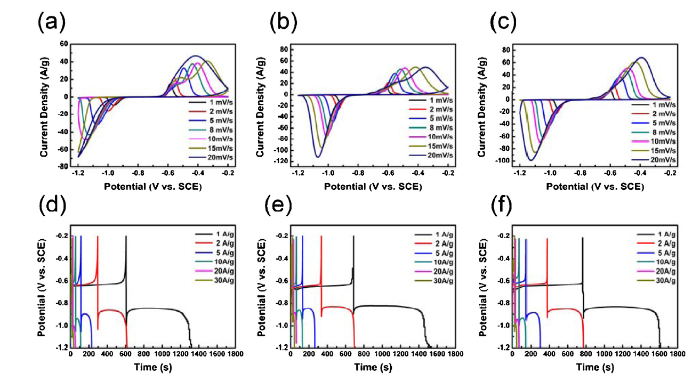
Fig. 4(a-c) shows the CV curves of the Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT electrodes from -1.2 to -0.2 V at 1-20 mV s-1 scan rates in an aqueous 6 M KOH electrolyte solution. A couple of typical characteristic redox peaks of Bi2O3 is observed between -1.2 V and -0.2 V, following the Faradaic reactions below [33].
Fig. 4.
Fig. 4.
(a, b, c) CV curves of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT at different rates. (d, e, f) GCD curves of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT at various current density.
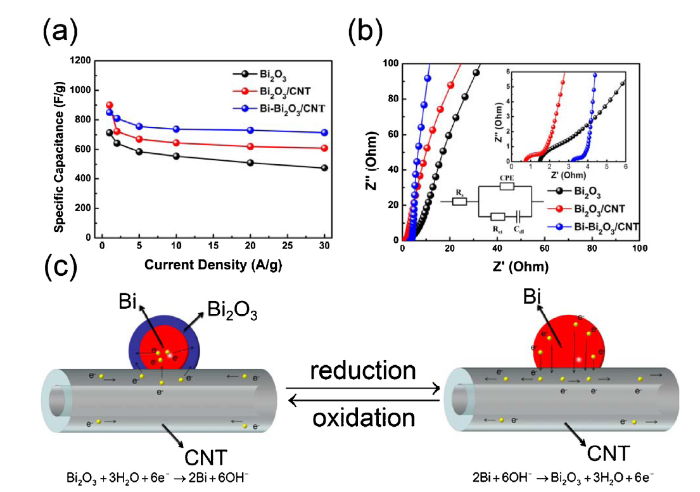
Typically, the current response increases and redox peaks shift with increasing scan rate. Compared with pure Bi2O3, the redox processing of Bi2O3/CNT and Bi-Bi2O3/CNT show higher specific capacitance and better reversibility because both Bi2O3/CNT and Bi-Bi2O3/CNT show higher peak current density and smaller shift of redox peaks, which can be attributed to the introduction of CNT. In addition, the oxidation peak current of Bi-Bi2O3/CNT increases with the increasing scan rate, while the Bi2O3/CNT increases slightly at high scan rate suggesting better rate performance of Bi-Bi2O3/CNT. Fig. 4(d-f) show GCD curves of pure Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT electrodes at a various current density from 1 A g-1-30 A g-1. The GCD curves are asymmetrical with voltage plateaus, which is attributed to a pseudocapacitive signature of Bi2O3. The discharge time of Bi2O3/CNT, Bi-Bi2O3/CNT are longer than pure Bi2O3, which indicates a high specific capacitance due to the presence of CNT. The calculated corresponding gravimetric capacitance based on GCD as a function of the current densities according to Eq. (1) are shown in Fig. 5(a). The specific capacitance of pure Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT at 1 A g-1 are calculated to be 713 F g-1, 900 F g-1, and 850 F g-1. Compared with pure Bi2O3 (474 F g-1) and Bi2O3/CNT (609 F g-1) at 30 A g-1, the specific capacitance of Bi-Bi2O3/CNT still retains 714 F g-1. It indicates that there is still a higher specific capacitance than the pure Bi2O3, even though the mass of the active material decreases with CNT added. The specific capacitance of Bi-Bi2O3/CNT is the highest specific capacitance value compared with Bi2O3 based pseudocapacitor electrodes ever reported such as Bi2O3 nanowire (691 F g-1) [15], Bi2O3 nanosheet (447 F g-1) [16], Bi2O3 nanoflower/CNF (51 F g-1) [17], Bi2O3/GN/BC (681 F g-1) [18], Bi2O3 film (98 F g-1) [35], Bi2O3 nanobelt (250 F g-1) [36], Bi2O3 flower (29 F g-1) [37], Bi2O3 nanosheet/CNF/CC (396 F g-1) [19]. In addition, it shows excellent rate performance of Bi-Bi2O3/CNT that the specific capacitance calculated at 30 A g-1 is 84% of the specific capacitance calculated at 1 A g-1, which is higher than pure Bi2O3 (66%) and Bi2O3/CNT (68%). In order to clarify the excellent electrochemical performance of Bi-Bi2O3/CNT, the Nyquist plots of all electrodes in the 100 kHz to 0.01 Hz frequency range are presented in Fig. 5(b). All Nyquist plots consist of a straight line in the low-frequency region and an arc in the high-frequency region. As shown in the equivalent circuit diagram in Fig. 5(b), the internal resistance of the electrode includes series resistance (Rs) and charge transfer resistance (Rct). The Rs of electrodes, which is related to the impedance of electrolyte depends on Nyquist plots intersection with X-axis, and the Rct of electrodes, which is related to the current exchange defined by the Butler-Volmer equation depends on the arc diameter of Nyquist plots. In addition, the straight slope in the low-frequency range corresponds to ion diffusion from the electrolyte solution to the electrode interface [38]. The fitting results of equivalent circuit diagram reveal that the charge-transfer resistance of pure Bi2O3, Bi2O3/CNT and Bi-Bi2O3/CNT are 9.65 Ω, 1.88 Ω and 0.98 Ω respectively, which indicated that the Rct of Bi2O3 can be reduced significantly with CNT added, and the Rct of 3-dimentinal neural network of Bi-Bi2O3/CNT could be lower than Bi2O3/CNT. Moreover, the straight slope of Bi-Bi2O3/CNT is also steeper than pure Bi2O3 and Bi2O3/CNT, indicating more efficient ion transport of Bi-Bi2O3/CNT. The above analysis shows that the specific surface area of Bi-Bi2O3/CNT is lower than pure Bi2O3 and Bi2O3/CNT, but it has higher specific capacitance, better rate performance. and smaller Rct, which reveals that there is a great improvement in electrochemical performance by forming core-shell Bi-Bi2O3/CNT with 3-dimensional neural network structure. Fig. 5(c) shows the scheme illustrating the benefits of Bi-Bi2O3/CNT neural network nanostructure. The metal Bi core of Bi-Bi2O3/CNT is reduced by CNT, so the contact between the metal Bi core and the CNT is great. In the electrochemical reduction reaction, electrons can be transferred to the Bi2O3 shell through CNT and metal Bi cores where Rct can be reduced greatly by two electron transfer paths. Similarly, electrons can be transferred from the metal Bi on the surface of nanosphere to CNT through the metal Bi nanosphere in the electrochemical oxidation reaction.
Fig. 5.
Fig. 5.
(a) SC valve of Bi2O3, Bi2O3/CNT, Bi-Bi2O3/CNT at various current density. (b) EIS of Bi2O3/CNT, Bi-Bi2O3/CNT. (c) Scheme illustrating the benefits of core-shell Bi-Bi2O3/CNT neural network nanostructure.
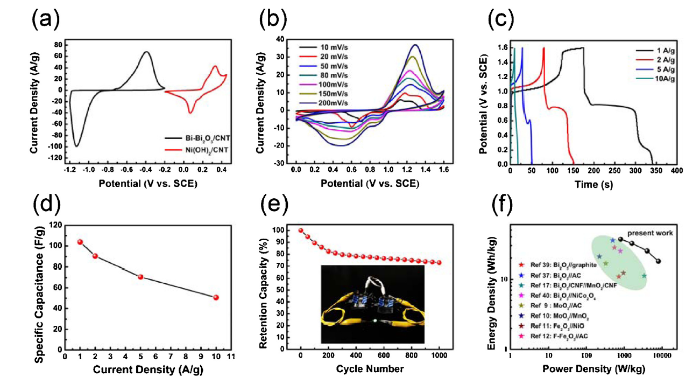
Fig. 6(a) shows the CV curves of negative electrode (Bi-Bi2O3/CNT) and positive electrode (Ni(OH)2/CNT), which shows higher operating potential extended to 1.6 V due to different potential ranges of the positive and the negative electrodes. It should be noted that the area of the CV curve and current density peak of the negative electrode are bigger than the positive electrode at 20 mV s-1 indicating the high capacitance of Bi-Bi2O3/CNT. The CV curves of ASC device were measured at 10 mV s-1 - 200 mV s- shown in Fig. 6(b). Clearly, well defined redox reaction peaks are observed, suggesting the capacitance of ASC device attributed to pseudocapacitance. The galvanostatic charge/discharge (GCD) curves at 1 A g-1 - 10 A g-1 are shown in Fig. 6(c) showing nonlinear profiles and discharge plateau at lower current densities which confirm the contribution of metal compound agreed with CV curves. The specific capacitance of the ASC device as a function of current density is shown in Fig. 6(d), which display a high capacitance with 104 F g-1 (1 A g-1) and 51 F g-1 (10 A g-1). Meanwhile, as is shown in Fig. 6(e), the ASC device exhibits acceptable electrochemical stability that the capacitive retention is still as high as 72.9% even after 1000 cycles at 1 A g-1. The inset image in Fig. 6(e) provides the evidence that this ASC device consisting of Bi-Bi2O3/CNT//Ni(OH)2/CNT could be applied to practice, in which the green LED can light at least 10 min. The Ragone plot is shown in Fig. 6(f) which reveal the energy density and power density value of this ASC device, confirming as high as 36.7 Wh kg-1 (800 W kg-1) and 18 Wh kg-1 (8000 W kg-1). In addition, the energy density and power density value of other similar system of ASC devices reported previously are observed in Fig. 6(f) and Table S1 [[9], [10], [11], [12],17,37,39,40].
Fig. 6.
Fig. 6.
(a) Comparison CV curves of Bi-Bi2O3/CNT electrode and Ni(OH)2/CNT electrode at a scan rate of 20 mV/s. (b) CV curves at different scan rates (10-200 mV/s). (c) GCD curves at different current densities. (d) Specific Capcitance at different current densities. (e) Cycle performance of ASC device(inset: photograph of the ignited LED from Bi-Bi2O3/CNT//Ni(OH)2/CNT ASC device). (f) Ragone plots of the Bi-Bi2O3/CNT//Ni(OH)2/CNT ASC device. The values reported for other ASCs are added for comparison.
4. Conclusion
In summary, we have prepared Bi2O3 nanosheets growing on the surface of CNT by an simple solvothermal method and Bi-Bi2O3/CNT with 3-dimensional neural network structure by annealing treatment of Bi2O3/CNT. The Bi-Bi2O3/CNT shows a high specific capacitance of 850 F g-1 (1 A g-1) and good rate performance with the 714 F g-1 at 30 A g-1, which is attributed to the benefits of double contact of Bi2O3 shell with CNT and metal Bi core. Compared with pure Bi2O3, Bi2O3/CNT and other Bi2O3 electrodes ever reported, Bi-Bi2O3/CNT possesses the highest specific capacitance at various current density except at 1 A g-1. The ASC device included Bi-Bi2O3/CNT negative electrode and Ni(OH)2/CNT positive electrode shows a high voltage window of 1.6 V, a high energy density of 36.7 Wh kg-1 and a maximum power density of 8000 W kg-1. This work verifies that Bi-Bi2O3/CNT with 3-dimensional neural network structure serve as an ideal candidate for advanced energy storage device with high performance.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jmst.2020.02.007.
Reference
DOI
URL
PMID
[Cited within: 2]

Supercapacitor with ultrahigh energy density (e.g., comparable with those of rechargeable batteries) and long cycling ability (>50000 cycles) is attractive for the next-generation energy storage devices. The energy density of carbonaceous material electrodes can be effectively improved by combining with certain metal oxides/hydroxides, but many at the expenses of power density and long-time cycling stability. To achieve an optimized overall electrochemical performance, rationally designed electrode structures with proper control in metal oxide/carbon are highly desirable. Here we have successfully realized an ultrahigh-energy and long-life supercapacitor anode by developing a hierarchical graphite foam-carbon nanotube framework and coating the surface with a thin layer of iron oxide (GF-CNT@Fe2O3). The full cell of anode based on this structure gives rise to a high energy of approximately 74.7 Wh/kg at a power of approximately 1400 W/kg, and approximately 95.4% of the capacitance can be retained after 50000 cycles of charge-discharge. These performance features are superior among those reported for metal oxide based supercapacitors, making it a promising candidate for the next generation of high-performance electrochemical energy storage.
DOI
URL
PMID
[Cited within: 3]

For aqueous nickel/metal batteries, low energy density and poor rate properties are among the limiting factors for their applications, although they are the energy storage systems with high safety, high capacity, and low production cost. Here, we have developed a class of active materials consisting of porous nanoflakes of Ni-Co hydroxides and Bi2O3 that are successfully assembled on carbon substrates of carbon cloth/carbon nanofiber 3D network (CC/CNF). The combination of the porous Ni-Co hydroxides/Bi2O3 nanoflakes with carbon substrate of 3D network is able to provide a large surface area, excellent conductivity, and promote synergistic effects, as a result of the interaction between the active materials and the carbon matrix. With the porous Ni-Co hydroxides and Bi2O3 nanoflakes, the Ni/Bi battery can deliver a high capacity of approximately 110 mA h g(-1) at a current density of 2 A g(-1). About 80% of its capacity (85 mA h g(-1)) can be retained when the current density increases to 20 A g(-1). The full cell can also maintain 93% of the initial capacity after 1000 charge/discharge cycles, showing great potential for Ni/Bi battery.





 WeChat
WeChat