1. Introduction
With the ever-growing concerns over the global energy crisis and environmental pollution, it is of great importance to develop sustainable and clean energy technologies. Recently, hydrogen has attracted great attention due to its environmental friendliness and renewability as an energy carrier [1,2]. Electrochemical water splitting has been considered as the most promising technique for large-scale hydrogen production, due to the abundance of H2O and low carbon emission of the process. However, the overall energy conversion efficiency of a water splitting unit is largely limited by the sluggish kinetics of oxygen evolution reaction (OER), which creates very high overpotentials. To address this issue, noble metal oxides (such as RuO2 and IrO2) are commonly used to improve the kinetics [[3], [4], [5]]. However, their wide industrial applications are hampered by their high price and rarity. Therefore, high efficiency OER electrocatalysts with earth-abundant materials and elements have become a research focus in recent decade [6].
Till now, extensive research has been devoted to the design of earth-abundant transition-metal-based catalysts, such as alloys, metal sulphides, metal oxides/hydroxides, metal phosphides, and metal nitrides [4,5,[7], [8], [9]]. In particular, Ni-based sulphides (eg. NiS [10,11], Ni3S2 [12], Ni3S4 [13] and Ni9S8 [14]) have drawn huge interest owing to their excellent OER activity, which can be even better than the benchmark catalysts. To optimize electrical conductivity and specific surface area of the prepared catalysts, a conductive substrate with 3D porous architecture is essential. For instance, Zhou et al. fabricated Ni3S2 nanorods on nickel foam (NF) structure through a one-step hydrothermal process, which exhibited excellent OER activity with an onset potential of 1.39 V vs. RHE in 0.1 M KOH [15].
Recent studies have also shown that the incorporation of additional metal elements, such as Fe [[16], [17], [18]], Co [19], Mo [20,21] or Zn [22], could effectively boost the OER performance of catalysts based on nickel sulphide by tuning their electronic structures. Doped with Co, a NiCo2S4/NF structure showed enhanced electrocatalytic activity and long-term stability compared to Ni3S2/NF, with the overpotential decreased from 300 to 260 mV at 10 mA/cm2, and current density loss of 15 % after 50 h in comparison of 20 % after 10 h [19]. Cheng synthesized Fe-doped Ni3S2 particle film on NF with an overpotential of only 253 mV at 100 mA/cm2 [23]. An electrocatalyst containing nickel iron sulphides on nickel foam (NiFeS/NF) showed a high water-splitting efficiency. This bi-functional catalyst led to a low OER overpotential of 231 mV at 100 mA/cm2 [24]. Furthermore, ultrathin Fe hydroxides were electrodeposited on V-doped nickel sulphide nanowires to achieve enhanced OER property and stability, due to the strong interactions between nickel-based sulphides and Fe-based hydroxides [25]. Despite the significant progresses so far, it is still challenging to use low-cost material to prepare efficient OER electrocatalysts with ideal activity and durability.
As one of the most abundant agricultural resources in the world, cotton fibres, which mainly consist of cellulose microfibres, are being widely used in textile, fashion and biomedical industries. In the last few decades, owing to the steady increase of cotton consumption, a vast amount of cotton textile waste has been generated [26]. Similar to other wastes, textile wastes are usually disposed of through landfill or incineration, which have led to serious economic loss and environment pollution. Therefore, many strategies have been exploited to regenerate values from textile wastes. Inspired by the widespread application of porous carbon materials, lots of attention have been paid to transform cotton textiles into carbon structures due to their low cost, high carbon yields and sustainability [[27], [28], [29], [30]]. Many encouraging achievements have been made on the carbonization of cotton materials for the application such as supercapacitor electrode [[31], [32], [33]]. A few studies on OER electrocatalytic behaviour of fibrous cellulose derived carbon materials have also been reported. However, their fabrication methods either involve highly corrosive gas environment (NH3) or tedious fabrication procedure, and more importantly, most of them demonstrated unsatisfying OER performance [[34], [35], [36], [37], [38]]. For example, Zhang et al. synthesized a nitrogen-doped carbon catalyst through a one-step carbonization process of polyacrylonitrile coated cotton cloth, which exhibited an OER overpotential of 351 mV at 10 mA/cm current density [34]. In another study, tissue paper was hydrothermally treated and carbonized into porous carbon with cobalt, nitrogen and sulphur doping. This tri-doped catalyst showed an overpotential of 373 mV at 10 mA/cm2 for OER, and this number increased by 2% after 20 h operation [39].
In this work, we use cotton fabric offcuts as the raw material to fabricate highly active and durable OER electrocatalyst through a simple two-step carbonization process. With a CO2 activation carbonization, cotton fabrics are transformed into a hierarchically porous carbon architecture with a high specific surface area of 1226 m2/g. During the second step of the carbonization process in N2, Fe3O4/NiS nanoparticles are formed on the cotton carbon matrix to obtain fibrous catalyst of Fe3O4/NiS@CC. This composite catalyst exhibits excellent OER performance with a low overpotential of 310 mV at a current density of 10 mA/cm2, along with a small Tafel slop of 82 mV/dec in a 1.0 M KOH solution. Additionally, this ternary catalyst exhibits remarkable long-term stability with no obvious loss in current density after continuous operation for 26 h. It is believed that the outstanding OER performance is contributed by the synergistic effect between the iron oxides and nickel sulphides, as well as the hierarchically micro-meso porous structure of the cotton carbon matrix. This work provides a new strategy to transform cotton wastes into highly active and stable OER electrocatalysts for water-splitting application.
2. Experimental section
2.1. Materials
Plain woven cotton cloth offcuts (140 g/m2) were obtained from Anhui Huamao Textile Co. Ltd, China. All chemicals, including iron (III) nitrate nonahydrate, nickel (II) nitrate hexahydrate, thiourea, 2-propanol, Nafion® perfluorinated resin solution, potassium hydroxide pellet and commercial RuO2 catalyst, were purchased from Sigma-Aldrich (Australia) and used as received.
2.2. Synthesis of M/S/C electrodes
Before the carbonization processes, all cotton fabrics were washed with DI water several times to remove surface impurities. To convert the plain cotton fabrics into porous cotton carbon, the cleaned cotton fabrics were carbonized in a HTFS60-300/12 split tube furnace at 900 °C for 2 h at a temperature ramp rate of 5 °C/min with CO2 as the gas atmosphere, and the obtained cotton carbon is denoted as CC. Then the as-prepared carbon fabric (∼10 mg) was soaked overnight in 20 mL of mixed aqueous solutions containing 1000 ppm of Fe3+, 1000 ppm of Ni2+ and 30 g/L of thiourea. Afterwards, the samples were removed from the solution and inserted into the furnace to go through a second carbonization process at three different carbonization temperatures (800, 900 and 1000 °C) in N2 flow for 1 h, and the final product is denoted as Fe3O4/NiS@CC.
For comparison, S@CC, Fe3O4/S@CC, NiS@CC and Fe3O4/Ni@CC samples were also prepared with the same procedures mentioned above, except that the cotton carbon fabric was impregnated with solutions containing thiourea, Fe3+/thiourea, Ni2+/thiourea or Fe3+/Ni2+ respectively, at the same concentrations before the second carbonization in N2.
2.3. Characterizations
2.3.1. Physical characterizations
To understand the surface area and pore features of different cotton carbon samples, nitrogen sorption isotherms were measured at 77 K on an Autosorb iQ3 instrument (Quantachrome Instruments). X-ray photoelectron spectroscopy (XPS) measurements were collected on a Thermo Scientific EscaLab 250Xi (UK) instrument, equipped with a monochromatic Al Kα source. Raman spectroscopy was performed using a Renishaw Raman spectrometer with a laser wavelength of 514.5 nm. The X-ray diffraction (XRD) patterns were obtained on a PANalytic X-ray diffractometer with Cu radiation of 1.54 Å. The surface morphologies of cotton fabrics and cotton carbon materials were observed on a scanning electron microscope (SEM, Zeiss Supra 55 V P). Transmission electron microscopy (TEM) images and energy dispersive spectroscopy (EDS) mapping were obtained using a JEOL 2100 F instrument at an accelerating voltage of 200 kV.
2.3.2. Electrochemical measurements
The electrochemical activities of different carbonized materials were evaluated on a CHI760D electrochemical workstation with a typical three-electrode cell at room temperature. A pre-polished and cleaned glassy carbon electrode (GCE, 5 mm in diameter), an Ag/AgCl electrode and a Pt wire were used as the working electrode, reference electrode (RE) and counter electrode (CE), respectively. All the recorded potentials were converted to reversible hydrogen electrode (RHE) potentials according to the Nernst equation (ERHE=EAg/AgCl+0.059pH+0.197).
For the rotating disk electrode (RDE) tests, the catalyst inks were prepared by mixing 5 mg of well-ground cotton carbon material or commercial RuO2, 1 mL of 2-propanol and 20 μL of Nafion solution (0.5 wt%). After sonication for 1 h to obtain a homogeneous suspension, 10 μL of the ink was pipetted onto the working electrode, giving a catalyst loading density of ∼250 μg cm-2 after air drying. Before the OER tests, the electrolyte was bubbled with O2 for 1/2 h, and purged continuously during the tests. Cyclic voltammetry (CV) was firstly performed between 1.0 and 1.8 V vs. RHE at a scan rate of 50 mV/s in 1.0 or 0.1 M KOH electrolyte for 20 cycles. Then three cycles of linear sweep voltammetry (LSV) curves were measured from 1.0-1.8 V vs. RHE with a scan rate of 10 mV/s, and the GCE was rotated at 1600 rpm to alleviate the accumulation of evolved oxygen bubbles on the surface. All the LSV curves were iR corrected unless otherwise specified and the data were collected at the second sweep to get a stable current. The onset potential for OER was determined by the joint point between the tangent line of the fast-rising current portion of the current wave and the background current line.
The electrochemical impedance spectroscopy (EIS) measurements were recorded in a frequency range from 100 kHz to 0.1 Hz, with an amplitude of 5 mV at an applied potential of 1.6 V vs. RHE. The lifetime tests were performed on a piece of 1 × 1 cm2 Ni foam loaded with 1 mg/cm2 of the as-prepared catalyst samples. The chronoamperometic (i-t) curves were recorded at 1.58 V vs. RHE in O2-saturated 1.0 M KOH at 1600 rpm.
3. Results and discussion
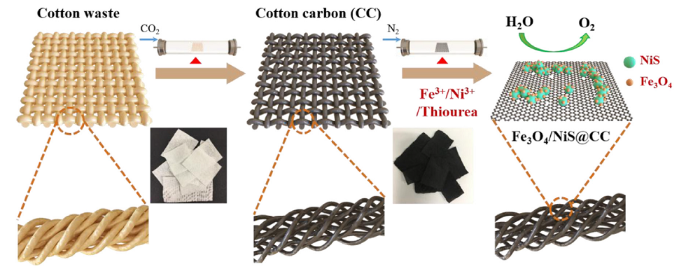
The simple two-step carbonization process to prepare the Fe3O4/NiS@CC catalyst is shown in Fig. 1. After the first CO2 activation carbonization, cotton fabric is transformed into a hierarchically porous carbon material which still inherits its original free-standing structure. After loading iron nitrate, nickel nitrate and thiourea as dopant precursors, iron oxide and nickel sulphide hybrid nanoparticles are formed on the porous carbon substrate via a second carbonization process under N2.
Fig. 1.
Fig. 1.
Schematic illustration of the synthesis route for Fe3O4/NiS@CC.
The SEM images shown in Figs. 2a and S1 reveal the surface morphology of the different carbonized cotton fibres. Compared with the rather smooth surface of cotton carbon doped with sulphur only (S@CC), the surface of the metal-doped fibres is much rougher. Without using sulphur, the catalyst with bimetallic doping (Fe3O4/Ni@CC) shows defective fibre surface, demonstrating the serious etching effect of metal ions to carbon substrate during the carbonization process. However, with sulphuration, this kind of surface defects is rarely observable. The structure of carbonized cotton fibre was further investigated by TEM. The images in Figs. 2b and S2 demonstrate that doping with metal ions can lead to the formation of nanoparticles on the fibres. In comparison to the homogenous carbon structure of S@CC without any nanoparticle, these nanoparticles are uniformly distributed within Fe3O4/NiS@CC, not only anchoring on the fibre surface. It can also be found that the average size (∼100 nm) of the nanoparticles on Fe3O4/NiS@CC is much bigger than that (∼40 nm) of the nanoparticles NiS@CC, and even smaller nanoparticles (∼12 nm) can be observed on Fe3O4/S@CC. From the corresponding EDS elemental mapping results shown in Fig. 2c, it is clear that the catalyst contains four elements of Ni, Fe, S, and O, demonstrating successful doping of the metal and hetero atoms into Fe3O4/NiS@CC. The oxygen mapping result also reveals that O atoms are contained in both carbon matrix and nanoparticles (Figure S3).
Fig. 2.
Fig. 2.
(a) SEM, (b) TEM and (c) TEM-EDX images of Fe3O4/NiS@CC, (d) XRD patterns of Fe3O4/NiS@CC and CC, (e) SAED image of Fe3O4/NiS@CC.
The crystal structure of the CC and Fe3O4/NiS@CC samples was examined by XRD. The broad diffraction peaks at 2θ = 22.1° and 43.6° can be observed on both samples (Fig. 2d), which is the typical structure of activated carbon materials [40,41]. In comparison, five extra small peaks were found on the Fe3O4/NiS@CC catalyst. The peaks at 2θ of 29.4°, 34.0°, 45.2° and 53.0° correspond to (100), (101), (102) and (110) planes of NiS (JCPD: 01-089-1956), and the peak at 2θ of 30.5° is related to (220) plane of iron oxides [42,43]. Similar crystal features can be also observed on the TEM corresponding SAED patterns shown in Fig. 2e.
XPS is generally used to characterize the elemental composition and bonding configuration of different materials. Six elements of C, S, O, N, Ni, and Fe are found from the full survey XPS spectrum of Fe3O4/NiS@CC (Fig. S4). Table 1 summarizes the atomic percentage of these elements on different carbon samples. It can be seen that ∼2% sulphur can be doped into the cotton carbon with the thiourea sulphuration. Moreover, it should be noted that even though oxygen was found on all the examined samples, its content is higher on those samples with iron doping, indicating chemical interactions may exist between iron and oxygen atoms.
Table 1 XPS atomic percentages of different catalysts.
| Samples | S@CC | Fe3O4/Ni@CC | Fe3O4/S@CC | NiS@CC | Fe3O4/NiS@CC |
|---|---|---|---|---|---|
| C 1 s | 94.75 | 91.11 | 88.22 | 92.65 | 87.23 |
| S 2p | 1.64 | 0.13 | 2.58 | 1.98 | 2.09 |
| N 1 s | 0.58 | 0.48 | 0.79 | 0.55 | 0.84 |
| O 1 s | 2.7 | 6.03 | 6.71 | 2.95 | 7.28 |
| Fe 2p | 0.17 | 1.26 | 1.43 | 0.13 | 1.18 |
| Ni 2p | 0.16 | 0.99 | 0.27 | 1.74 | 1.38 |
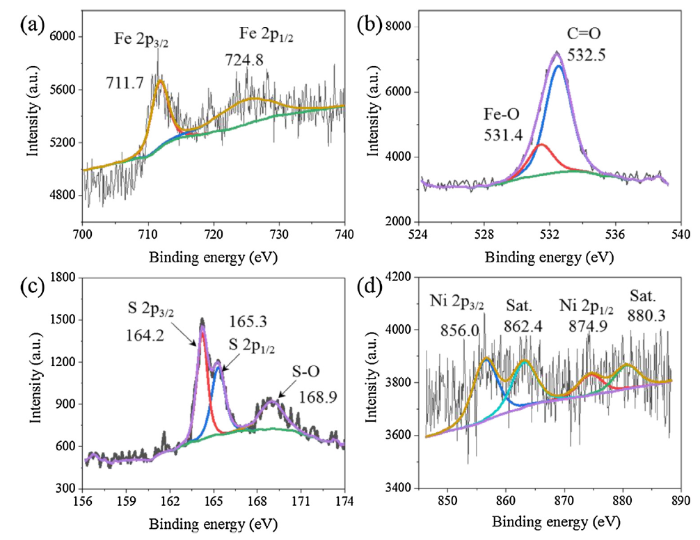
To further explore its chemical bonding details, Fig. 3a shows a high resolution Fe 2p XPS spectrum of Fe3O4/NiS@CC. The peaks at the binding energies of 711.5 and 724.8 eV are finely fitted with two spin-orbit coupling arising from Fe 2p3/2 and Fe 2p1/2 doublets, in which the 2p3/2 peak of Fe at 711.5 eV can be ascribed to a mixed oxidation state of +2 and +3 in Fe3O4 nanoparticles [[43], [44], [45]]. Also, the presence of a shakeup satellite at 719 eV indicates that Fe is mostly in the Fe3+ oxidation state, which is in good agreement with the O 1s spectrum in Fig. 3b, where the peak located at 531.4 eV belongs to the Fe-O bonding in Fe3O4 [46]. In comparison to Fe3O4/NiS@CC, identical findings are observed from the Fe 2p spectrum of Fe3O4/Ni@CC which is doped only by metal ions (Figure S5a). However, the binding energies of Fe3O4/S@CC (Fig. S5b) shift to higher levels, which might be ascribed to the sulphuration effect.
Fig. 3.
Fig. 3.
High resolution XPS spectra of (a) Fe 2p, (b) O 1s, (c) S 2p and (d) Ni 2p of Fe3O4/NiS@CC.
For the high-resolution S 2p spectrum of Fe3O4/NiS@CC in Fig. 3c, the two peaks at 164.2 and 165.3 eV are assigned to the S 2p3/2 and 2p1/2 orbital of divalent sulphide (S2-) in metal-S and C-S units. Moreover, the peak located at 168.9 eV would be related to the sulphur species bonded to oxygen (S—O) from Fe3O4 nanoparticles [47]. Notably, this S—O species are not found on either S@CC or NiS@CC samples, but exists in Fe3O4/S@CC (Figure S6). Similar results can be obtained from the O 1s spectra (Figure S7), implying that oxygen atoms are only bonded to the iron species of these metal doped cotton carbon catalysts. Finally, the Ni 2p spectrum of Fe3O4/NiS@CC catalyst (Fig. 3d) can be deconvoluted into four peaks, in which the peaks at 856.0 and 874.8 eV can be assigned to Ni3+ 2p3/2 and Ni3+ 2p1/2, accompanied by their satellite peaks at 862.4, and 880.3 eV [48,49]. Based on the XPS and element mapping results, it can be concluded that the nanoparticles observed on the TEM images are most likely to be the mixed compounds of Fe3O4 and NiS. From the element weight of Fe (1.18 %), the loading of the Fe3O4/NiS nanoparticles can be estimated to be ∼2.27 % according to the chemical formula.
Fig. 4a shows the nitrogen adsorption-desorption isotherm curves of different cotton carbon samples. All the curves show typical features of mixed І/IV isotherm, with a steep nitrogen adsorption value at very low relative nitrogen pressures (P/P0), and H4 hysteresis loop between the adsorption and desorption curves at P/P0 between 0.4 and 1 due to capillary condensation in mesopores (2-50 nm) [33]. The Barrett Joyner-Halenda (BJH) model was used to obtain the pore size distribution curves (Fig. 4b). Notably, micropores (pore size < 2 nm) with an average size of 1.8 nm can be observed on CC, S@CC, Fe3O4/S@CC and Fe3O4/NiS@CC. Moreover, another pore size peak at 3.0 nm is also shown on Fe3O4/NiS@CC. For NiS@CC and Fe3O4/S@CC samples, the peak value moves to 3.7 nm, and the broader pore size region (3-10 nm) of Fe3O4/Ni@CC is consistent with its more obvious hysteresis loop, suggesting a more severe metal etching effect. These results confirm that the as-prepared cotton carbon materials have hierarchically porous structures with mixed micro and meso pores. From the BET specific surface area (SSA) results shown in Fig. 4c, with the first CO2 activation carbonization, a remarkably high SSA value of 1226 m2/g can be achieved. This large surface area contributes to an excellent porous carbon substrate for hosting precursor compounds of different doping elements. After the second carbonization, the SSA increases gradually in the order of S@CC, Fe3O4/Ni@CC, NiS@CC, Fe3O4/S@CC and Fe3O4/NiS@CC, with the highest value of 1796 m2/g.
Fig. 4.
Fig. 4.
(a) Nitrogen adsorption and desorption isotherm, (b) pore size distribution, (c) BET specific surface area and (d) Raman spectra of different catalysts.
To gain more insights into the carbon structure, Raman spectra of different carbon cotton materials were collected and analysed, as shown in Fig. 4d. Two dominant peaks are present at 1338 cm-1 (D band) and 1590 cm-1 (G band), corresponding to the amorphous and graphitic carbon structure, respectively. Generally, the relative intensity of these two peaks (ID/IG) indicates the overall degree of structural defects, with a higher value suggesting more defects in the porous carbon matrix. Except for a much larger value of NiS@CC, the other samples show similar ID/IG values, implying that the pure NiS nanoparticles may have more interactions with the graphitic carbon, leading to a higher degree of disorder in the carbon structure of this catalyst.
3.1. OER performance
The electrocatalytic OER activity of all the catalysts was investigated in alkaline media (0.1 or 1.0 M KOH) with a standard three-electrode system. After stabilizing with 20 cycles of cyclic voltammetry (CV) scans, the linear sweep voltammetry (LSV) measurements at a scan rate of 5 mV/s was conducted to compare the OER activity of as-prepared carbon materials and commercial RuO2 catalyst.
To optimize the experimental parameters, the effects of doping element precursor and carbonization temperature on the OER performance of S@CC were firstly examined (Figure S8a). The results demonstrate that a thiourea concentration of 30 g/L, Ni2+ concentration of 1000 ppm, Ni2+/Fe3+ ratio of 5:4 and a carbonization temperature of 900 ℃ are the optimal conditions to produce this catalyst with the best performance. These parameters were then used for preparing different cotton carbon catalysts in the following experiments.
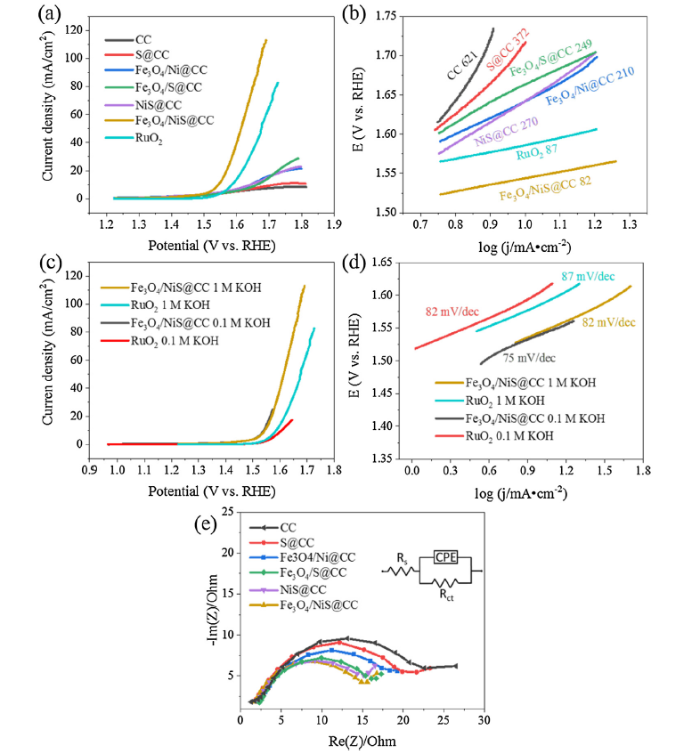
Fig. 5a displays the OER polarization curves of different catalysts in 1 M KOH. The current density of all the synthesized catalysts starts to rise when the scanning potential reaches ∼1.45 V versus RHE, and this value has a negative shift to ∼1.25 V in 0.1 M KOH (Figure S9a), which is not observed on RuO2. Once the potential is higher than 1.50 V, the current density increases rapidly due to the oxygen evolution reaction. This potential is defined as the onset potential, and this value is 1.54 V for RuO2 (Table S1). The overpotential (η) at a current density (j) of 10 mA/cm2 was also calculated to evaluate the OER activity. This potential for Fe3O4/NiS@CC is 310 mV, which is significantly lower than 434 mV of Fe3O4/Ni@CC, 413 mV of NiS@CC, 430 mV of Fe3O4/S@CC, and also 356 mV of RuO2. Excellent results of low onset potential and overpotential@10 demonstrate that our new cotton carbon material overperforms the benchmark OER catalyst. Moreover, this result stands out in the reported OER catalysts derived from cellulose materials, as shown in Table S2. It is deducted that the excellent OER overpotential of the Fe3O4/NiS@CC sample should originate from the synergistic effect of Ni-based sulphides and Fe oxides, and the high current density is ascribed to the ideal porous structure created by CO2 activation carbonization and metal ion etching. The OER kinetics, determined by the Tafel slopes, of the as-synthesized catalysts are demonstrated in Figs. 5b and S9b. The Tafel slope of Fe3O4/NiS@CC is only 82 mV/dec, which is much smaller than that of all the other catalysts including RuO2 (87 mV/dec), suggesting more effective kinetics for water oxidation with more rapid increase of current density by increasing overpotential.
Fig. 5.
Fig. 5.
(a) OER polarization curves of different catalysts in 1 M KOH, (b) Tafel slopes derived from the polarization curves in (a), (c) OER polarization curves of Fe3O4/NiS@CC catalyst in 0.1 and 1 M KOH, (d) Tafel slopes derived from the polarization curves in (c), (e) EIS Nyquist plots of different samples at in 1 M KOH.
For a more profound comparison, the LSV curves of the catalysts obtained with 0.1 M and 1.0 M KOH electrolyte are shown in Figs. 5c and S9. The LSV curves of the Fe3O4/NiS@CC catalyst in 0.1 M and 1.0 M KOH after iR correction almost overlap in the potential range of 1.0-1.6 V, while a lower current density is found on RuO2 catalyst in 0.1 M KOH. The overpotential gap between the as-prepared catalysts and the commercial one in 0.1 M KOH at 10 mA/cm2 increases from 46 to 66 mV. In addition, the Tafel slope shows an even lower value of 75 mV/dec in 0.1 M KOH.
Electrochemical impedance spectroscopy (EIS) is also an important indicator of charge transfer ability of an electrocatalyst. Fig. 5e shows the Nyquist plots of the different catalysts in 1.0 M KOH and the inset Randles circuit was obtained by fitting the EIS spectra with the related electrochemical parameters of Rs (solution resistance), Rct (charge-transfer resistance) and CPE (constant phase element). The calculated Rct values are 13.5, 14.6, 15.1, 17.2, 19.1 and 21.8 Ω for Fe3O4/NiS@CC, NiS@CC, Fe3O4/S@CC, Fe3O4/Ni@CC, S@CC and CC, respectively. The results indicate that Fe3O4/NiS@CC has the highest electrical conductivity, which benefits charge transfer during the OER process.
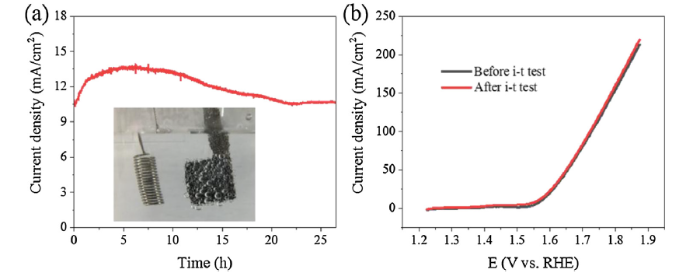
The long-term electrochemical stability of a catalytic electrode is of particular importance for practical applications. Therefore, chronoamperometry measurements at a static potential of 1.58 V vs. RHE were performed on the ternary Fe3O4/NiS@CC catalyst loaded on a Ni foam (1 mg/cm2) in 1 M KOH aqueous solution. As observed in Fig. 6a, the initial current density at 1.58 V is ∼10.5 mA/cm2. In the first five hours, the current density increases to ∼13.7 mA/cm2, which indicates that increasing number of active sites have been functioning for OER reaction. This may be caused by the surface hydrophobicity and oxidation of the nickel species on the cotton carbon materials. [18,[50], [51], [52], [53]] As the electrochemical OER reaction goes on, the air cathode becomes fully wetted by the electrolyte and the maximum amount of OER active sites are accessible with the highest current density. Then in the following 17 h continuous operation (Video S1), the current density gradually goes down before reaching a constant value. There might be two reasons for this decrease [4]: 1) O2 bubbles generated from the Ni foam surface (inset picture in Fig. 5a) can block some active sites on the surface of the catalyst, 2) coated catalyst peels off from the air cathode due to oxygen gas generation. Finally, a stable current density of ∼10.6 mA/cm2 can be retained for at least 4.5 h. In addition, from the OER polarization curves before and after the i-t test, there is no degradation. These results demonstrate the new catalyst has excellent durability for OER in alkaline condition, indicating its great potential for water-splitting application.
Fig. 6.
Fig. 6.
(a) Current-time curve of Fe3O4/NiS@CC catalyst at 1.58 V, (b) OER polarization curves for Fe3O4/NiS@CC catalyst before and after i-t measurements without iR compensation.
4. Conclusions
In this work, we have used a simple two-step carbonization process to turn cotton fabric offcuts from textile industry into highly active and durable OER electrocatalysts. The results demonstrate our carbonization method can effectively introduce iron, nickel and nitrogen elements into fibrous carbon matrix to form Fe3O4/NiS nanoparticles on cotton carbon (Fe3O4/NiS@CC) structure. The composite structure has sufficient active sites, a high specific surface area and an ideal porous structure to achieve a very low overpotential and high durability, both superior to that of commercial RuO2 catalyst. It is believed that its outstanding OER performance is ascribed to the synergistic effect between the iron oxides and nickel sulphides, as well as the micro-meso hierarchical porous carbon structure. Our results not only demonstrate the feasibility of using fibrous materials to prepare low-cost and effective oxygen electrocatalyst, but also indicate a new way of turning waste textiles into valuable products.
Acknowledgements
The authors would like to thank the support from Australian Research Council (ARC) through ARC Centre of Excellence for Electromaterials Science (CE140100012) and ARC Research Hub for Future Fibres (IH140100018). Deakin University’s Advanced Characterization Facility is acknowledged for use of the EM instrument and assistance from Dr Pavel Cizek. This work was performed in part at the Deakin node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and micro-fabrication facilities for Australia’s researchers.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jmst.2020.04.055.
Reference
DOI
URL
PMID
[Cited within: 1]

Searching for the highly active, stable, and high-efficiency bifunctional electrocatalysts for overall water splitting, e.g., for both oxygen evolution (OER) and hydrogen evolution (HER), is paramount in terms of bringing future renewable energy systems and energy conversion processes to reality. Herein, three-dimensional (3D) Ni3FeN nanoparticles/reduced graphene oxide (r-GO) aerogel electrocatalysts were fabricated using precursors of (Ni,Fe)/r-GO alginate hydrogels through an ion-exchange process, followed by a convenient one-step nitrogenization treatment in NH3 at 700 degrees C. The resultant materials exhibited excellent electrocatalytic performance for OER and HER in alkaline media, with only small overpotentials of 270 and 94 mV at a current density of 10 mA cm(-2), respectively. The good performance was attributed to abundant active sites and high electrical conductivity of the bimetallic nitrides and efficient mass transport of the 3D r-GO aerogel framework. Furthermore, an alkaline electrolyzer was set up using Ni3FeN/r-GO as both the cathode and the anode, which achieved a 10 mA cm(-2) current density at 1.60 V with durability of 100 h for overall water splitting. Density functional theory calculations support that Ni3FeN (111)/r-GO is more favorable for overall water splitting since the surface electronic structure of Ni3FeN is tuned by transferring electrons from Ni3FeN cluster to the r-GO through interaction of two metal species. Thus, the currently developed Ni3FeN/r-GO with superior water-splitting performance may potentially serve as a material for use in industrial alkaline water electrolyzers.
DOI
URL
PMID
[Cited within: 1]

Water electrolysis is considered as the most promising technology for hydrogen production. Much research has been devoted to developing efficient electrocatalysts for hydrogen production via the hydrogen evolution reaction (HER) and oxygen production via the oxygen evolution reaction (OER). The optimum electrocatalysts can drive down the energy costs needed for water splitting via lowering the overpotential. A number of cobalt (Co)-based materials have been developed over past years as non-noble-metal heterogeneous electrocatalysts for HER and OER. Recent progress in this field is summarized here, especially highlighting several important bifunctional catalysts. Various approaches to improve or optimize the electrocatalysts are introduced. Finally, the current existing challenges and the future working directions for enhancing the performance of Co-implicated electrocatalysts are proposed.
DOI
URL
PMID
[Cited within: 1]

The activities of the oxygen evolution reaction (OER) on iridium-oxide- and ruthenium-oxide-based catalysts are among the highest known to date. However, the OER activities of thermodynamically stable rutile iridium oxide (r-IrO2) and rutile iridium oxide (r-RuO2), normalized to catalyst mass or true surface area are not well-defined. Here we report a synthesis of r-IrO2 and r-RuO2 nanoparticles (NPs) of approximately 6 nm, and examine their OER activities in acid and alkaline solutions. Both r-IrO2 and r-RuO2 NPs were highly active for OER, with r-RuO2 exhibiting up to 10 A/goxide at 1.48 V versus reversible hydrogen electrode. When comparing the two, r-RuO2 NPs were found to have slightly higher intrinsic and mass OER activities than r-IrO2 in both acid and basic solutions. Interestingly, these oxide NPs showed higher stability under OER conditions than commercial Ru/C and Ir/C catalysts. Our study shows that these r-RuO2 and r-IrO2 NPs can serve as a benchmark in the development of active OER catalysts for electrolyzers, metal-air batteries, and photoelectrochemical water splitting applications.
DOI
URL
PMID
[Cited within: 2]

There is still an ongoing effort to search for sustainable, clean and highly efficient energy generation to satisfy the energy needs of modern society. Among various advanced technologies, electrocatalysis for the oxygen evolution reaction (OER) plays a key role and numerous new electrocatalysts have been developed to improve the efficiency of gas evolution. Along the way, enormous effort has been devoted to finding high-performance electrocatalysts, which has also stimulated the invention of new techniques to investigate the properties of materials or the fundamental mechanism of the OER. This accumulated knowledge not only establishes the foundation of the mechanism of the OER, but also points out the important criteria for a good electrocatalyst based on a variety of studies. Even though it may be difficult to include all cases, the aim of this review is to inspect the current progress and offer a comprehensive insight toward the OER. This review begins with examining the theoretical principles of electrode kinetics and some measurement criteria for achieving a fair evaluation among the catalysts. The second part of this review acquaints some materials for performing OER activity, in which the metal oxide materials build the basis of OER mechanism while non-oxide materials exhibit greatly promising performance toward overall water-splitting. Attention of this review is also paid to in situ approaches to electrocatalytic behavior during OER, and this information is crucial and can provide efficient strategies to design perfect electrocatalysts for OER. Finally, the OER mechanism from the perspective of both recent experimental and theoretical investigations is discussed, as well as probable strategies for improving OER performance with regards to future developments.
DOI
URL
PMID
[Cited within: 1]

Nickel(II) sulfide (NiS) nanosheets with a thickness of 10 nm and a size of 200 nm were facilely grown on stainless steel (SLS) meshes via a one-pot hydrothermal method. This unique construction renders an excellent electrical contact between the porous film of active NiS sheets and the highly conductive substrate, which exhibits a superior catalytic activity toward oxygen evolution reaction (OER). The NiS@SLS electrocatalyst exhibits an unusually low overpotential of 297 mV (i.e., 1.524 V vs RHE) at a current density of 11 mA.cm(-2), and an extra small Tafel slope of only 47 mV.dec(-1) proves an even more competitive performance at high to very high current densities. This performance compares very favorably to other Ni-based catalysts and even to the precious state-of-the-art IrO2 or RuO2 catalyst.






 WeChat
WeChat