1. Introduction
Renewable energy technologies, mainly based on wind and solar energy, are essential for realizing sustainable development. As these renewable energy sources are intermittent, it is vital to store energy produced by these technologies for future applications [[1], [2], [3]]. Therefore, efficient devices are required for energy storage. Supercapacitors (SCs) are promising candidates for such applications due to their fast charge-discharge characteristics, long cycle life, and excellent safety features [[4], [5], [6]]. However, the low energy density is still a bottleneck for the wide application of SCs [[7], [8], [9]]. Therefore, the development of efficient electrode materials is critical for enhancing the energy densities of SCs. So far, a wide range of materials, including porous carbon, metal hydroxides, metal oxides, and conducting polymers, have been explored for the development of supercapacitor electrodes [10,11]. However, the development of high-performance supercapacitor electrodes still remains very challenging.
Supercapacitor electrodes based on 2D materials, such as graphene oxide (GO), and Ni-Co layered double hydroxides (NiCo-LDH) and their analogs, have attracted significant attention in the recent years due to their superior electrochemical performance [3,[12], [13], [14], [15], [16], [17], [18], [19]]. GO shows high surface area, good conductivity, and stability. The NiCo-LDH exhibits excellent electrochemical performances in alkali solution. Given the unique properties of GO and NiCo-LDH nanosheets, the preparation of a hybrid electrode based on GO and NiCo-LDH nanosheets may open new possibilities to improve the performance of supercapacitors. In order to realize high-performance GO/NiCo-LDH electrodes, it is necessary to prevent the aggregation of GO and NiCo-LDH nanosheets as the aggregation of these components reduces the surface area of the electrodes [20,21]. Incorporation of a spacer between the RGO and NiCo-LDH nanosheets may prevent the aggregation of these 2D materials.
In this study, we develop a sandwich-like composite based on GO, polypyrrole (PPy), and NiCo-LDH by incorporating PPy between RGO and NiCo-LDH nanosheets as a spacer. The incorporation of PPy not only decreases the aggregation of GO and NiCo-LDH nanosheets but also improves the stability of the composites. Moreover, PPy also contributes to the pseudocapacitance of the composite electrodes. Due to the presence of NiCo-LDH, the developed RGO/PPy/NiCo-LDH electrode shown a high specific capacitance. On the other hand, the presence of RGO/PPy makes the RGO/PPy/NiCo-LDH electrode very conductive and stable by supporting NiCo-LDH. The specific capacitance of RGO/PPy/NiCo-LDH is 2534 F g-1 at 1 A g-1.
2. Experimental
2.1. Synthesis of RGO/PPy/NiCo-LDH
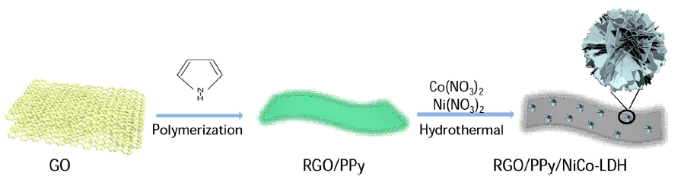
All chemicals used were of analytical grade. GO for the preparation of Sandwich-like RGO/PPy/NiCo-LDH composites was synthesized via the modified Hummers’ method [22]. Sandwich-like RGO/PPy/NiCo-LDH composites were prepared by a two-step process (Fig. 1). In the first step, RGO/PPy was prepared by in-situ polymerization of pyrrole on the surface of GO, based on a previous report [23]. During this step, the GO can be reduced by pyrrole [24]. That is the reason for denoting the composite of GO and PPy as RGO/PPy. In the second step, LDH was coated on RGO/PPy by a simple hydrothermal process. For this, RGO/PPy (0.01 g) was first dispersed in 30 mL deionized water by ultrasonication for 0.5 h. Then, 1.5 mmol Co(NO3)2·6H2O, 4.5 mmol Ni(NO3)2·6H2O, 0.09 g urea, and 0.21 g hexamethylenetetramine were added into the RGO/PPy dispersion. The resulting solution was then transferred into a 100 mL Teflon-lined autoclave and heated at 120 °C for 6 h. After the hydrothermal process, the obtained samples were washed and dried. After drying, a black colored powder (RGO/PPy/NiCo-LDH) was obtained.
Fig. 1.
Fig. 1.
Schematic illustration of the fabrication procedure for RGO/PPy/NiCo-LDH composite.
2.2. Characterization methods
The prepared materials were characterized by scanning electron microscope (SEM), transmission electron microscope (TEM), selected area electron diffraction (SAED), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and BET surface area measurements. The detailed information of the equipment can be found in our previous work [5].
2.3. Electrochemical measurements
The electrochemical tests were performed using IM6e electrochemical workstation (Zahner-Elektrik, Kronach, Germany). The detailed information regarding the preparation of working electrodes and the electrode configuration can be founded in our previous work [25].
An asymmetric cell (ASC) was assembled using RGO/PPy/NiCo-LDH and reduced graphene oxide as the positive and negative electrodes, respectively. The parameters required for the fabrication of the supercapacitor were calculated based on previous work [7].
3. Results and discussion
3.1. Characteristics of composite samples
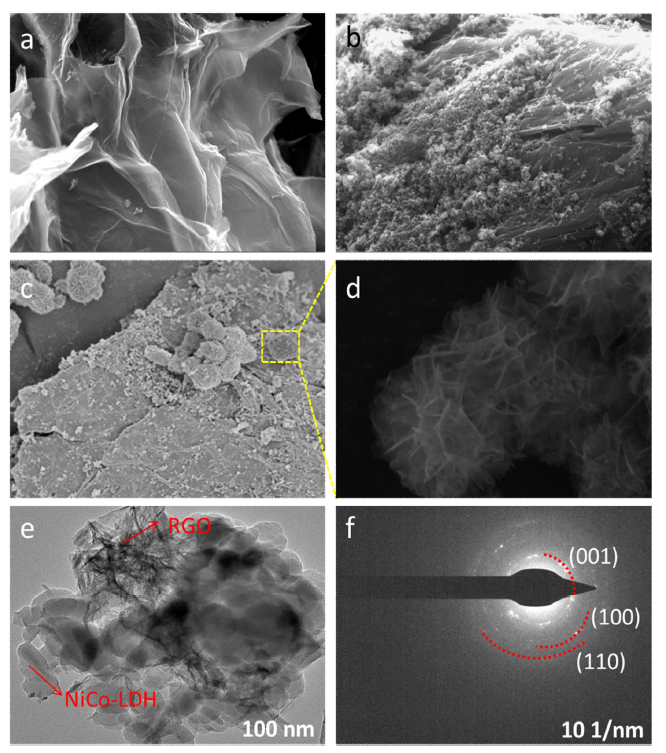
SEM and TEM were used for the morphological and structural characterizations of the developed composites. SEM image of the GO is shown in Fig. 2(a). The SEM shows the the typical wrinkled structure of the GO. Fig. 2(b) shows the SEM image of RGO/PPy composite with a rough surface that prepared by the in-situ polymerization of Pyrrole on RGO. NiCo-LDH was further deposited on the RGO/PPy composite by a hydrothermal method. SEM image RGO/PPy/NiCo-LDH shown in Fig. 2(c) displays the flat surface of the composite. The magnified image shows the interlaced flake-like structure of the NiCo-LDH. (Fig. 2(d)). The TEM image of RGO/PPy/NiCo-LDH was shown in Fig. 2(e). For comparison, the TEM images of RGO and RGO/PPy were also recorded (Fig. S1 in supporting information). It can be seen the RGO/PPy/NiCo-LDH was composed of RGO, PPy, and NiCo-LDH. The SAED pattern shows well-defined diffraction rings and crystal plane one-to-one correspondence. The (001), (100) and (110) of NiCo-LDH can be identified from the rings (Fig. 2(f)). Moreover, EDS mapping for C, O, N, Co, and Ni elements in the composite clearly shows that N, Co, and Ni element homogeneously distributed on the carbon substrate (Fig. S2).
Fig. 2.
Fig. 2.
SEM images of GO (a), RGO/PPy (b), and RGO/PPy/NiCo-LDH (c, d), TEM image of RGO/PPy/NiCo-LDH (e) and SAED pattern of RGO/PPy/NiCo-LDH (f).
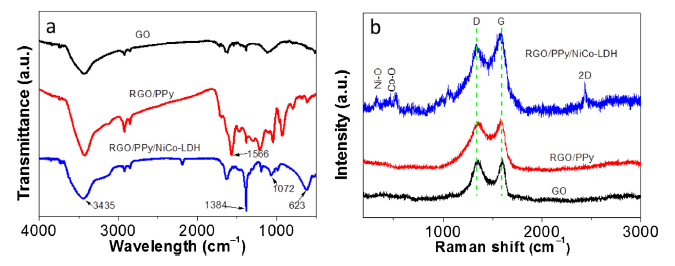
The FT-IR spectra of the GO, RGO/PPy, and RGO/PPy/NiCo-LDH are shown in Fig. 3(a). The broad absorption at 3435 cm-1 represents the stretching of OH bonds in the samples [26]. The band at 1630 cm-1 can be indexed to the stretching vibrations of C=O groups in GO [27]. The absorption peaks at 1566 cm-1 corresponds to the N-H stretching vibrations in PPy ring [28]. The peak at 623 cm-1 in RGO/PPy/NiCo-LDH sample can be indexed to the δ(Ni-O-H) stretching vibrations, while the peak at 1384 cm-1 represents the NO3- vibrations in the NiCo-LDH lattice [29]. These results confirm the presence of NiCo-LDH in the RGO/PPy/NiCo-LDH composite.
Fig. 3.
Fig. 3.
FT-IR spectra (a) and Raman spectra (b) of GO, RGO/PPy, and RGO/PPy/NiCo-LDH.
Fig. 3(b) shows the Raman spectra of the GO, RGO/PPy, and RGO/PPy/NiCo-LDH. The two peaks located at 1350 and 1580 cm-1 represent the D-band and the G-band peak, respectively [30,31]. The intensity ratios of D-band and G-band peak (ID/IG) are generally used to describe the degree of graphitization of carbon. The degree of graphitization increases as ID/IG value lowers. The ID/IG of RGO/PPy/NiCo-LDH is 0.94, which is lower than that of RGO-PPy (0.97) and GO (1.0), indicating a high degree of graphitization of RGO/PPy/NiCo-LDH due to the catalytic reaction of Ni and Co species during the carbonization. Furthermore, an additional peak was found at 2341 cm-1 in RGO/PPy/NiCo-LDH, which is the 2D band owing to the GO [7]. The characteristic peaks at 450 cm-1 and 520 cm-1 represent the Ni-O and Co-O vibrational modes [32], respectively.
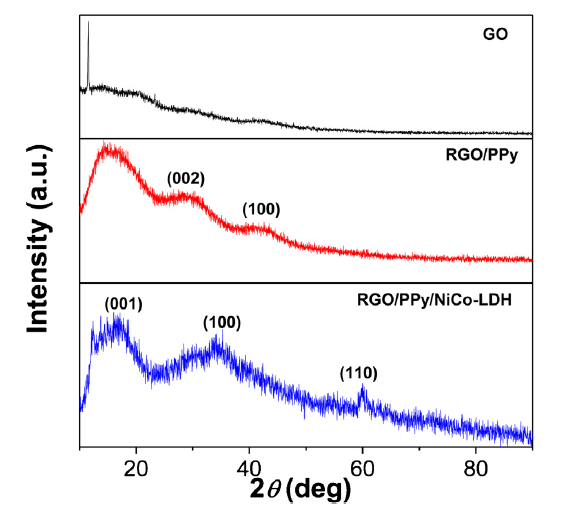
Fig. 4 shows the XRD patterns of the GO, RGO-PPy and RGO/PPy/NiCo-LDH. A peak located at 11.5° in the pattern of GO indicates the presence of a few layers of GO [29]. Two diffraction peaks at around 28° and 43° observed in the GO and RGO-PPy correspond to the (002) and (100) lattice in amorphous carbon structure [33]. The peaks in the XRD pattern of RGO/PPy/NiCo-LDH at 18.3°, 32.6° and 59.6° can be indexed as (001), (100) and (110) planes of hexagonal NiCo-LDH (PDF, #74-1057) [34], which is idential to the SEAD results.
Fig. 4.
Fig. 4.
XRD patterns of the GO, RGO-PPy, and RGO/PPy/NiCo-LDH.
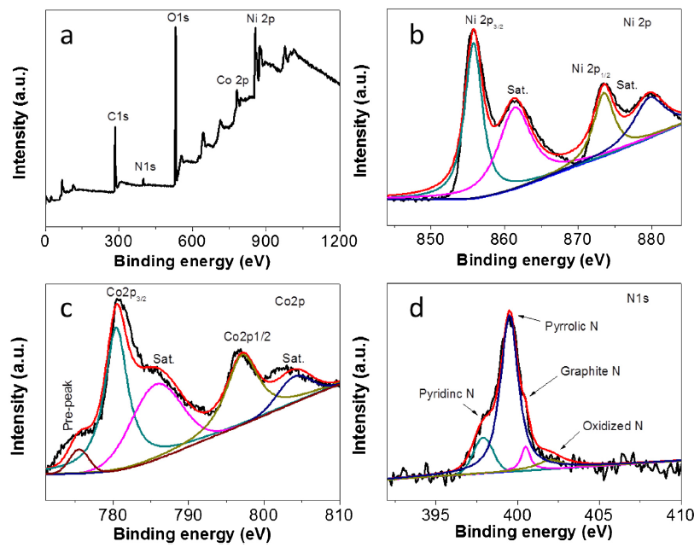
The elemental chemical states of RGO/PPy/NiCo-LDH were investigated using XPS, and shown in Fig. 5. The survey spectrum of RGO/PPy/NiCo-LDH (Fig. 5(a)) demonstrates the presence of Ni, Co, N, O, and C elements. A predominant peak is observed at 531.1 eV, which can be assigned to O 1s, indicating the presence of oxygen on the surface of RGO/PPy/NiCo-LDH. The fitting peaks at 855.8 and 873.6 eV in the Ni 2p spectrum (Fig. 5(b)) represents the Ni 2p3/2 and Ni 2p1/2 spin orbits [35], respectively. Two additional peaks at 861.5 and 880.1 eV can be ascribed to the shake-up satellites of Ni 2p3/2 and Ni 2p1/2, respectively. These results confirm the presence of Ni2+ in the composite [36]. In the same manner, two peaks at 780.4 and 797.1 eV in the Co 2p spectrum corresponds to the Co 2p3/2 and Co 2p1/2, and their shake-up satellites are located at 785.8 and 803.9 eV (Fig. 5c). These results indicate that Ni and Co ions are in the divalent state in the RGO/PPy/NiCo-LDH composite. The XPS spectra of N 1s is shown in Fig. 5(d). Four peaks can be identified including pyridine N (397.9 eV), pyrrole N (399.5 eV), graphite N (401.0 eV) and oxidize N (420.0 eV) [[37], [38], [39]].
Fig. 5.
Fig. 5.
XPS survey spectrum of RGO/PPy/NiCo-LDH (a), high-resolution XPS of Ni 2p (b), Co 2p (c), and N 1s (d).
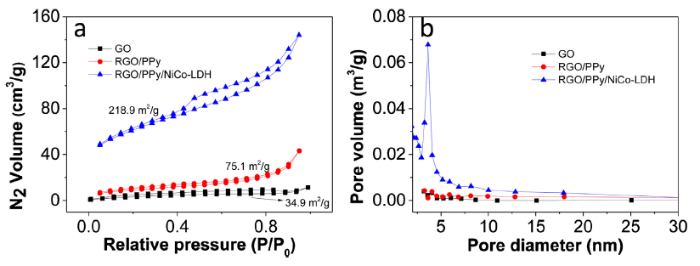
The nitrogen adsorption-desorption isotherms of GO, RGO/PPy, and RGO/PPy/ NiCo-LDH are shown in Fig. 6(a). The N2 adsorption-desorption isotherm of RGO/PPy/NiCo-LDH exhibits an apparent hysteresis loop in the range from 0.40 to 1.00 P/P0 and a sharp increase of N2 adsorption at about 1.00 P/P0, indicating the existence of mesopores and macropores. The specific surface area of RGO/PPy (75.1 m2 g-1) is two times higher than GO (34.9 m2 g-1), indicating PPy increases the surface area of GO substantially. The surface area was further boosted to 218.9 m2 g-1 after the growth of NiCo-LDH on RGO/PPy. The pore-size distribution of RGO/PPy/NiCo-LDH in Fig. 6(b) shows a sharp peak at about 4.0 nm, confirming the existence of mesopores. Due to the porous structure, RGO/PPy/NiCo-LDH possesses a large surface area, which is favorable for the charge transfer and ion transportation [40].
Fig. 6.
Fig. 6.
N2 adsorption-desorption isotherms (a) and pore-size distribution curves (b) of GO, RGO/PPy, and RGO/PPy/NiCo-LDH.
3.2. Electrochemical characterization of RGO/PPy/NiCo-LDH
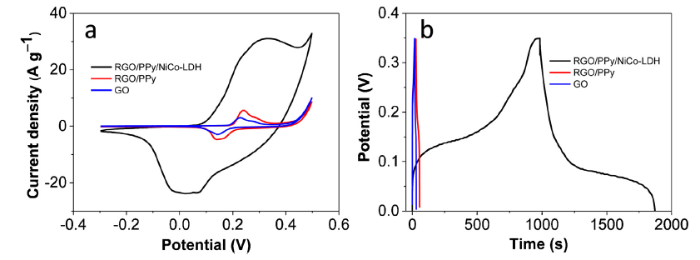
The electrochemical performance of the samples was assessed by cyclic voltammograms (CV) and galvanostatic charge-discharge (GCD) in a three-electrode cell with 6 M KOH. All electrodes show a pair of redox peaks around 0.2 V (Fig. 7(a)), which originate from the nickel foam [41]. RGO/PPy electrode demonstrates larger CV area than that of GO electrode, which is mainly due to the pseudocapacitance of the PPy and large surface area of RGO/PPy [42]. The area of the CV curve dramatically increased after the introduction of NiCo-LDH, indicating excellent pseudocapacitive behavior. The anodic and cathodic peaks of RGO/PPy/NiCo-LDH are symmetric, indicating that the electrochemical reactions occurring on the electrode are highly reversible. The chemical reactions occurring on NiCo-LDH of RGO/PPy/NiCo-LDH can be described by the following equations [29].
Fig. 7.
Fig. 7.
Electrochemical characterization of GO, RGO/PPy, and RGO/PPy/ NiCo-LDH electrodes: (a) CV curves at 10 mV s-1; (b) GCD curves at 1 A g-1.
The galvanostatic charge-discharge (GCD) curves of GO, RGO/PPy, and RGO/PPy/ NiCo-LDH electrodes at 1 A g-1 are shown in Fig. 7(b). The non-linerarity in the GCD curves indicates the contribution of redox reaction from the composite. The specific capacitance calculated form GCD curves of RGO/PPy/NiCo-LDH is 2534 F g-1, which is much higher than the values obtained for the RGO/PPy (72 F g-1) and GO (35 F g-1) at the same current density. The impedances of the electrodes were also recorded (Fig.S3). The calculated charge transfer resistance (Rct) for the GO, RGO/PPy, and RGO/PPy/NiCo-LDH electrodes were 0.4, 5, 6 ohm, respectively, which further proves the RGO/PPy/NiCo-LDH electrode possesses excellent electrochemical performance. Table 1 compares the specific capacitances of some of the recently reported materials with the results of this work, which shows the superior energy storage characteristics of the electrodes based RGO/PPy/NiCo-LDH composites. The outstanding performance of RGO/PPy/NiCo-LDH electrode can be attributed to three reasons: First, The short ion diffusion path of the sandwich-like composite electrodes due to the presence of plenty of mesopores and macropores. Second, the large surface area of RGO/PPy that provide lots of active sites for the growth of NiCo-LDH. Third, the efficient utilization of NiCo-LDH for charge storage.
Table 1 Comparison of the specific capacitance of RGO/PPy/NiCo-LDH electrode with some of the recent reports.
| Electrode materials | Current density (A g-1) | Specific capacitance (F g-1) | Ref. |
|---|---|---|---|
| Co(OH)2/Ni-Co LDH | 1 | 825 | [20] |
| Co9S8@Ni-Co LDH | 1.25 | 1020 | [34] |
| Ni(OH)2/Co(OH)2/GO | 1 | 2050.6 | [43] |
| Ni(OH)2/PNTs | 1 | 864 | [44] |
| PPy/Ni(OH)2/SGO | 2.8 | 1632.5 | [45] |
| Ni(OH)2/graphene sheets | 0.5 | 1335 | [46] |
| Co(OH)2 nanowires | 5 | 358 | [47] |
| Ni(OH)2/CNTs hybrids | 3 | 1244.2 | [48] |
| Ni-Co binary hydroxides | 5 | 1030 | [49] |
| Ni(OH)2/3D graphene | 1 | 1450 | [50] |
| RGO/Ni-Co-OH | 1 | 1622 | [51] |
| Carbon cloth/N-doped-LDH | 1 | 1817 | [52] |
| NiCo2O4/RGO | 0.5 | 947.4 | [53] |
| NiCo-LDH/RGO | 2 | 1911.1 | [54] |
| RGO/Ag/NiCo2S4 | 2 | 2438 | [55] |
| RGO/PPy/NiCo-LDH | 1 | 2534 | This work |
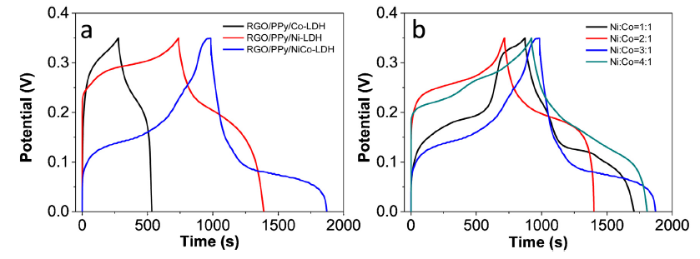
To understand the role of PPy in the electrochemical performance of RGO/PPy/NiCo-LDH composites, the composite without PPy (RGO/NiCo-LDH) was also prepared for comparison. The RGO/NiCo-LDH electrode shows low specific capacitance (1130 F g-1) (Fig. S4), compared to the specific capacitance of RGO/PPy/NiCo-LDH (2534 F g-1), which indicates that PPy plays vital role in the electrochemical performance of RGO/PPy/NiCo-LDH. Moreover, PPy is a hydrophilic conducting polymer. PPy not only increase the surface area by separating the nanosheets but also provides active sites for the growth of NiCo-LDH. Also, PPy also contributes to the capacitance of the composite electrodes. The composition of the RGO/PPy/NiCo-LDH was further optimized to obtain the best electrochemical performance. It can be seen from Fig. 8(a) that the RGO/PPy/NiCo-LDH demonstrates longer discharge time than RGO/PPy/Co-LDH and RGO/PPy/Ni-LDH, indicating the combination of Co-LDH and Ni-LDH are beneficial to their electrochemical performance. The synergistic effect between the Co-LDH and Ni-LDH reduces the thermodynamic barrier and thus facilitates the electron transfer in the composite [56]. The influence of Ni/Co ratio on the electrochemical properties of RGO/PPy/NiCo-LDH was also investigated (Fig. 8(b)). The composite shows largest specific capacitance when Ni/Co ratio is 3:1. Therefore, the Ni/Co ratio of 3:1 was selected for the preparation of the RGO/PPy/NiCo-LDH composite.
Fig. 8.
Fig. 8.
(a) GCD curves of RGO/PPy/Co-LDH, RGO/PPy/Ni-LDH, and RGO/PPy/ NiCo-LDH at 1 A g-1 and (b) GCD curves of RGO/PPy/NiCo-LDH with various Ni/Co ratios at 1 A g-1.
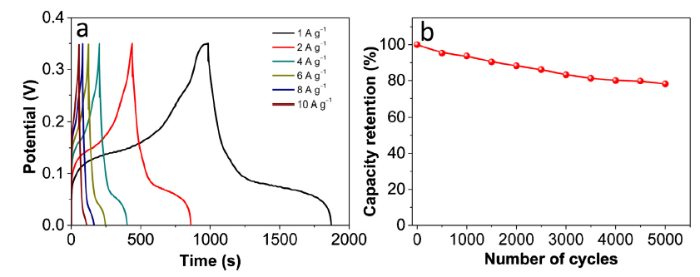
Fig. 9(a) shows GCD curves of the RGO/PPy/NiCo-LDH electrode at different current densities. The specific capacitances of the electrode are 2534, 2411, 2278, 2084, 1928 and 1837 F g-1 at current densities of 1, 2, 4, 6, 8 and 10 A g-1, respectively. When the current density increased to 10 A g-1, the electrode can retain 72.5 % of the initial specific capacitance. The high surface area, short ion diffusion path, and high electrical conductivity of the electrodes are mainly responsible for this superior electrochemical performance.
Fig. 9.
Fig. 9.
Electrochemical performance of RGO/PPy/NiCo-LDH: (a) GCD curve at various current densities; (b) cycling performance at 1 A g-1.
Cycling stability is a crucial factor in deciding the practical applications of the supercapacitors. As shown in Fig. 9(b), the RGO/PPy/NiCo-LDH electrode retains 78 % of its capacitance after 5000 cycles, which is competitive to reported pseudocapacitive electrode (Table S1). This superior performance indicates the excellent electrochemical stability of sandwichlike RGO/PPy/NiCo-LDH composite electrodes.
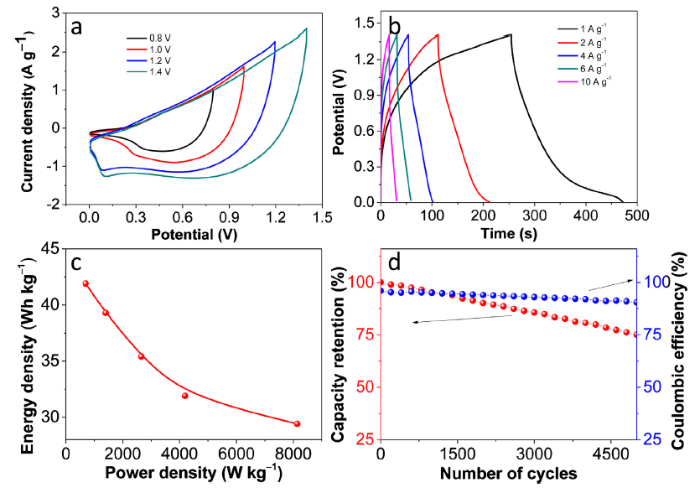
The electrochemical performance of the RGO/PPy/NiCo-LDH electrode was further accessed by fabricating an asymmetric supercapacitor (ASC). RGO/PPy/NiCo-LDH and RGO were used as the positive and negative electrodes, respectively. The loading masses of RGO/PPy/NiCo-LDH and RGO were 9 and 45.8 mg, respectively. Fig. 10(a) shows the CV curves of the RGO/PPy/NiCo-LDH//RGO asymmetric cell at various potential windows (0.8-1.4 V). No severe polarization was observed up to 1.4 V. Therefore; this value was selected for performance analysis of the developed ASC. Fig. 10(b) shows the rate performance of the ASC. The specific capacitance of the ASC is 154 F g-1 at 1 A g-1, and it retains 71 % its capacitance (108 F g-1) when current density increases to 10 A g-1.
Fig. 10.
Fig. 10.
Electrochemical performance of PPy/NiCo-LDH//RGO ASC: (a) CV curves at 5 mV s-1; (b) GCD curves; (c) Ragone plots; (d) cycling test and coulombic efficiency at 1 A g-1.
Fig. 10(c) shows the Ragone plots of the RGO/PPy/NiCo-LDH//RGO ASC. This device shows the energy density of 41.9 Wh kg-1 at a power density of 698 W kg-1. The energy density is 29.4 Wh kg-1 at a power density of 8141 W kg-1. The energy density of RGO/PPy/NiCo-LDH//RGO ASC is comparable when compared to some of the recent reports, such as NiCo-MOF//AC ASC (49.4 Wh kg-1 at 562.5 W kg-1) [1], RGO@NiMn-LDH@NF//AC (22.5 Wh kg-1 at 700 W kg-1) [19], RGO-MnNiCo//RGO (35.6 Wh kg-1 at 699.9 W kg-1) [22], Ni - Co LDH@RGO//RGO (35 Wh kg-1 at 750 W kg-1) [29], CoOx//graphene (44.06 Wh kg-1 at 800 W kg-1) [57], NiCo2O4-RGO//AC (23.3 Wh kg-1 at 324.9 W kg-1) [58]. Furthermore, RGO/PPy/NiCo-LDH//RGO shown an excellent cycle life. It can retain 72 % of the original capacitance (with 90 % coulombic efficiency) after 5000 charge-discharge cycles (Fig. 10(d)), indicating the excellent energy storage characteristics.
4. Conclusion
A sandwich-like hybrid composite containing reduced graphene oxide, polypyrrole and NiCo-LDH was prepared for the development of high-performance supercapacitors. The hybrid RGO/PPy/NiCo-LDH electrode shows a high specific capacitance (2534 F g-1 at 1 A g-1), a good rate capability and an excellent cycling stability (78 % after 5000 cycles). Furthermore, an ASC based on RGO/PPy/NiCo-LDH as the anode and RGO as the cathode exhibits a superior specific capacitance of 154 F g-1 at 1 A g-1 with a stable voltage window of 1.4 V. The energy density of the ASC is 41.9 Wh kg-1 at 698 W kg-1 and still maintains 29.4 Wh kg-1 at 8141 W kg-1. The ASC also exhibits excellent cyclic stability with 72 % retention of its initial capacitance after 5000 cycles. These results highlight the prospect of the developed supercapacitor based on RGO/PPy/NiCo-LDH.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51861005 and 51861004), the Innovation Project of Guangxi Graduate Education (No. YCSW2019149) and Guangxi Natural Science Foundation (No. 2017AD23029).
Appendix A. Supplementary data
Supplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.jmst.2019.10.030.
Reference
DOI
URL
PMID
[Cited within: 1]

By changing the mixed metal sulfide composition, morphology tuning of an active electrode material can be possible, which can have a huge impact on its electrochemical performance. Here, effective morphology tuning of Ni-Co layered double hydroxide (LDH)/MMoS x (M = Co, Ni, and Zn) heteronanostructures is demonstrated by varying the composition of MMoS x. Taking advantage of the benefits associated with Kirkendall growth and Ostwald ripening, tunable morphologies were successfully achieved. Among the Ni-Co LDH/MMoS x (M = Co, Ni, and Zn) heteronanostructures, a Ni-Co LDH/NiMoS x core-shell structured electrode delivered a high specific capacity of 404 mAh g(-1) at 3 mA cm(-2) and an extraordinary cycling stability (after 10 000 cycles) of 93.2% at 50 mA cm(-2). In addition, an asymmetric supercapacitor (ASC) device coupled with Ni-Co LDH/NiMoS x as the cathode and Fe2O3/reduced graphene oxide as the anode exhibited excellent cell capacity and extraordinary cycling stability. Moreover, the ASC device provided a very high specific energy of 72.6 Wh kg(-1) at a specific power of 522.7 W kg(-1) and maintained the specific power of 23.5 Wh kg(-1) at 5357.6 W kg(-1), demonstrating its high applicability to energy storage devices.
DOI
URL
PMID
[Cited within: 2]

Hybrid reduced graphene oxide (RGO) nanosheet supported Mn-Ni-Co ternary oxides (MNCO) are prepared through a facile coprecipitation reaction with a subsequent calcination process as electrodes for supercapacitors. Electrochemical measurements prove that RGO can significantly improve the supercapacitive behaviors, compared with the pure MNCO electrode. A high specific capacity of 646.1 C g(-1) at 1 A g(-1) can be achieved and about 89.6% of the capacity can be remained at 30 A g(-1) relative to that of the low-current capacity, indicating attractive rate capability of the RGO-MNCO electrode. Moreover, an asymmetric supercapacitor (ASC) device is fabricated with nitrogen-enriched RGO as the negative electrode and the synthesized RGO-MNCO as the positive electrode. Electrochemical performances investigated at different potential range reveal that the ASC device presents excellent capacitive behavior and reversibility. A maximum energy density of 35.6 Wh kg(-1) at power density of 699.9 W kg(-1) can be delivered. Furthermore, stable cycle capability with 100% Coulombic efficiency and 77.2% the capacitance retention is also achieved after 10000 cycles. The achieved outstanding electrochemical properties indicate that the obtained RGO-MNCO electrode materials are fairly ideal for progressive supercapacitors.
DOI
URL
PMID
[Cited within: 4]

The discovery of graphene oxide (GO) has made a profound impact on varied areas of research due to its excellent physicochemical properties. However, surface engineering of these nanostructures holds the key to enhanced surface properties. Here, we introduce surface engineering of reduced GO (rGO) shells by radially grafting Ni-Co layered double hydroxide (LDH) lamella on rGO shells to form Ni-Co LDH@rGO. The morphology of synthesized Ni-Co LDH@rGO mimics dendritic cell-like three-dimensional (3D) hierarchical morphologies. Silica nanospheres form self-sacrificial templates during the reduction of GO shells to form rGO shells during the template-assisted synthesis. The radial growth of LDH lamellae during hydrothermal process on GO shells provides access to a significantly larger number of additional active redox sites and overcompensates the loss of pseudocapacitive charge storage centers during the reduction of GO to form rGO shells. This enables in the synthesis of novel surface-engineered rGO nanoshells, which provide large surface area, enhanced redox sites, high porosity, and easy transport of ions. These synthesized 3D dendritic cell-like morphologies of Ni-Co LDH@rGO show a high capacitance of approximately 2640 F g(-1). A flexible hybrid device fabricated using this nanomaterial shows a high energy density of approximately 35 Wh kg(-1) and a power density of 750 W kg(-1) at 1 A g(-1). No appreciable compromise in device performance is observed under bending conditions. This synthesis strategy may be used in the development of functional materials useful for potential applications, including sensors, catalysts, and energy storage.
DOI
URL
PMID
[Cited within: 2]

CoNi layered double hydroxides (LDH) and related monometallic hydroxides (Ni(OH)2 and Co(OH)2) were synthesized by a facile, simple and inexpensive method under mild condition (50 degrees C). The resulting products displayed a unique honeycomb-like nanoflakes array assembled two-dimensional (2D) thin sheets structure. Among them, CoNi-LDH thin sheets delivered higher specific capacity (394.5Cg(-1) at 1Ag(-1)) with superior cyclic performance (92.3% capacity retention over 10,000 cycles) than Co/Ni monometallic hydroxides owing to the synergistic effect of cobalt and nickel. Afterward, hybrid supercapacitors (HSC) devices were fabricated using the as-obtained products (CoNi-LDH, Co(OH)2 and Ni(OH)2 thin sheets) and activated carbon (AC) as the positive and negative electrode, respectively. The operating voltage of the devices can be extended to 1.6V. What's more, the assembled CoNi-LDH HSC device exhibited a maximum energy density of 20.38Whkg(-1) at the power density of 800Wkg(-1). Consequently, these outstanding electrochemical performances of the CoNi-LDH thin sheet endow it with great potential to be implemented in HSCs or other energy storage systems.
DOI
URL
PMID
[Cited within: 1]

Highly porous nanostructures with large surface areas are typically employed for electrical double-layer capacitors to improve gravimetric energy storage capacity; however, high surface area carbon-based electrodes result in poor volumetric capacitance because of the low packing density of porous materials. Here, we demonstrate ultrahigh volumetric capacitance of 521 F cm(-3) in aqueous electrolytes for non-porous carbon microsphere electrodes co-doped with fluorine and nitrogen synthesized by low-temperature solvothermal route, rivaling expensive RuO2 or MnO2 pseudo-capacitors. The new electrodes also exhibit excellent cyclic stability without capacitance loss after 10,000 cycles in both acidic and basic electrolytes at a high charge current of 5 A g(-1). This work provides a new approach for designing high-performance electrodes with exceptional volumetric capacitance with high mass loadings and charge rates for long-lived electrochemical energy storage systems.
DOI
URL
PMID
[Cited within: 1]

A new class of nitrogen-doped ordered mesoporous carbon/silica (N-OMC/SiO2) nanocomposites was successfully fabricated via a multi-constituent co-assembly strategy. The N-OMC/SiO2 nanocomposite presented a unique interpenetrating carbon/silica structure whose carbon/silica interface is highly uniform, and thus demonstrated high capacity, good cycling and excellent rate properties.
DOI
URL
PMID
[Cited within: 1]

Ni(OH)(2) nanocrystals grown on graphene sheets with various degrees of oxidation are investigated as electrochemical pseudocapacitor materials for potential energy storage applications. Single-crystalline Ni(OH)(2) hexagonal nanoplates directly grown on lightly oxidized, electrically conducting graphene sheets (GS) exhibit a high specific capacitance of approximately 1335 F/g at a charge and discharge current density of 2.8 A/g and approximately 953 F/g at 45.7 A/g with excellent cycling ability. The high specific capacitance and remarkable rate capability are promising for applications in supercapacitors with both high energy and power densities. A simple physical mixture of pre-synthesized Ni(OH)(2) nanoplates and graphene sheets shows lower specific capacitance, highlighting the importance of direct growth of nanomaterials on graphene to impart intimate interactions and efficient charge transport between the active nanomaterials and the conducting graphene network. Single-crystalline Ni(OH)(2) nanoplates directly grown on graphene sheets also significantly outperform small Ni(OH)(2) nanoparticles grown on heavily oxidized, electrically insulating graphite oxide (GO), suggesting that the electrochemical performance of these composites is dependent on the quality of graphene substrates and the morphology and crystallinity of the nanomaterials grown on top. These results suggest the importance of rational design and synthesis of graphene-based nanocomposite materials for high-performance energy applications.




 WeChat
WeChat