1. Introduction
Metallic glasses (MGs) or amorphous alloys with uniquely disordered atomic structure have already been known to researchers as advanced alternatives of crystalline alloys [1]. The initial research enthusiasm of MGs originates from their superior mechanical properties, thereby having promising structural applications [2,3]. On the other hand, their functional applications, e.g. excellent corrosion resistance [4] and wear resistance [5], tunable magnetic properties [6], and superhydrophobicity [7] have been successively investigated and developed. Very recently, MGs have also been exploited as advanced catalysts in environmental and energy fields owing to their amazing metastable nature induced far-from-equilibrium state [[8], [9], [10]], which is believed to enable more catalytic active sites for highly efficient catalysis. Until now, MG catalysts have been widely employed for dyes degradation [[11], [12], [13], [14]], removal of heavy metals [15], nitrate and alkyl halides [15,16], hydrogen evolution reaction and oxygen evolution reaction [[17], [18], [19]], methanol and ethanol oxidation [20,21], aiming to remediate the environmental and energy concerns. In addition, introducing amorphous characteristics into highly ordered structure now has attracted increasing interests in fabricating novel catalysts [[22], [23], [24]], further demonstrating the advantages of MGs used in catalytic applications.
Heat treatment induced structural relaxation and further crystallization is generally applied to investigate the thermal stability and structural dynamic heterogeneity of MGs [25], [26], [27], [28]. Indeed, many researchers gradually start to focus on the effect of heat treatment on MGs for dye degradation in order to study their unique properties in catalysis and develop new catalysts. For example, the residual stress relaxation and the elimination of stress gradient during low temperature annealing (300 and 400 °C) were responsible for the decline in catalytic ability of (Fe73.5Si13.5B9Nb3Cu1)91.5Ni8.5 MG ribbons [29]; the loss of Fe-rich clusters and Fe-poor region in Fe76B12Si9Y3 MG powder after annealing led to a weaker galvanic cell effect in amorphous structure [30]; a faster electron transfer in Fenton-like process due to a higher volume fraction of amorphous phase had been confirmed by annealing (515-580 °C) Fe78Si9B13 MG ribbons [31]. Notably, some interesting catalytic phenomena were also found during the investigation of heat treatment on MGs. An introduction of a small amount of nanocrystals in Fe80P13C7 MG ribbons by annealing close to the glass transition temperature Tg was reported to produce a better catalytic performance than the MG counterparts [12] and this was further confirmed by the partial crystallization of Fe82.65Si4B12Cu1.35 MG ribbons [32]. Moreover, a full crystallization of (Fe73.5Si13.5B9Nb3Cu1)91.5Ni8.5 MG ribbons contributed to a better catalytic performance in dye degradation [33]. As such, the heat treatment of MGs indeed helps to study their intrinsic catalytic nature, and the crystallization of MGs seems to present a high promising potential to exploit new environmental catalysts in wastewater treatment.
Previously our research group have demonstrated the fast electron transfer efficiency in the MGs, leading to fast activation of H2O2 in the Fenton-like process [31]. Very recently, we found that the rejuvenation of catalytic behavior in the fully crystallized counterparts of MGs is attributed to a significant grain size effect and the generation of crystalline phases with a large electrochemical potential difference [34]. However, an effective rejuvenation of catalytic behavior only achieves when surface oxide layer on crystallized ribbons can be properly removed (such as recycling of ribbons with weak surface layer binding effect) [34]. Currently the annealing treatment of MGs still has its limitation for catalytic improvement, especially for surface oxidation effect. The homogeneous amorphous structure can effectively avoid the production of thick oxide layer on the surface [35], and such layer effect also contributes to a superior reusability of MGs in wastewater treatment [36,37]. In contrast, surface oxidation effect of crystallized ribbons or even structural-relaxed ribbons (without crystallization) becomes more pronounced after heat treatment, exerting an inhibited catalytic activity [38,39]. Therefore, how to effectively solve the surface oxidation of crystallized ribbons remains a critical problem before developing novel heterostructured catalysts from MGs. Herein, three partially crystallized Fe-based ribbons were firstly obtained by annealing of Fe78Si9B13 MG ribbons at 520, 560 and 600 °C. In order to overcome the surface oxidation effect, the short-time electrochemical etching in partially crystallized ribbons was employed to fabricate nanoporous structure. The optimal processing conditions including etching time, electrolyte concentrations and electrolyte types have also been fully investigated.
2. Experimental
2.1. Materials preparation
As-spun MG ribbons with nominal composition of Fe78Si9B13 (at.%) were manufactured by as-reported melt-spinning technique [34]. The as-spun MG ribbons were cut into ~5 × 20 mm (width and length, respectively) and then treated by conventional annealing (at a heating rate of 20 K/min) in a tube furnace for 5 min with protection of Argon gas. The after-annealed ribbons were then fast cooled down with Argon gas. According to our previous differential scanning calorimetry (DSC) results [34], the onset crystallization temperature of Fe78Si9B13 MG ribbons is located at 514 °C. In order to obtain partially crystallized Fe-based ribbons, the annealing temperatures were set at 520, 560 and 600 °C. Hereafter, the Fe78Si9B13 ribbons annealed at 520, 560 and 600 °C were denoted as FeSiB-A520, FeSiB-A560 and FeSiB-A600, respectively.
Partially crystallized ribbons were applied as precursor materials for subsequent electrochemical etching treatment by an electrochemical station (PARSTAT 2273). The electrochemical etching in the traditional three-electrode cell with a saturated calomel electrode (SCE) and a platinum sheet counter electrode was conducted at a constant electric potential (i.e. 0.0 V). The initial electrolyte was selected as 0.3 M H3PO4 solution under predetermined etching time (i.e. 0, 10, 45, 90, 180 and 720 s). The investigation of electrochemical etching conditions was based on different concentrations of H3PO4 (i.e. 0.05, 0.1, 0.3, 0.5, 1.0 M) and different electrolytes (i.e. acids for HCl, H3PO4, H2SO4 and HNO3, and sodium salts for NaCl, Na3PO4, Na2SO4 and NaNO3). In order to maintain equivalent H+ in the electrolyte (compared to 0.3 M H3PO4), 0.9 M HCl, 0.45 M H2SO4 and 0.9 M HNO3 were used. The sodium salt solutions were obtained using the same concentration (i.e. 0.3 M with the equivalent anion) of acids and the titration by NaOH until pH 6.0. The after-etched ribbons at the working electrode were rinsed with Milli-Q water (18.2 MΩ·cm) and absolute ethanol by three times, and then put into a drying cabinet for further materials characterization.
Cibacron brilliant red 3B-A (BR3B-A) dye, tert-butanol (TBA, ≥99 %), and HCl (37 % w/w) were purchased from Sigma-Aldrich. Hydrogen peroxide (H2O2, 30 % w/w), H3PO4 (85 % w/v) and H2SO4 (0.5 M) were supplied by Rowe Scientific Pty Ltd. Other chemicals (absolute ethanol, HNO3 and NaOH) were all in the analytical grade. All the chemicals were used without any purification.
2.2. Characterization
The microstructures of as-spun and partially crystallized Fe-based ribbons before and after electrochemical etching were characterized by X-ray diffraction (XRD) recorded on a PANalytical Empyrean diffractometer with monochromated Cu-Kα radiation (λ = 0.15406 nm). The surface morphologies of ribbons before/after electrochemical etching and after catalytic degradation of dye were observed by scanning electron microscopy (SEM, FEI Verios 460). The surface chemical states of as-annealed Fe-based ribbons were characterized by X-ray photoelectron spectroscopy (XPS) using a Kratos AXIS Ultra DLD instrument with Al-Kα X-ray.
2.3. Electrochemical measurements
Electrochemical measurements of partially crystallized ribbons were performed in an electrochemical station with a three-electrode cell, which was the same as the electrochemical etching. The H3PO4 solution with pH 3.0 was used as the electrolyte. The potentiodynamic polarization curves were recorded with a sweep rate of 0.1667 mV/s ranging from -0.88 V to +0.8 V after a stabilization of open circuit potential (OCP) at 10 h. Electrochemical impedance spectroscopy (EIS) was carried out with an AC amplitude of 5 mV at a frequency range from 104 Hz to 0.01 Hz.
2.4. Catalytic analyses
All the catalytic degradation tests of BR3B-A dye (20 ppm) were performed using the freshly prepared ribbons. That is, the after-etched ribbons were immediately employed for catalytic tests after rinsed with Milli-Q water and absolute ethanol. 0.5 g/L ribbons were typically used for the activation of 1 mM H2O2 in the Fenton-like process for dye (pH 3.0) degradation. The thermostatic water bath was used to control the constant reaction temperature at 25 °C and the reaction was conducted under the mechanical stirring (300 rpm). At predetermined time intervals, the dye solution was extracted for characterization using a UV-vis spectrometer (Lambda 35, PerkinElmer) at the absorbance peak (λmax) of 517 nm. In recycling tests, the after-used FeSiB-A520 ribbons were rinsed with Milli-Q water and absolute ethanol before performing next run of dye degradation test. Different concentrations of TBA (i.e. 10 mM, 50 mM, 0.1 M, 0.5 M, and 1.0 M) were used to investigate the quenching effect in the Fenton-like process using after-etched FeSiB-A520 ribbons as a catalyst.
3. Results and discussion
3.1. Characterization of partially crystallized Fe-based ribbons
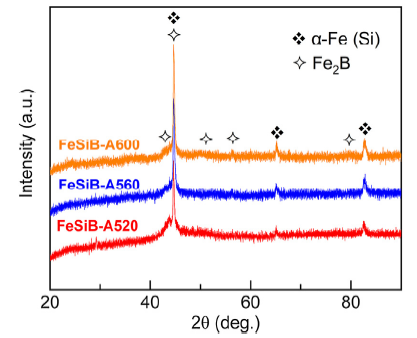
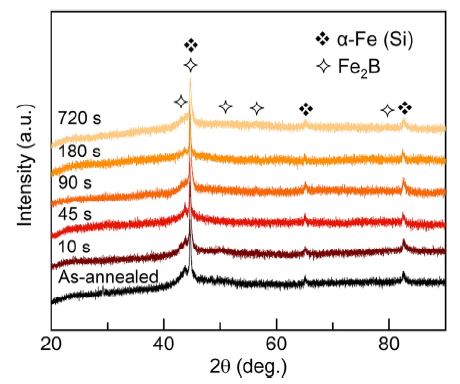
The as-spun Fe78Si9B13 MG ribbons in this work present a typical broad diffraction peak at 2θ between 40° and 50°, and no intense crystallization peak is observed (Fig. S1), suggesting a standard amorphous feature in their structure [40,41]. After the conventional annealing at temperatures of 520, 560 and 600 °C, all the ribbons (FeSiB-A520, FeSiB-A560 and FeSiB-A600) show the crystallization with formation of α-Fe (Si) and Fe2B phases, as illustrated in Fig. 1, which is in good agreement with the results reported in our previous study [34]. In addition, an amorphous feature still exists on the XRD patterns when the annealing temperatures are between 520-600 °C, indicating that a partially crystallized state (or amorphous/crystalline structure) has been obtained from as-spun Fe78Si9B13 MG ribbons. With increasing the annealing temperature, a slight increase in peak intensity at 2θ of ~56°, 65° and 83° reveals that the crystallization rate from amorphous structure has increased.
Fig. 1.
Fig. 1.
XRD patterns of as-annealed FeSiB-A520, FeSiB-A560 and FeSiB-A600 ribbons.
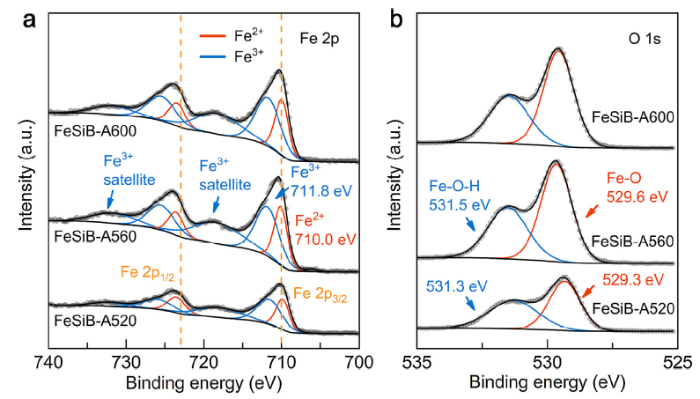
It is well accepted that the as-spun Fe78Si9B13 MG ribbons generally have a very smooth surface morphology [31,42]. In this work, although MG ribbons were annealed to be partially crystallized state, their surface morphologies were not significantly changed (Fig. S2). The small bright points on the annealed ribbon surfaces are supposed to be the nanosized oxide aggregates. Further investigation of surface chemical states of three annealed ribbons can be seen from XPS studies (Fig. 2). Fe 2p spectra in Fig. 2(a) show that the surfaces of all annealed ribbons are mainly composed of a mixture of Fe2+ and Fe3+. For example, peaks at binding energies of 710.0 eV and 723.6 eV on the FeSiB-A560 surface can be assigned to be Fe2+ at Fe 2p3/2 and Fe 2p1/2, respectively [15,37], while peaks at 711.8 eV and 725.5 eV correspond to Fe3+ at Fe 2p3/2 and Fe 2p1/2, respectively [43,44]. The existence of two shake-up satellite peaks at 718.3 eV and 731.6 eV also confirms the Fe3+ on the annealed ribbons surface [43]. Slight peak shifting can be observed on FeSiB-A520 and FeSiB-A600. In addition, O 1s spectra of e.g., FeSiB-A560 in Fig. 2(b) show the existence of Fe-O bond (529.6 eV) and Fe-O-H bond (531.5 eV) [[43], [44], [45]] on the surface, indicating that the surfaces of annealed ribbons mainly consist of FeO, Fe2O3 and FeOOH. It is noteworthy that the as-spun Fe78Si9B13 MG ribbons usually contain a high intensity of Fe0 peak signal (at ~706.0 eV) in the XPS spectrum [31,34]. Although binding energy at ~531.5 eV (Fig. 2(b)) enhances the adsorption function for hydroxides (usually indicating the active surface sites), the loss of Fe0 after annealing also contributes to a decline in catalytic ability of partially crystallized ribbons.
Fig. 2.
Fig. 2.
High resolution (a) Fe 2p and (b) O 1s spectra of as-annealed FeSiB-A520, FeSiB-A560 and FeSiB-A600 ribbons.
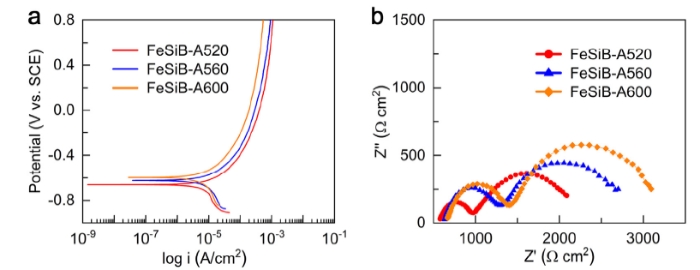
Fig. 3 shows electrochemical measurements of as-annealed FeSiB-A520, FeSiB-A560 and FeSiB-A600 ribbons. The OCP of three annealed ribbons were measured in the H3PO4 solution with pH 3.0 at room temperature and all of them stabilized after 4 h (Fig. S3). The OCP of FeSiB-A600 (-0.60 V) is relatively higher than that of FeSiB-A560 (-0.62 V) and of FeSiB-A520 (-0.66 V). After stabilization of OCP, the corrosion behavior of FeSiB-A520, FeSiB-A560 and FeSiB-A600 were investigated by potentiodynamic polarization curves in Fig. 3(a). The estimated corrosion current densities follow the below order: FeSiB-A600 (9.90 × 10-6 A/cm2) < FeSiB-A560 (1.04 × 10-5 A/cm2) < FeSiB-A520 (1.19 × 10-5 A/cm2), indicating that a higher annealing temperature results in a better corrosion resistance in partially crystallized ribbons, which is further confirmed by EIS measurement (Fig. 3(b)). FeSiB-A520 ribbons present the smallest semicircular diameter with a weakest corrosion resistance [46], compared to FeSiB-A560 and FeSiB-A600. From electrochemical measurements, apparently, an increase in annealing temperature at the partially crystallized state of Fe78Si9B13 ribbons enhances the corrosion resistance. In contrast, a lower annealing temperature (FeSiB-A520) contributes to a lower electron transfer resistance, which is expected to present a better catalytic behavior.
Fig. 3.
Fig. 3.
(a) Potentiodynamic polarization curves and (b) electrochemical impedance spectroscopy of as-annealed FeSiB-A520, FeSiB-A560 and FeSiB-A600 ribbons.
3.2. Electrochemical etching of partially crystallized ribbons in H3PO4
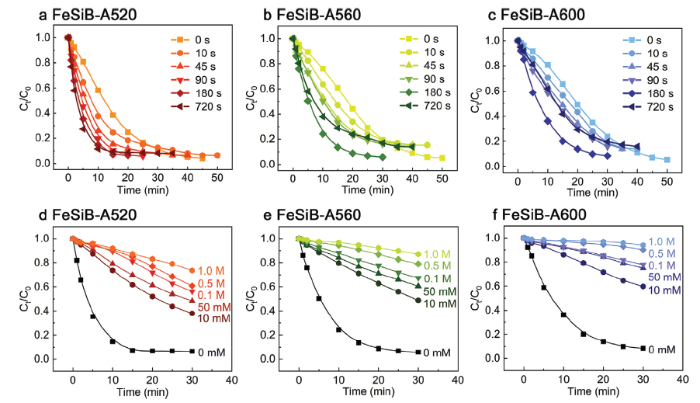
From our previous studies [31,34,47], it has been known that partially crystallized ribbons in the dye decolorization by Fenton-like process generally have a lower catalytic activity than MG counterparts. In this work, the correlation of catalytic performance and the annealing temperature of partially crystallized ribbons can be further observed from Fig. 4(a)-(c). The dye color removal rate over 95 % generally requires 40 min for as-annealed FeSiB-A520 without electrochemical etching (0 s), while more than 50 min is needed for FeSiB-A560 (0 s) and FeSiB-A600 (0 s), indicating that the as-annealed FeSiB-A520 presents the best catalytic behavior among three ribbons, which is in good agreement with the electrochemical measurements in Fig. 3. The higher catalytic efficiency of FeSiB-A520 mainly originates from a higher volume fraction of amorphous phase [31,48]. It is worthy to note that the catalytic efficiency of FeSiB-A520 is largely improved when an external potential of 0.0 V was applied for electrochemical etching at different time (Fig. 4(a)). The 720s-etched FeSiB-A520 exhibits an over 2 times higher efficiency than those of as-annealed counterparts. In comparison, both FeSiB-A560 and FeSiB-A600 ribbons in Fig. 4(b) and (c) show a similar improvement tendency with increasing etching time, respectively. However, their best catalytic efficiencies occur at 180 s etching. Further increasing to 720 s leads to a significant decline of color removal rate.
Fig. 4.
Fig. 4.
BR3B-A dye decolorization by electrochemical-etched (a) FeSiB-A520, (b) FeSiB-A560 and (c) FeSiB-A600 varying from 0 s to 720 s in 0.3 M H3PO4 solution. Quenching effect by 0.01 - 1.0 M TBA on 180s-etched (d) FeSiB-A520, (e) FeSiB-A560 and (f) FeSiB-A600.
Fig. S4 summarizes their reaction rate constants kobs and the catalytic efficiencies for all range of etching time apparently follow the order: FeSiB-A520 > FeSiB-A560 > FeSiB-A600. kobs is obtained from: ln (C0/Ct) = kobst, where C0 and Ct are the initial concentration and the concentration at the time t of BR3B-A dye. In order to investigate the activation mechanism of H2O2 by electrochemically etched ribbons, Fig. 4(d)-(f) show the quenching effect by various concentrations of TBA on the dye decolorization. Apparently, all the decolorizations by 180s-etched ribbons present an inhibitive effect by TBA within 30 min and it becomes more significant when increasing the concentration of TBA. Therefore, it is believed that hydroxyl radical (·OH) acts as the main reactive species during the activation of H2O2 by electrochemically etched Fe-based ribbons and ·OH dominates the role for dye degradation, which was also reported by previous studies [34,49,50].
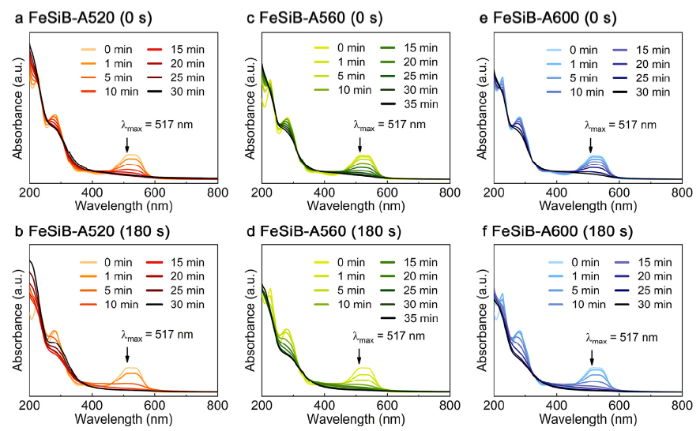
Fig. 5 illustrates a comparison of UV-vis spectra of as-annealed and 180s-etched partially crystallized ribbons for BR3B-A dye degradation. The maximum absorbance peak of BR3B-A dye located at 517 nm indicates the chromophore (-N=N-) of the azo bond [42,51], while peaks at 227 nm and 283 nm generally represent the aromatic structures in the dye molecules [52,53]. Apparently, as-annealed FeSiB-A520 ribbons present a relatively strong degradation ability to BR3B-A dye, where all the characteristic peaks in UV-vis spectrum are progressively invisible within 30 min (Fig. 5(a)), indicating that as-annealed FeSiB-A520 can not only decolorize the dye solution but also intrinsically destroy the aromatic structure of dye into smaller inorganic molecules [54,55]. In comparison, 180s-etched FeSiB-A520 ribbons suggest an aggressive degradation efficiency with a complete decolorization in 15 min, which corresponds to the decolorization efficiency in Fig. 4(a). In addition, Fig. 5(c)-(f) compare the degradation efficiency in UV-vis spectra of FeSiB-A560 and FeSiB-A600 at 0 s and 180 s etching, which are also closely related to Fig. 4(b) and (c). As shown in Fig. 5, it is known that all ribbons could activate H2O2 to generate active ·OH so as to completely decompose dye molecules and the activation efficiency has been strongly promoted by electrochemical etching of partially crystallized ribbons. Given that the structural change with annealing temperature largely dominates the electron transfer efficiency [34,39], the electrochemical etching in this work shows a high promising potential to promote catalytic activity of partially crystallized ribbons by altering their microstructure and surface morphology, which will be further discussed.
Fig. 5.
Fig. 5.
UV-vis spectra of BR3B-A dye decolorization by: (a) as-annealed and (b) 180s-etched FeSiB-A520; (c) as-annealed and (d) 180s-etched FeSiB-A560; (e) as-annealed and (f) 180s-etched FeSiB-A600.
In order to reveal the effect of electrochemical etching on the partially crystallized ribbons, we selected FeSiB-A520 ribbons to investigate the structural and morphology changes during the etching in the 0.3 H3PO4 solution. Phase identification of FeSiB-A520 has been discussed in Fig. 1. In Fig. 6, there is no additional peak generation during the potentiostatic etching, indicating that electrochemical etching does not induce further crystallization for partially crystallized ribbons. In addition, peak intensities corresponding to Fe2B become lower with increasing etching time. Noting that the preferential dissolution of active α-Fe was confirmed in the α-Fe/amorphous matrix composite [56], while Fe2B has been known to obtain a lower OCP than α-Fe [57]. In this work, the effect of galvanic cell among α-Fe (Si), Fe2B and residual amorphous matrix would be accelerated with the external applied potential of 0.0 V and therefore more active Fe2B has a higher tendency to lose electron in the galvanic cell. In addition to the peak evolution in Fig. 6, it is believed that Fe2B has been selectively dissolved during the potentiostatic etching in the H3PO4 solution.
Fig. 6.
Fig. 6.
XRD patterns of FeSiB-A520 after electrochemical etching from 0 s to 720 s in 0.3 M H3PO4.
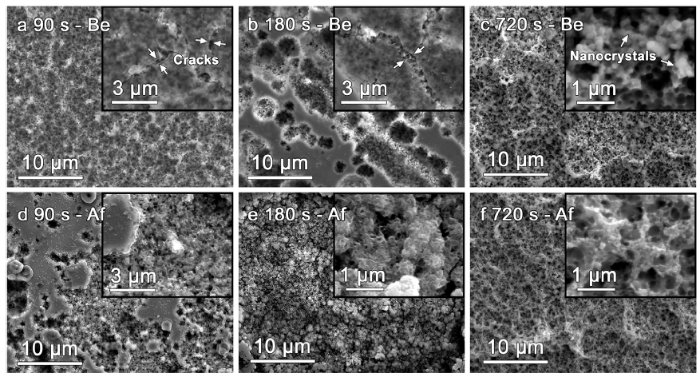
On the other hand, the evolution of surface morphologies of FeSiB-A520 during electrochemical etching before and after dye degradation has been investigated. Fig. 7(a) shows that there are many small cracks on the surface layer after 90 s etching before dye degradation. According to XPS studies in Fig. 2, the surface layer mainly consists of Fe-rich oxides. After 180 s, those surface cracks are largely extended (Fig. 7(b)), thereby leading to an observation of nanocrystalline phases covered by Fe-rich oxide layer. In addition, the surface layer seems to be dissolved during the electrochemical etching. Increasing to 720 s etching clearly shows the full exposure of nanocrystals, which are formed from annealing treatment (Fig. 7(c)). The selective dissolution of Fe2B phase contributes to the formation of a honeycomb-like nanoporous structure and therefore it is expected to highly promote the catalytic active sites of electrochemically etched FeSiB-A520 ribbons. From the surface morphologies after electrochemical etching and subsequent dye degradation (Fig. 7(d)-(f)), it is found that nanocrystals mainly dominate the surface active sites for fast capturing and activating H2O2. The loose and thin surface layer after electrochemical etching seems to slough off during the reaction. According to a high catalytic efficiency of 180s-etched FeSiB-A520, Fig. 7(e) exhibits many flocculent-like precipitates covered on the nanocrystals, which are observed in other studies [33,58]. Fig. 7(f) also shows the precipitation of by-products on the nanocrystals.
Fig. 7.
Fig. 7.
Surface morphologies of FeSiB-A520 electrochemically etched at (a) 90, (b) 180 s and (c) 720 s before BR3B-A dye degradation, which are correspondingly denoted as: xxx (etching time) - Be. (d) (e) (f) surface morphologies of (a) (b) (c) after dye degradation, which are correspondingly denoted as: xxx (etching time) - Af.
From the studies in Fig. 7, it is known that Fe-rich surface oxides, to some extent, inhibit the catalytic reaction during Fenton-like process. The decline in catalytic efficiency of partially crystallized ribbons from annealing of MG ribbons is not only because of structural change [33,39,57], but also due to the thicker surface layer formation [38,59]. However, the electrochemical etching highly modifies the ribbon surface morphologies into nanoporous structure, which leads to easy contact between catalysts and H2O2/dye and brings galvanic cell effect into significant position. In fact, a modified surface layer may also provide more active reaction sites and help to capture H2O2/dye.
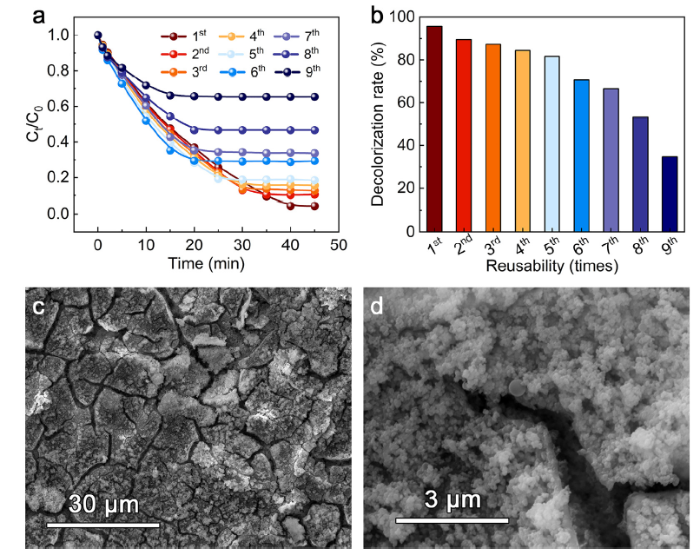
In order to investigate the natural surface layer effect, Fig. 8 shows the reusability of as-annealed FeSiB-A520 ribbons without electrochemical etching. The reusability of 9 times is illustrated in Fig. 8(a). Our previous investigation of Fe78Si9B13 ribbons annealed at 900 °C has indicated that the reusability could effectively activate the surface with surface layer shedding, leading to highly enhanced catalytic efficiency [34]. In contrast, as-annealed FeSiB-A520 ribbons in this work present progressively decline in total dye decolorization rate with increasing reusability. Fig. 8(b) clearly shows the total decolorization rate after stabilization in 45 min. The FeSiB-A520 ribbons after reused for 9 times only achieve less than 40 % of decolorization. Fig. 8(c) displays the surface morphology of 9th-reused FeSiB-A520 ribbons. It can be seen that a surface layer with many large cracks still exists even for a long-time reusability. These cracks seem to be observed in many MGs and crystalline counterpart after dye degradation [58,[60], [61], [62]]. But the zoom-in view (Fig. 8(d)) suggests that the surface of after-reused FeSiB-A520 ribbons has been fully covered by many nanosized precipitation. Previously, the self-reconstructed hierarchical gradient structure has been demonstrated for the origin of robust reusability of MGs [36]. In this work, the stronger binding effect of surface layer of FeSiB-A520 leads to the persistence of layer even recycling for 9 times. The exposure of cracks only slightly enhances the catalytic activity in the initial stage and the following active sites are fully covered by many nanosized intermediates and products. As such, it provides a clear guidance that the electrochemical etching could not only dissolve the surface layer but also provide more active sites on the surface, leading to highly enhanced catalytic efficiency in the Fenton-like process.
Fig. 8.
Fig. 8.
(a) Reusability of FeSiB-A520 and (b) corresponding decolorization rate after stabilization. (c) and (d) Surface morphologies of 9th-reused FeSiB-A520 ribbons.
3.3. Electrochemical etching conditions
Aforementioned electrochemical etching is based on the preselected 0.3 M H3PO4 solution as an electrolyte with variation of etching time, and 180 s has been demonstrated to be the most effective etching time for all partially crystallized ribbons. Accordingly, we also considered other important conditions, i.e. electrolyte concentration and types of electrolyte, which will be demonstrated as following.
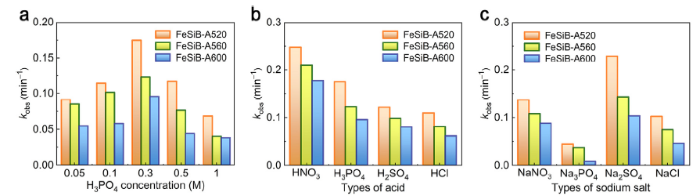
Fig. 9(a) shows the reaction rate constants kobs of FeSiB-A520, FeSiB-A560 and FeSiB-A600 for dye degradation, which are obtained from Fig. S5. The ribbons were electrochemically etched at H3PO4 solution varying from 0.05 to 1.0 M. It can be seen that 0.3 M H3PO4 solution has the best etching effect on the promotion of catalytic efficiency for these three ribbons. The lower or higher concentration than 0.3 M is not favorable for improving the catalytic ability of partially crystallized ribbons, which may be caused by the generation of inefficient surface morphologies according to previous discussion in Fig. 7, Fig. 8. From Fig. S5, the total decolorization rate has also been largely inhibited when 1.0 M H3PO4 solution is applied.
Fig. 9.
Fig. 9.
Reaction rate constants kobs of partially crystallized ribbons (FeSiB-A520, FeSiB-A560 and FeSiB-A600) 180s-etched in (a) 0.05 - 1.0 M H3PO4 solution, (b) HNO3, H3PO4, H2SO4 and HCl solution (with equivalent H+) and (c) NaNO3, Na3PO4, Na2SO4 and NaCl solution (with equivalent anion at pH 6.0).
Fig. 9(b) and Fig. S6 show the effect of various types of acid as electrolytes on the catalytic ability of partially crystallized ribbons. The concentrations of H+ for different acids were controlled to be consistent. Notably, HNO3 solution, which has a better effect than H3PO4 solution, seems to be an effective electrolyte for electrochemical etching of partially crystallized Fe78Si9B13 ribbons. The corresponding kobs for FeSiB-A520, FeSiB-A560 and FeSiB-A600 ribbons in HNO3 solution achieves 0.248, 0.210 and 0.177 min-1, respectively. In fact, it has been reported that HNO3 solution generally exerts a significant effect on the surface activation of Fe-based MGs not only in dye degradation [63], but also in water splitting [64]. Recently, the surface activation of Fe-based MGs by ball milling has also been reported, which is attributed to uneven topography of surface and deformation energy induced by ball milling [65]. In this work, the catalytic efficiencies of all ribbons follow the order in the electrolyte: HNO3 > H3PO4 > H2SO4 > HCl.
However, the catalytic enhancement of ribbons by electrochemical etching in aforementioned acids may be caused by the synergic effect of H+ and paired anion. The anion indeed influences the corrosion of materials. For example, the corrosion behavior of alloys by Cl- has been widely studied [4,66,67]. In order to investigate their function in the electrochemical etching, NO3-, PO43-, SO42- and Cl- with an equivalent concentration at pH 6.0 have been employed as electrolytes. Fig. 9c and Fig. S7 show the effect of anion as electrolytes on the catalytic ability of partially crystallized ribbons. Regardless of concentration variation in HNO3 (0.9 M) and NaNO3 (0.3 M), the kobs shows that NO3- indeed plays an important role in the etching of ribbons, and the highly enhanced catalytic ability of ribbons when using HNO3 as an electrolyte is attributed to synergic effect of H+ and NO3-. In contract, sole PO43- has a negligible effect on the ribbons, but it promotes the entire etching effect of H3PO4. It is worthy to note that SO42- also presents a very strong ability for surface etching of partially crystallized ribbons, which is even better than the coexistence of H+ and SO42-. In addition, the etching effects of both NaCl and HCl mainly rely on Cl-. At the same concentration of anion, SO42- presents the optimal improvement of catalytic ability of partially crystallized ribbons.
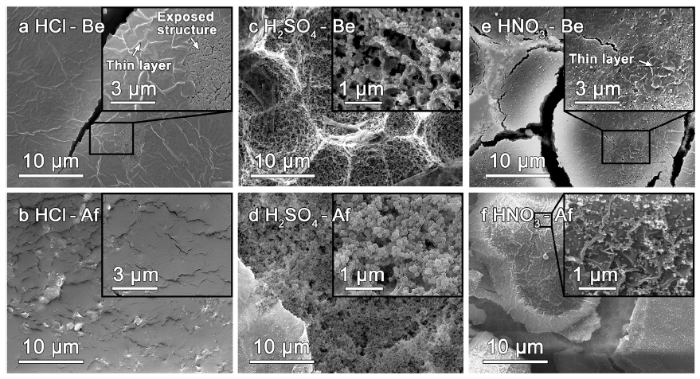
Typically, surface morphologies of FeSiB-A520 after etching in various acids (Fig. 10) help to reveal the origin of catalytic improvement in dye degradation. Fig. 10(a) shows a relatively smooth and thin layer covered on the exposed structure after potentiostatic etching in HCl solution. Corresponding surface morphology after dye degradation (Fig. 10(b)) only exhibits a small variation on the surface without generation of nanostructure and precipitation, indicating that the etched surface by HCl only provides a small number of active sites on the activation of H2O2 during the Fenton-like process, thereby leading to a low dye decolorization efficiency. In Fig. 10(c), the surface suffers a typical pitting corrosion (average ~10 μm) during electrochemical etching in H2SO4 solution. However, the strong pitting corrosion seems to largely destroy internal galvanic cell structure. Compared to the inset of Fig. 7(c), only nanostructured skeleton is left on the surface and most nanocrystals are missing in the inset of Fig. 10(c). The losing of numerous nanocrystals leads to a low electron transfer efficiency and a low dye decolorization efficiency. After dye degradation, the nanostructured skeleton has been coarsened by products (Fig. 10(d)). For HNO3 solution, the pitting corrosion with large cracks also occurs (Fig. 10(e)). According to inset of Fig. 10f, the numerous nanostructured precipitates indicate the active surface characteristics during the etching in HNO3 solution, which confirms the surface activation function of HNO3 [63,64]. Here, it is believed that a proper activation of surface oxide layer also increases the active sites for Fenton-like process.
Fig. 10.
Fig. 10.
Surface morphologies of 180s-etched FeSiB-A520 in (a) (b) 0.9 M HCl, (c) (d) 0.5 M H2SO4 and (e) (f) 0.9 M HNO3 solution before and after dye degradation. Surface morphologies before and after dye degradation are denoted as: xxx (acid) - Be and xxx (acid) - Af, respectively.
On the other hand, the surface etching in NaCl solution is not effective (Fig. 11(a)). The main surface is still covered by the thick Fe-rich oxide. Only a small portion of area has been destroyed with exposure of nanocrystals. Compared to H3PO4, a minor surface modification can be seen when electrochemically etched by sole PO43- (Fig. 11(b)). The dye degradation efficiency after etching in PO43- is even lower than that before etching, which may be caused by the growth of oxide layer in the current conditions. In Fig. 11(c), the similar pitting corrosion as H2SO4 can be seen, suggesting the main pitting corrosion function by SO42-. Here, considering the strong enhancement of catalytic ability after etching in sole SO42-, the remaining oxide layer provides a good protection for nanocrystals so that they can present a better galvanic cell effect during activation of H2O2, which is distinct from Fig. 10(c) in H2SO4. Fig. 11(d) shows a smooth surface morphology after electrochemical etching in NaNO3. In this situation, the surface activation of etched ribbons may play the major role in the dye degradation, which also demonstrates the synergistic effect of Fe-rich oxide layer and nanocrystals in this work.
Fig. 11.
Fig. 11.
Surface morphologies of 180s-etched FeSiB-A520 in (a) NaCl, (b) Na3PO4, (c) Na2SO4 and (d) NaNO3 solution at pH 6.0. The concentrations of all electrolytes are 0.3 M.
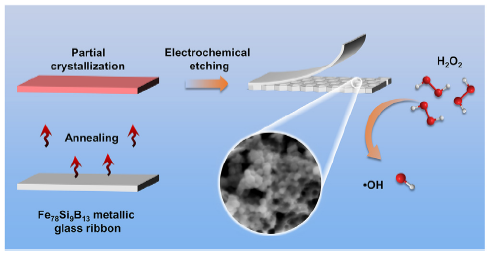
According to aforementioned results and discussion, a schematic illustration has been demonstrated in Fig. 12 for the H2O2 activation process by partially crystallized Fe78Si9B13 ribbons. The annealing of Fe78Si9B13 MG ribbons between 520 and 600 °C induces partial crystallization with generation of α-Fe (Si) and Fe2B phases. The novel surface treatment of partially crystallized ribbons combining external potentiostatic etching strongly promote the catalytic activation efficiency of H2O2, which originates from highly modified surface structure. The electrochemically etched surface produces an efficient honeycomb-like nanoporous structure, where exposed nanocrystals serving as catalytic active sites easily capture free H2O2 in the solution and the galvanic cell effect from α-Fe (Si), Fe2B and residual amorphous phase promotes the electron transfer. Meanwhile, the slightly dissolved Fe-rich oxide layer also acts as effective active sites for the activation of H2O2 to fast generate ·OH, thereby leading to a highly efficient dye degradation.
Fig. 12.
Fig. 12.
Schematic illustration of H2O2 activation process by partially crystallized Fe78Si9B13 ribbons with electrochemical etching.
4. Conclusion
In this work, a facile electrochemical etching method is employed to highly promote the catalytic efficiency of partially crystallized Fe78Si9B13 ribbons for brilliant red 3B-A dye degradation. The annealing process of Fe78Si9B13 MG ribbons induces partial generation of α-Fe (Si) and Fe2B from amorphous phase in their structure, and generation of Fe-rich oxide layer on their surface. Having the best catalytic ability among three different crystallized ribbons (i.e. FeSiB-A520, FeSiB-A560 and FeSiB-A600), FeSiB-A520 by electrochemical etching suggests over 2 times higher reaction rate constants kobs than without electrochemical etching, and the time for complete dye degradation decreases from 45 min to 15 min. Structural analysis indicates that Fe2B phase is gradually dissolved due to electrochemical potential difference of various phases, and nanostructure can be produced by electrochemical etching. With the investigation of optimal processing conditions, the application of 0.3 M H3PO4 as an electrolyte under 180 s electrochemical etching provides effective honeycomb-like nanoporous structure for highly enhanced catalytic ability of partially crystallized ribbons. The employment of other electrolytes such as HNO3 and Na2SO4 solution also strongly improves the catalytic ability, showing the important function of activated Fe-rich oxide layer in the Fenton-like process. The reusability of partially crystallized ribbons also demonstrates that the strong binding effect of surface leads to a low catalytic efficiency with increasing reused times. This work holds the promise to employ electrochemical etching to develop highly efficient environmental catalysts from partially crystallized ribbons.
Acknowledgements
Financial supports from Australian Research Council through Discovery Project (DP130103592) and National Natural Science Foundation of China (Grant No. 51771103) are also gratefully acknowledged.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jmst.2020.06.016.
Reference
DOI
URL
PMID
[Cited within: 1]

With an intrinsically disordered atomic structure and a widely tunable atomic constituent, metallic glasses (MGs) have been extensively studied as promising catalysts in different catalytic fields. Particularly, Fe-based MGs with high catalytic activity, relatively low material cost, and environmental friendly compatibility also emerge as advanced catalysts. This review systematically discusses the recent advances of Fe-based MGs in catalytic applications, including wastewater remediation based on reductive degradation by multicomponent Fe-based MGs, oxidative degradation by introduction of advanced oxidation processes (AOPs) and nanocrystallization applied in Fe-based MGs up to date, and renewable energy conversion, with purposes of revealing Fe-based MG catalysts in the further improvement of catalytic performance and exploiting their promising catalytic abilities in a widely catalytic field.
DOI
URL
PMID
[Cited within: 1]

A multicomponent metallic glass (MG) with highly efficient and anomalous durability for catalyzing water splitting is reported. The outstanding performance of the MG catalyst contributed by self-optimized active sites originates from the intrinsic chemical heterogeneity and selective dealloying on the disordered surface; thus, a new mechanism for improving the durability of catalysts is uncovered.
DOI
URL
PMID
[Cited within: 1]

Distinct corrosion behavior was reported in multiphased titanium alloys prepared by additive manufacturing and by traditional technologies because of different phase constituents formed during processing. An open question is therefore raised: is there always different corrosion behavior of materials prepared by different methods? This work reports resemble corrosion behavior of selective laser melted and wrought single beta-phase Ti-24Nb-4Zr-8Sn (Ti2448) in both NaCl solution and Hank's solution. The electrochemical measurements showed that both samples have close calculated polarization resistance and corrosion potential in NaCl solution, i.e., 4.99 +/- 0.63 MOmega cm(2) and -0.26 +/- 0.01 V for the selective laser-melted Ti2448, and 4.42 +/- 0.71 MOmega cm(2) and -0.25 +/- 0.01 V for the wrought Ti2448, respectively. Both samples reveal the same variation in weight change after 180-day immersion test in Hank's solution. Such resemblance in corrosion behavior without pitting morphologies is attributed to the formation of monolithic beta-phase during processing, which demonstrates that titanium alloys with single phase show comparable corrosion behavior regardless of the manufacturing methods adopted.





 WeChat
WeChat