1. Introduction
High-entropy alloys (HEAs), which were primarily designed as multicomponent alloys with equi-molar atomic ratio of constituent elements, have attracted enormous attention due to their interesting microstructure and properties [[1], [2], [3], [4]]. HEAs usually contain a single solid solution phase, such as the face-centered cubic (FCC) and body-centered cubic (BCC) phases [[5], [6], [7], [8], [9]]. However, the simple microstructure of a single solid-solution phase limits engineering applications of HEAs [10]. For example, the inferior castability and compositional segregation of single-phase HEAs needs to be improved in case of bulk casting production [11,12]. In addition, single-phase HEAs still exhibit the general trade-off trend regarding the mechanical properties, depending on their crystal structure, i.e. BCC-structured HEAs possess a high strength, but low plasticity, while FCC-structured HEAs show excellent ductility, but limited strength [2,13,14].
As a breakthrough to overcome these disadvantages of single-phase HEAs, Lu et al. [12] proposed a new design concept of eutectic high entropy alloys (EHEAs), and developed AlCoCrFeNi2.1 EHEA consisting of FCC (L12) and BCC (B2) eutectic phases, which gives rise to an improvement of the castability and strength-plasticity trade-off. For example, the AlCoCrFeNi2.1 EHEA has a superior combination of ultimate tensile strength of 1,050 MPa and elongation of 17 % [14]. Moreover, CoCrFeNiTa0.395 EHEA consisting of FCC and Laves eutectic phases shows relatively high compression strength of 2,432 MPa and plastic strain of 27 %, and the high-temperature stability due to the excellent high-temperature resistance of the Laves phases [15].
Various EHEAs have been developed and investigated via experimental approaches, such as AlCoCrFeNiNbx [16], CoFeNixVMoy [17], CoCrFeNiZrx [18], and CoCrFeNiNbx [19] EHEAs. For instance, the CoCrFeNiNbx EHEA [19] was developed based on combining elements possessing eutectic reaction in binary phase diagrams. However, the experimental ways to develop EHEAs require a high amount of experimental trial-and-error. Recently, thermo-physical approaches have been proposed to more scientifically and efficiently design EHEAs, as compared to the experimental manners. The CoCrFeNiTax EHEA [20] was designed based on thermodynamic calculation of thermo-physical parameters, such as valence electron concentration (VEC), atomic size difference (δr), mixing enthalpy (ΔHmix), and mixing entropy (ΔSmix). After that, Lu et al. [21] proposed that the formation principle of constitutive phases in the AlCoCrFeNi2.1 EHEA is strongly dependent upon mixing enthalpy, which in turn results in the formation of different atomic groups: Al-Ni group and Co-Cr-Fe group. According to this strategy, the Ta0.76CoCrFeNi2.1 EHEA made of FCC and Laves phases was successfully designed by substituting Al element with Ta element having molar ratio of 0.76, which is obtained from the ratio of the mixing enthalpy of Al and Ni (-22 kJ mol-1) to that of Ta and Ni (-29 kJ mol-1) [21].
In this work, we systematically fabricated (AlTa0.76)xCoCrFeNi2.1 samples with various values of x to design eutectic high entropy alloys and investigate the microstructure and mechanical properties. The molar ratio of Al and Ta was fixed at a ratio of 1/0.76, considering the mixing enthalpy with Ni. Moreover, Al and Ta contents were adjusted with x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5. Furthermore, the thermo-physical parameters (ΔSmix, ΔHmix, VEC, and δr) of (AlTa0.76)xCoCrFeNi2.1 EHEAs were calculated and analyzed for understanding phase formation and alloy design.
2. Experimental
The alloy ingots were prepared by vacuum-arc-melting with high purity Al, Ta, Co, Cr, Fe and Ni elements (99.95 wt.% elemental purity) under a Ti - gettered high purity argon atmosphere (99.99 %). The ingots were re-melted more than five times for homogeneity and solidified on a water-cooled copper hearth. The as-cast rod samples were fabricated by suction casting into a cylindrical copper mold with 50 mm in length and 3 mm in diameter. The phase identification of the as-cast sample was investigated using X-ray diffraction (XRD, PANalytical/Empyrean/PC) with Cu Kα radiation (λ = 1.5406 Å) and transmission-electron-microscopy (TEM, Tecnai-F20) with energy-dispersive-spectrometry (EDS). The thin specimens for TEM analysis were prepared by ion milling under liquid-nitrogen cooling. The microstructural analysis of as-cast samples was performed by scanning-electron-microscopy (SEM, JEOL JSM-6390; JEOL) and energy-dispersive X-ray (EDX, Hitachi/S-4700/PC). The mechanical properties of as-cast samples were examined by compression tests (at a strain rate of 1 × 10-3 s-1) using a cylindrical sample with an aspect ratio of 2:1.
3. Results and the discussion
3.1. Microstructure and phase identification
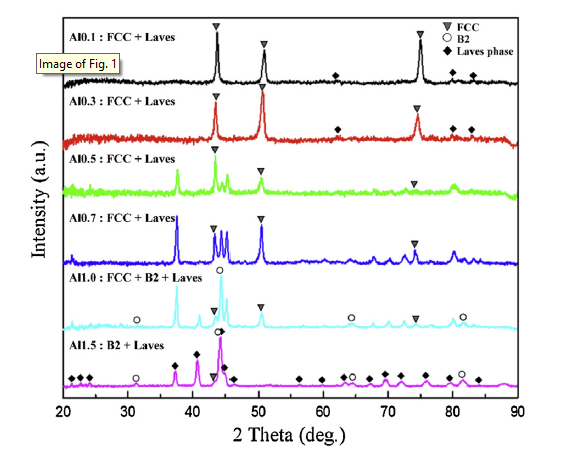
The crystal structures of phases were identified by XRD. The XRD patterns for the as-cast (AlTa0.76)xCoCrFeNi2.1 EHEAs (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5, denoted as Al0.1, Al0.3, Al0.5, Al0.7, Al1.0, and Al1.5) are shown in Fig. 1. Al0.1 and Al0.3 exhibit a high intensity of a face-centered-cubic (FCC) structure and low intensity of a Laves phase. The intensity of the Laves phase continues to increase with increasing x value up to Al1.5, while the intensity of the FCC phase decreases. In addition, reflections of a B2 structure appear in Al1.0 and Al1.5, and become stronger as x value increases.
Fig. 1.
Fig. 1.
XRD patterns of as-cast (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5) alloys.
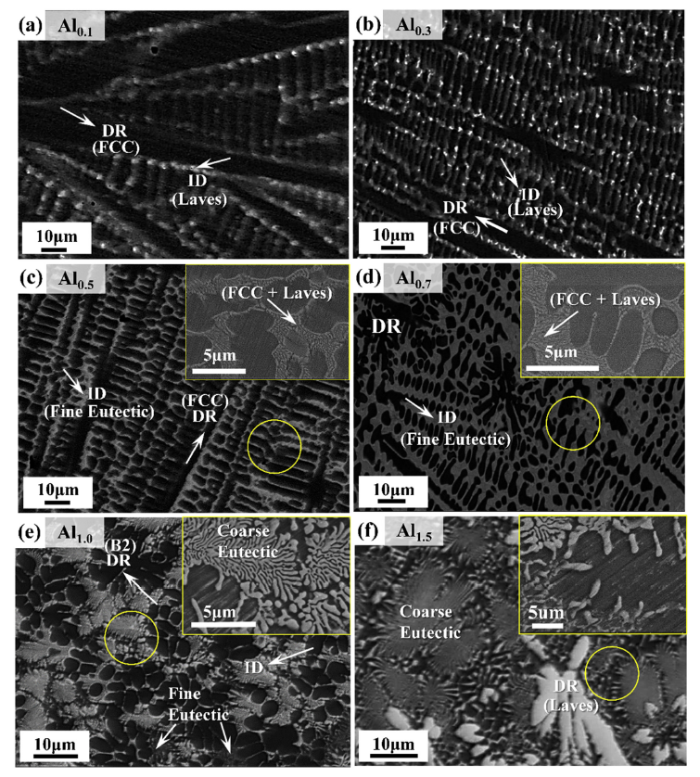
Fig. 2 presents the microstructures of the as-cast (AlTa0.76)xCoCrFeNi2.1 alloys (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5). As can be seen from Fig. 2, all the alloys exhibit dendritic morphology. The Al0.1 alloy in Fig. 2(a) mainly contains the dark dendrites of the FCC phase and bright inter-dendrite of a Laves phase. The microstructures of the Al0.3, Al0.5 and Al0.7 alloys in Fig. 2(b-d) seem similar to that of the Al0.1 alloy, but the careful examination at higher magnification (inset) reveals a fine lamellar structure comprising of the FCC and Laves phases along with the micro-scale FCC dendrites. A further increase of Al and Ta contents, i.e. x = 1.0 and 1.5, leads to a clear transition of the microstructure, as observed in Fig. 2(e) and (f). Specifically, The Al1.0 alloy is composed of two different sized lamellar structures (bimodal eutectic structures), fine one with a lamellar spacing of about 50-150 nm, and the other with that of about 250-600 nm. Moreover, the dark dendritic phase changes to the Laves phase when the value of x increases from 1.0-1.5, as can be seen in Fig. 2(f).
Fig. 2.
Fig. 2.
Back-scattered electron (BSE) SEM images of as-cast (AlTa0.76)xCoCrFeNi2.1 alloys with x = 0.1 (a), 0.3 (b), 0.5 (c), 0.7 (d), 1.0 (e), and 1.5 (f). Insets of (b), (c) and (d) show secondary-electron (SE) SEM images, and (e) and (f) are BSE SEM images at a higher magnification. The dendrite and interdendrite region are marked in DR and ID, respectively.
The chemical analysis on the microstructure of the as-cast (AlTa0.76)xCoCrFeNi2.1 alloys (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5) was conducted by SEM-EDX, which are listed in Table 1. It should be noted that due to the fine length-scale of the lamellar structure, the compositional measurements using the SEM-EDX were made only on the micro-scale dendrites for all the alloys, and more careful examination of the lamellar structures was carried out by TEM, which will be shown in the following section. For Al0.1, Al0.3, Al0.5, and Al0.7 alloys, the FCC dendritic phase is rich in Co, Cr, Fe, and Ni, whereas the dendrite region of Al1.0 is enriched in Al and Ni. Particularly, the compositional stoichiometry of Al and Ni in the dendrite for the Al1.0 indicates the formation of a B2-type NiAl phase, as observed in the XRD results (Fig. 1) and previous reports [12,14,22]. The Laves phase in Al1.5 alloy contains high amount of Ta.
Table 1 Chemical composition in dendrite region of as-cast (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5) alloys (at.%), measured by EDS.
| Alloys | Al | Ta | Co | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Al0.1 | 8.7 | 6.3 | 14.2 | 16.2 | 16.6 | 35.7 |
| Al0.3 | 5 | 5 | 18 | 17.5 | 18 | 36.5 |
| Al0.5 | 8.1 | 6 | 17 | 17 | 17.3 | 34.6 |
| Al0.7 | 13 | 5 | 15.9 | 15.5 | 16.4 | 34.2 |
| Al1.0 | 25.5 | 7.4 | 11.9 | 8.2 | 10.2 | 36.8 |
| Al1.5 | 4.6 | 29.4 | 15.9 | 17.7 | 18.1 | 14.4 |
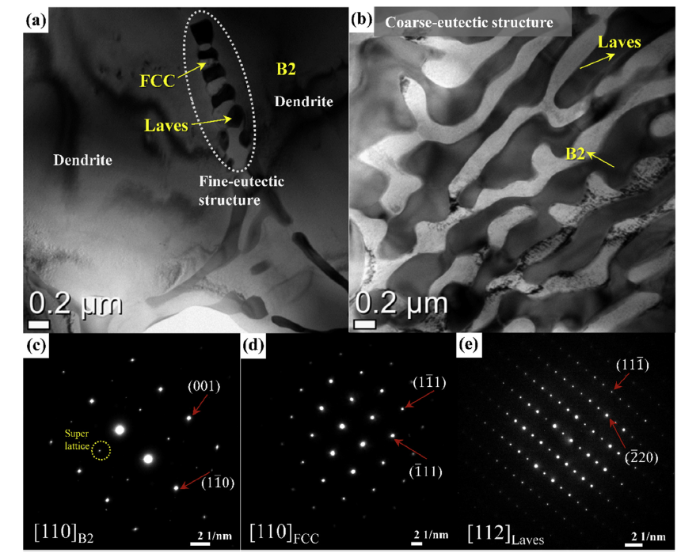
Based on the XRD and SEM results, it was difficult to analyze the dendrite and bimodal eutectic structures in the Al1.0 due to the microstructural complexity and fine length scales. Accordingly, TEM inspection was performed to further identify the crystal structures and compositions of the phases in the Al1.0. Fig. 3(a) and (b) show representative bright-field images of the fine-eutectic and coarse-eutectic structures, respectively. The fine-eutectic structures are mainly observed around inter-dendritic regions, and the TEM selected-area-electron-diffraction (SAED) analyses in Fig. 3(c-e) confirm that the fine eutectic lamellae consist of the Laves and FCC phases, whereas the dendrite is identified as a B2 phase. Furthermore, the coarse eutectic lamellae are composed of the B2 and Laves phases. The chemical compositions of the FCC, B2 and Laves phases were measured by TEM-EDS, and the results are summarized in Table 2. The EDS results clearly reveal that the B2 phase is enriched in Al and Ni, corresponding to the B2-type NiAl phase, while the Laves phase contains a high amount of Ta, Co, Cr, Fe, and Ni, which indicates the Cr, Fe, and Ni elements dissolved into Co2Ta-type HCP structure [20,23]. The FCC phase contains a high amount of Co, Cr, Fe and Ni. These TEM-EDS results are consistent with the SEM-EDX results. Consequently, the TEM results confirm that Al1.0 consists of the B2-structured dendrite, [B2 + Laves]-containing coarse-eutectic, and [FCC + Laves]-containing fine-eutectic structures.
Fig. 3.
Fig. 3.
(a) Bright-field image of the dendrite region and fine-eutectic structure, and (b) coarse-eutectic structure of Al1.0. SAED patterns corresponding to the B2 (c) and FCC (d), and Laves phases (e), respectively.
Table 2 Chemical compositions of the phases in Al1.0 alloy (at.%).
| Phase and microstructure | Al | Ta | Co | Cr | Fe | Ni | |
|---|---|---|---|---|---|---|---|
| Dendrite | B2 | 25.5 | 7.4 | 11.9 | 8.2 | 10.2 | 36.8 |
| Fine lamellar | FCC | 6.9 | 3.4 | 15.7 | 16.8 | 20.4 | 36.8 |
| Laves | 1.8 | 26 | 18.9 | 16.2 | 16.4 | 20.7 | |
| Coarse lamellar | B2 | 25.7 | 2.8 | 10.2 | 3.6 | 12.7 | 45 |
| Laves | 2.6 | 33.8 | 18.2 | 15.9 | 14.4 | 15.1 | |
Based on these microstructural examination, the microstructures of (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5) fall into four distinct groups: (1) Group 1: FCC dendrite + interdendrite of the Laves phase (Al0.1), (2) Group 2: FCC dendrite + fine-eutectic structure containing the FCC and Laves phases (Al0.3, Al0.5 and Al0.7), (3) Group 3: B2 dendrite + bimodal eutectic structure ([FCC + Laves]-containing fine eutectic + [B2 + Laves]-containing coarse eutectic) (Al1.0), (4) Group 4: Laves dendrite + coarse-eutectic structure consisting of the B2 and Laves phases (Al1.5). The microstructural attributes are summarized in Table 3.
Table 3 Microstructural attributes and constitutive phases for (AlTa0.76)xCoCrFeNi2.1 alloys.
| Alloys | Dendrite phase | Eutectic phases | Alloy groups |
|---|---|---|---|
| Al0.1 | FCC | - | 1 |
| Al0.3 | FCC | FCC + Laves | 2 |
| Al0.5 | FCC | FCC + Laves | 2 |
| Al0.7 | FCC | FCC + Laves | 2 |
| Al1.0 | B2 | [B2 + Laves] + [FCC + Laves] | 3 |
| Al1.5 | Laves | B2 + Laves | 4 |
3.2. Compression properties
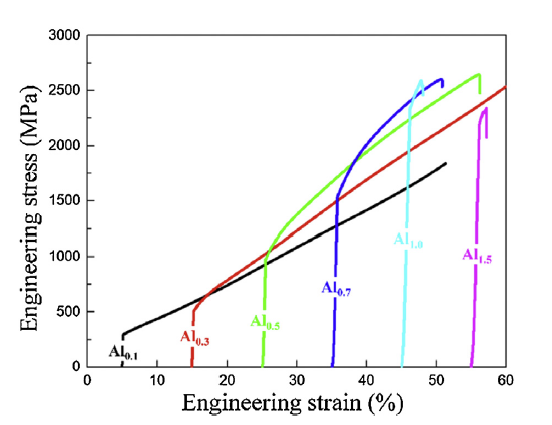
The compressive engineering stress-strain curves of the Al0.1, Al0.3, Al0.5, Al0.7, Al1.0 and Al1.5 alloys are given in Fig. 4, and the values of the yield strength (σ0.2), ultimate strength (σmax), and plastic strain (εp) are denoted in Table 4. The yield stress increases from 209 MPa for Al0.1 to 2336 MPa for Al1.0. The strength increase is associated with an increase in the volume fraction of the fine eutectic structure. However, the high fraction of the eutectic structure leads to a gradual reduction of the plasticity down to 2%. Especially, the Al1.0 exhibits an abrupt drop of the plasticity, as compared to Al0.1-Al0.7 alloys. It is believed that the limited plasticity of the Al1.0 is related to the B2 dendrite and the [B2 + Laves]-containing coarse eutectic structure. This trend can be also seen in the Al1.5 containing the coarse eutectic structure. A slight reduction of the yield strength for the Al1.5, as compared to that of the Al1.0, could imply that the B2 dendrite is harder than the Laves dendrite. According to the analysis of the microstructure and mechanical properties in this alloy series, it was concluded the formation of the coarse-eutectic structure is detrimental to the mechanical properties, and the fine-eutectic structure plays an important role in improving both strength and plasticity simultaneously.
Fig. 4.
Fig. 4.
Compressive engineering stress-strain curves of as-cast (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 1.5) alloys at room temperature (at a strain rate: 1 × 10-3 s-1). Note that the Al0.1 and Al0.3 did not fail under the compression strain up to 50 %.
Table 4 Mechanical properties of as-cast (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5) alloys.
| Alloys | σ0.2 (MPa) | σmax (MPa) | εp (%) |
|---|---|---|---|
| Al0.1 | 293 | No fracture | |
| Al0.3 | 503 | No fracture | |
| Al0.5 | 974 | 2646 | 30.6 |
| Al0.7 | 1533 | 2604 | 15 |
| Al1.0 | 2336 | 2566 | 2 |
| Al1.5 | 2208 | 2342 | 2 |
3.3. Thermo-physical parameters for phase prediction
The thermo-physical parameters have been considered as a factor to predict crystallographic structures of single-phase HEAs, and many researchers have studied the relationship between the phases and thermo-physical parameters, such as ΔSmix (mixing entropy), ΔHmix (mixing enthalpy), VEC (valence electron concentration), and δr (atomic size difference) [[24], [25], [26], [27], [28]]. It is generally believed that the high value of ΔSmix plays a crucial role in stabilizing the solid solution phase [25]. On the other hand, the intermetallic phases are formed when the mixing enthalpy (ΔHmix) of constituent elements has a large negative value, while the elements are separated when the mixing enthalpy is positive or less negative [29]. In this regard, the ΔHmix value could give rise to the formation of intermetallic phases, as observed in diverse eutectic HEAs [21,30]. Therefore, it is believed that the high values of positive ΔSmix and negative ΔHmix are necessary to form eutectic structures containing a solid solution and intermetallic phases in HEAs.
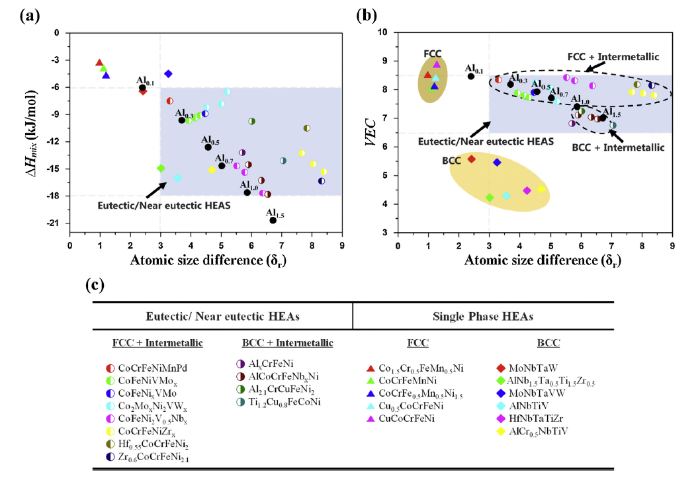
Liu et al. [31] used δr - VEC and δr - ΔHmix plots to evaluate the phase-formation tendency for diverse HEAs, and elucidate the formation of eutectic structures composed of BCC/FCC phases and/or intermetallic compounds. Recently, Chanda [32] proposed a criteria regarding the formation of eutectic structures in HEAs by evaluating the thermo-physical parameters plots, such as δr - ΔHmix and δr - VEC plots, as shown in Fig. 5. Specifically, the eutectic structures in various HEAs are formed when -18≤Hmix≤-6, 6≤VEC≤8.5, and δr>3 [32], as marked by blue rectangular regions in Fig. 5. Based on this previous study, we attempted to calculate the empirical parameters (ΔSmix,ΔHmix, VEC, and δr) for the present (AlTa0.76)xCoCrFeNi2.1 alloys. The ΔSmix,ΔHmix, VEC and δr are defined as following Eqs. (1)-(4), respectively.
where n is the number of elements in an alloy system, ci and cj are the molar fraction of the ith and jth element respectively, and ${{\Omega }_{ij}}=4\Delta H_{mix}^{ij}$, where $\Delta H_{mix}^{ij}$ is the mixing enthalpy of binary alloys. VECi is the valence electron concentration of ith element. ri is the atomic radius of the ith element, and $\bar{r}=\underset{i=1}{\overset{n}{\mathop \sum }}\,{{c}_{i}}{{r}_{i}}$ is the average atomic radius of constituent elements. The above-mentioned parameters for the (AlTa0.76)xCoCrFeNi2.1 alloys, calculated in accordance with the Eqs. (1)-(4), are given in Table 5. Additionally, thermo-physical parameters of the constituent alloying elements required for the calculation, specifically, values of mixing enthalpy, VEC and atomic radius are listed in Table 6 [32,33].
Fig. 5.
Fig. 5.
The Relationships between (a) the mixing enthalpy (ΔHmix) and atomic size difference (δr), (b) the valence electron concentration (VEC) and atomic size difference (δr) for the (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5) alloys, and (c) the table representing typically- reported HEAs with different compositions and microstructures, which are marked by various symbols in (a) and (b). Black circles represent the Alx alloys developed in the present study. Blue square areas represent ranges to form eutectic structures from previous EHEAs, and bright and dark yellow ellipses indicate regimes to form BCC or FCC single-phase HEAs, respectively [32].
Table 5 Calculated values of ΔSmix, ΔHmix, VEC and δr for (AlTa0.76)xCoCrFeNi2.1 alloys according to the Eqs. (1)-(4).
| Alloys | ΔSmix (J k -1 mol-1) | ΔHmix (kJ mol-1) | VEC | δr (%) |
|---|---|---|---|---|
| Al0.1 | 12.05 | -6.02 | 8.465 | 2.4 |
| Al0.3 | 13.09 | -9.63 | 8.183 | 3.7 |
| Al0.5 | 13.69 | -12.6 | 7.93 | 4.57 |
| Al0.7 | 14.05 | -14.66 | 7.71 | 5.02 |
| Al1.0 | 14.37 | -17.61 | 7.406 | 5.86 |
| Al1.5 | 14.54 | -20.67 | 7.01 | 6.71 |
Table 6 Values of VEC and atomic radius among the alloying elements and mixing enthalpies for atomic pairs between elements used in the present study [32,33].
| Al | Ta | Co | Cr | Fe | Ni | ||
|---|---|---|---|---|---|---|---|
| VEC | 3 | 5 | 9 | 6 | 8 | 10 | |
| Atomic radius (pm) | 118 | 146 | 125 | 128 | 126 | 124 | |
| ΔHmix (kJ mol-1) | Al | ‒ | -19 | -19 | -10 | -11 | -22 |
| Ta | ‒ | ‒ | -24 | -7 | -16 | -29 | |
| Co | ‒ | ‒ | ‒ | -4 | -1 | 0 | |
| Cr | ‒ | ‒ | ‒ | ‒ | -1 | -5 | |
| Fe | ‒ | ‒ | ‒ | ‒ | ‒ | -2 | |
| Ni | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
According to the careful consideration between the calculated thermo-physical parameters and microstructural formation, several consistencies between the criteria and experimental results are observed, as follows. First, all the ΔSmix values of (AlTa0.76)xCoCrFeNi2.1 alloys are higher than 12.471 J k-1 mol-1 (1.5R), implying that all the alloys are within a ΔSmix range required to become high entropy alloy [34]. Second, the ΔHmix values of (AlTa0.76)xCoCrFeNi2.1 alloys decrease, as the x value increases due to the large negative mixing-enthalpy between Al/Ta and the other constituent elements (Co, Cr, Fe, Ni) (Table 6). This trend is consistent with our microstructural evolution, for example, the volume fraction of the Laves phase in (AlTa0.76)xCoCrFeNi2.1 alloys increases, as x value increases (Fig. 2). Third, the transformation of the dendrite from FCC to B2 phases (Table 3) can be influenced by decreasing value of VEC (Table 5), since the high value of VEC affects the formation of FCC phase (in contrast, BCC or B2 phase becomes stable when VEC value is low [27]). Forth, the δr value increases as the Ta content of the alloys increases due to the largest atomic radius of Ta (146 pm), as can be seen in Table 5.
Fig. 5(a) and (b) display the relationships between constitutive phases and thermo-physical parameters in δr - ΔHmix and δr - VEC plots for (AlTa0.76)xCoCrFeNi2.1 alloys, respectively. For comparison, previously-reported HEAs are also indicated by different symbols in Fig.5(a) and (b), and their compositions and microstructures are listed in Fig. 5(c). The blue rectangular area represents the range of previously-reported EHEAs, whereas the bright and dark yellow ellipses represent the range of BCC and FCC single-phase HEAs [32]. Fig. 5(a) and (b) exhibit that the EHEAs containing FCC/B2 phases and the Laves phase, such as Al0.3, Al0.5, Al0.7 and Al1.0, simultaneously meet these two empirical criteria (δr - ΔHmix and δr - VEC plots, -18≤Hmix≤-6, 6≤VEC≤8.5, and δr>3). It has been reported that the EHEAs can further fall into two different kinds of eutectic structures, one with a FCC phase and an intermetallic compound (VEC > 7.5), and another with a BCC (B2) phase and an intermetallic compound (VEC < 7.5), as marked by two dotted-ellipses in Fig. 5(b) [32]. This previous trend is also consistent with the current experimental results. For example, the Al0.3, Al0.5, and Al0.7 (VEC > 7.5) consist of the FCC and Laves phases. The Al1.0 is located along the boundary of the blue area for δr - VEC plot, which could be responsible for the formation of the three phases, FCC, B2 and Laves phases.
The Al0.1 and Al1.5 alloys do not meet the two criteria (beyond the blue regimes in Fig. 5(a) and (b)), which seems to make it difficult to design the eutectic high-entropy alloys with excellent mechanical properties. For instance, all the parameters of the Al0.1 are not satisfied (a low value of ΔHmix, and a high value of VEC), which leads to a FCC single-solid solution microstructure with a small fraction of the Laves phase (Fig. 2(a)). This single phase microstructure is effective to improve the plasticity, but reduce the strength. The Al1.5 fulfills the δr - VEC criteria, but not the δr - ΔHmix one. Specifically, the reasonable value of the VEC seems to facilitate the formation of the eutectic structure in the Al1.5. However, the high negative value of the ΔHmix is believed to induce the transition from FCC to ordered B2 phase. Note that the Al1.0 contains FCC, B2, and Laves phase, which indicates the incomplete transition from FCC to B2 phase. As mentioned before, since the increase of the B2 and Laves phases leads to a considerable reduction of the plasticity, from the mechanical properties point of view, the transition from FCC to B2 phase in the present EHEAs containing hard intermetallic compounds seems detrimental.
Based on previous studies and our experimental results, the thermo-physical parameters of EHEAs are reliable and useful to predict the formation of eutectic structures in HEAs. Furthermore, in order to design eutectic high-entropy alloys with excellent mechanical properties via manipulating the constitutive phases, both δr - VEC and δr - ΔHmix should be considered simultaneously.
4. Conclusions
Based on the mixing enthalpy of Al and Ta, (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5) alloys consisting of soft FCC, hard B2 and the Laves phase were designed and investigated. The analyses of phase composition, microstructure, and the thermo-physical parameters (ΔSmix,ΔHmix, VEC and δr) were conducted for understanding complex phase formation and mechanical properties, and certain conclusions were drawn, as follows:
(1) (AlTa0.76)xCoCrFeNi2.1 (x = 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5) alloys were composed of FCC, B2, and the Laves phases. Al0.1 was composed of FCC-structured dendrite and the interdendrite region of Laves phase. As x value increases, interdendrite region changed to fine lamellar structure consisting of FCC and Laves phases (x = 0.3, 0.5, 0.7), exhibiting an increase in the yield stress up to 1533 MPa and a decrease in the plasticity down to 15 %. When x = 1.0, the coarse eutectic structure consisting of B2 and Laves phases appears in the interdendrite region, and the phase transition occurs from FCC to B2 phases, which causes an increase in the yield stress (2336 MPa) and a considerable reduction of the plasticity (2%). Moreover, complete transition occurs from FCC to B2, and the volume fraction of the Laves phase in Al1.5 increases, which deteriorates both yield stress and plasticity.
(2) The thermo-physical parameters (ΔSmix,ΔHmix, VEC and δr) of Alx alloys were calculated to evaluate the formation of the constitutive phases, FCC, B2 and Laves phases. Two criteria (δr -VEC and δr - ΔHmix) were used for empirical analysis on the formation of eutectic phases. Experimental and theoretical comparison shows a reasonable consistency, which demonstrates the reliability and usefulness of these criteria to predict the microstructure of the EHEAs and control a good combination of strength and plasticity for EHEAs.
Acknowledgements
This work was financially supported by the Basic Research Laboratory Program through the Ministry of Education of the Republic of Korea (No. 2019R1A4A1026125) and the Human Resources Development of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean government Ministry of Trade, industry & Energy (No. 20164030201340).
Reference
Two refractory high entropy alloys with compositions near Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20, were produced by vacuum arc-melting. Despite containing many constituents, both alloys had a single-phase body-centered cubic (BCC) structure that remained not only stable after exposure to 1400 degrees C, but also disordered, as confirmed by the absence of superlattice reflections in neutron diffraction data. Compressive flow properties and microstructure development of these alloys were determined from room temperature up to 1600 degrees C. Limited compressive plasticity and quasi-cleavage fracture at room temperature suggest that the ductile-to-brittle transition for these alloys occurs above room temperature. At 600 degrees C and above, both alloys showed extensive compressive plastic strain. The yield stress of both alloys dropped by 30-40% between room temperature and 600 degrees C, but was relatively insensitive to temperature above 600 degrees C, comparing favorably with conventional superalloys. (C) 2011 Elsevier Ltd.





 WeChat
WeChat