In this paper, we report a strain rate related glass transition in model SrCaYbMg(Li)Zn(Cu) metallic glasses at room temperature. A critical strain rate, equivalent to glass transition temperature, is found for the strain rate induced glassy state to liquid-like viscoplastic state translation. The results show that the observation time, equivalent to temperature and stress, is a key parameter for the transition between the glass and supercooled liquid states. A three-dimension glass transition diagram involved in time, temperature and stress in metallic glasses is established.
Metallic glasses (MGs) with atomic randomly packing structure, excellent glass-forming ability and wide supercooled liquid temperature region are model system for studying the fundamental issues in glass field [1], [2], [3], [4], [5] and [6]. Glasses are regarded as liquids that have lost their flow ability. Generally, it is recognized that the glass transitions between supercooled liquid and glassy states in metallic glasses (MGs) are induced by thermalization or by applied stress [7], [8], [9], [10], [11], [12] and [13]. Through simulation, Guan et al. [12] quantified the intimate coupling of temperature and shear stress in glass transition. In fact, the glass transition is observation time dependent [9]. For polymer glasses, their responses to stress can be observed both within a short time at high temperature and a sufficient long time at low temperatures, which is time-temperature superposition principle [14]. The physical similarity among MGs, colloidal and polymer glasses suggests that the glass transition in MGs could have the similar response to the key parameters of temperature ( T), stress as well as the observation time.
However, little work has been done on the effects of observation time on glass transition in MGs. This is because the MGs normally have high flow energy barrier below glass transition temperature ( Tg), and the homogeneous deformation of MGs generally is only observed at high T or in inaccessible long time scale under stress at low T [15] and [16]. For example, for Zr-based MG with a flow activation energy ∼1.3 eV, the room temperature (RT) uniaxial compression at 80% of its yield stress for 5 h only leads to homogeneous flow with irreversible strain of ∼2.0 × 10-4 [17]. The long time scale and very small homogeneous flow strain at low T make the study of the effects of strain rate and observation time on glass transition very difficult. Recently, some MGs such as Ce-, La- and Sr-based with low Tg have been developed [18], [19], [20] and [21]. Remarkable liquid-like homogeneous flow near room temperature and under relatively high strain rates can be realized in the Sr-based glasses due to their very low flow activation energy [18] and [22]. Importantly, the supercooled liquid state of the MGs can be realized solely by the homogeneous mechanical behavior, which provides a model system to study the roles of strain rate or observation time in glass transition, and to test the concept of glass transition diagram in MGs.
In this work, we report that the glass to supercooled liquid state transition can be solely induced by the strain rate
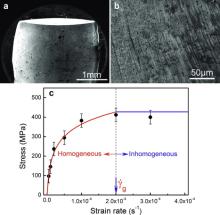
Cylindrical specimens of Sr20Ca20Yb20Mg20Zn20, Sr20Ca20Yb20Mg20Zn10Cu10, Sr20Ca20Yb20(Li0.55Mg0.45)20Zn20 MGs of 2 mm in diameter were prepared by copper mold casting method [22]. The Tg and shear modulus G of the MGs were presented in . Compression tests at RT were conducted on these MGs with a length-to-diameter ratio of 2 at different applied strain rates on an Instron 3384 machine (Norwood, MA). The temperature of the compression tests was controlled by a thermocouple and the tests were performed in pure Ar atmosphere to avoid the oxidation. The X-ray diffraction results showed that the temperature in the range of 310–350 K for compression tests did not induce the crystallization. The scanning electron microscopy (SEM) was conducted in a Philips XL30 instrument. According to the shear cooperative model [23], the barrier energy of plastic flow units WSTZ (also termed as shear transformation zones (STZs)) in MGs can be estimated by [24] WSTZ∝0.4 GVm, where Vm is average molar volume. These MGs have ultra low G (see ), and their flow barrier energy WSTZ is estimated to be 0.79, 0.75, and 0.52 eV, respectively. Clearly, these MGs have much lower activation energy of flow units compared with that of conventional MGs [24] and the activation of flow units is then much easier. We then could realize the homogenous flow or glass to supercooled liquid transition near RT in the MGs by stress in reasonable observation time scale, and characterize the effect of time on glass transition through their homogeneous mechanical behavior.
| Table 1. Values of Tg, shear modulus G, compression yield strength σy, the maximum flow stress σmax and steady flow stress σflow at the critical strain rate, the experimental critical strain rate |
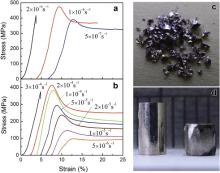
Fig. 1 shows the compressive stress–strain curves at different applied strain rates for as-cast rods of Sr20Ca20Yb20Mg20Zn20 ( Fig. 1(a)) and Sr20Ca20Yb20(Li0.55Mg0.45)20Zn20 ( Fig. 1(b)). For both of the MGs, at RT and above a critical strain rate
Similar to the T dependence of the homogenous deformation in supercooled liquids, a strain rate dependent homogeneous flow can be clearly seen in the MG below
Fig. 2(c) shows the maximum stress as a function of
The relationship of apparent viscosity η of homogeneous flow,
Maxwell firstly extrapolated the relation between liquid and solid behavior, and proposed the relation between the viscosity and relaxation time τ as: τ = η/ G∞, where G∞ is the instantaneous shear modulus. The Maxwell relaxation time τ provides a key to understanding the glass transition [31] and [32]. The general property of the relation indicates that liquid is solid-like on time scales much shorter than τ, and on a sufficiently long time scale any glass behaves like a liquid. The glass transition is observation time dependent, and difference between glasses and fluid liquid can be defined by a nondimensional number of the so-called Deborah number [31] and [32]: D = tr/ to, where tr is the time of relaxation, and to is the time of observation. If to is very large, the glass flowing can be seen. On the other hand, if tr >> to, the liquid is a glassy solid. A viscous liquid can be viewed as solid which flows in enough short time scale, and a glass can be regarded as viscous liquid in enough large time scale [32]. Actually, the inverse strain rate is related to action time scale of strain or stress on MGs, and is equivalent to the experimental observation time, and the effect of the strain rates on the glass transition is equivalent to that of the observation time on glass transition.
To determine the relation between the time scale, temperature and stress in glass transition process of MGs, we studied the relation between maximum yield stress and inverse strain rate at RT. Fig. 3(a) shows the σmax vs.
where νDis the Debye frequency, Δ Gmis the activation free energy of defect migration, Vis the activation volume and kis Boltzmann constant. At a given temperature of RT, the νD,Δ Gmand Vare constant, and the relation between the σmaxand
where
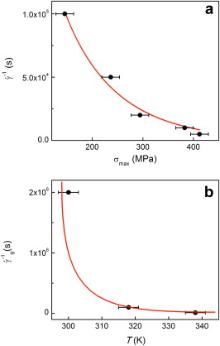 | Fig. 3. (a) Maximum yield stress vs. the inverse strain rate in the Sr20Ca20Yb20(Li0.55Mg0.45)20Zn20 MG. (b) Temperature vs. |
Fig. 3(b) shows the relation of T and
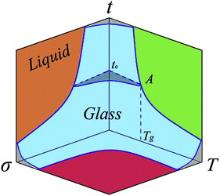
The diagrams involved in temperature and stress to describe the jamming and glass transition in different glasses are summarized [12], [13] and [35]. Based on previous and our above results, we propose a speculative 3D glass transition diagram involved in observation time t (equivalent to
A glass to liquid-like homogeneous flow translation at RT occurs in Sr-based MGs when the strain rate reaches a critical value, which indicates that the action time of strain which is equivalent to observation time, like temperature and stress, is another intrinsic parameter to understand the glass transition. A 3D diagram involved in the observation time, stress and temperature is established to describe the glass transition in MGs. The temperature, observation time, and stress are equivalent control parameters, and the change of any of them has equivalent role in glass transition. The glass transition is the interplay between thermal energy, applied stress and action time scale. The work might be helpful for understanding the nature of the glass transition and for providing a unifying link between the glass transition and jamming.
References
This work was supported by the National Natural Science Foundation of China (No. 51271195).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. |
|
| 42. |
|
| 43. |
|
| 44. |
|
| 45. |
|