1. Introduction
Utilization of resources in a rational and effective manner has gained great attention. Presently, thermal insulation materials are used popularly in energy conservation and pollution reduction [1,2]. According to the regulations of the Ministry of Public Security of China, in future, class-A fireproof materials shall be employed as out-wall of civil buildings for heat insulation and energy conservation [ref]. Class-A fireproof materials mainly include: foam cement, foam glass, rock wool, inorganic insulation mortar, phenolic composite insulation board, and foam ceramic. Foam ceramics have been paid much attention, for their low thermal conductivity, high fire resistance, and excellent compatibility with cement mortar; low water absorption, high strength, and reliable durability [[3], [4], [5], [6], [7]]. At the same time, with the development of economy, a larger quantity of solid wastes such as fly ash and so on has been generated in China and it remains increasing at a high speed annually. Dealing with such wastes is not only an economic problem, but also a significant environmental and ecological problem [[8], [9], [10]].
In this study, solid wastes were used as main raw material to prepare a new kind of CaO-Al2O3-SiO2 foam ceramic through melt-foaming [11,12] which is the special (only) way to prepare foam ceramics with high strength and thermal insulation (pore rate) property as building heat insulation and energy conservation materials. The properties of prepared foam ceramic were characterized, and the possible relationships between the properties and the unique microstructure of this material were revealed.
2. Experimental procedures
2.1. Raw materials
Fly ash was supplied from Tangshan Power Station with a nominal chemical composition of SiO2 (about 55.0 wt%), Al2O3 (about 30.0 wt%) and CaO (about 5.0 wt%). Furnace slag was supplied from Tangshan Iron & Steel Group Co., Ltd. Waste glass was supplied from China Building Materials Academy. Kaolin was supplied from Beijing Lanning Company with a chemical composition of SiO2 (about 63.0 wt.%), Al2O3 (about 35.0 wt.%) and CaO (about 1.0 wt.%). SiC was obtained from waste grinding materials, supplied from Shandong Qingzhou Micro powder Co., Ltd, which SiC content is about 95.0 wt%. The chemical compositions of the raw materials were obtained by X-ray fluorescence (XRF) spectroscopy (Model XRF-1800, Shimadzu).
2.2. Preparation of foam ceramic
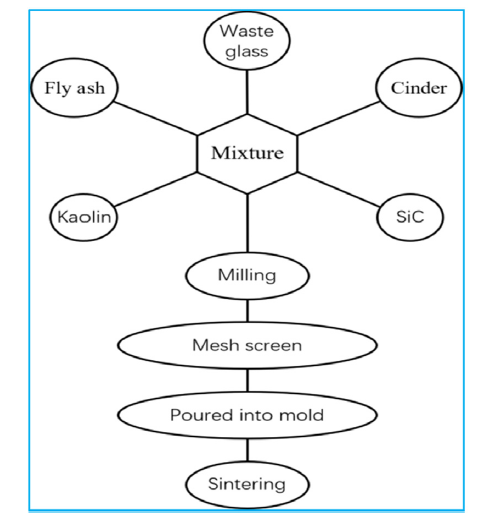
Fig. 1 shows the process flow chart. Raw materials were mixed according to mass ratio (fly ash: cinder): (waste glass: kaolin): SiC = (50:50): ((18-x) : x) : (y), where y is 2-9, then the mixture was rigidly milled in a ball mill at the speed of 300 r/min for 2 h, the mixture was sieved through a 30 mesh screen, then poured into a recrystallization silicon carbide mold up to 1/3 of the mold. The mold was put in a kiln, and heated up to 1120-1200 °C with the heating rate of 1.7 °C/min, holding for 0.5-3 h. Thus, the foam ceramic was prepared.
Fig. 1.
Fig. 1.
Flow chart of the process of making foam ceramic.
2.3. Microstructure characterization
Microstructure was observed using a HITACHI S-4800 cold field emission scanning electron microscope. Flexural strength was tested under an MTS CMT5205 electronic universal testing machine. Foam ceramic volume was measured through the Archimedes method, according to the theoretical density of CaO-Al2O3-SiO2, and the density and porosity of the foam ceramic were calculated [13,14].
3. Results and discussion
3.1. Effects of foaming temperature and holding time on microstructure
The microstructure of foam ceramic is formed by close packing pores. According to the Laplace equation:
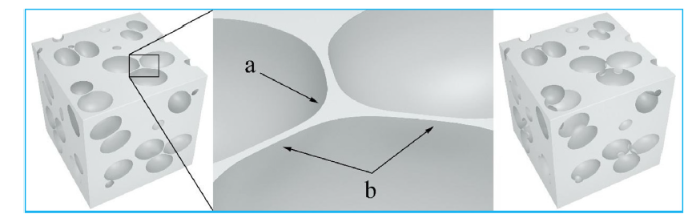
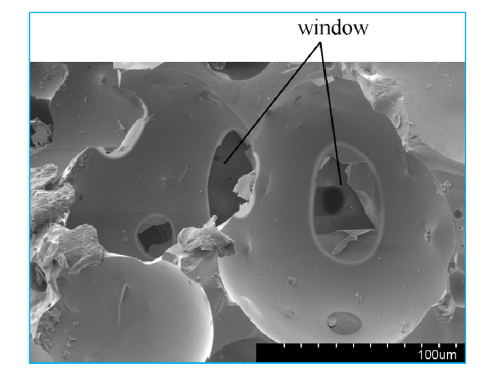
Fig. 2 demonstrates the change process of adjacent bubbles of liquid film. The curvature liquid membrane ‘b’ (two bubbles adjacent) is nearly zero, while the curvature liquid membrane ‘a’ is negative, internal pressure of ‘b’ is greater than that of a, therefore the materials in ‘b’ tend to flow into the area of ‘a’, and then ‘b’ is thinner. In the melt-foaming process, when foam is in the conditions of higher temperature or a longer holding time, windows might appear in place ‘b’ to connect two bubbles. SEM microphotograph for the structure is shown in Fig. 3.
Fig. 2.
Fig. 2.
Schematic illustration of the adjacent bubbles change process.
Fig. 3.
Fig. 3.
SEM micrograph for foam ceramic.
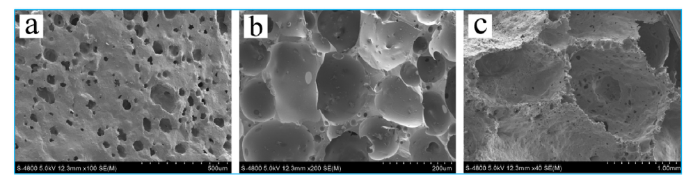
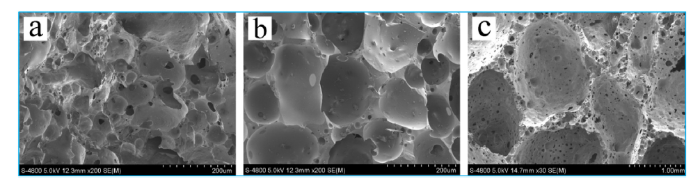
Fig. 4 shows microstructure of foam ceramic at different foaming temperatures. If the foaming temperature is too low, foaming agent does not yet begin to response or the reaction is not sufficient, it will lead to low porosity, uneven aperture and incomplete pore structure (Fig. 4(a)). If foaming temperature is too high, the foaming process is too violent, it will lead to overlarge holes, unconsolidated hole wall and over burnt (Fig. 4(c)). In this article, the suitable foaming temperature is 1160 ℃ (Fig. 4(b)).
Fig. 4.
Fig. 4.
SEM of foam ceramic at different temperatures: (a) 1100 ℃, (b) 1160 ℃, and (c) 1200 ℃.
Fig. 5 represents foam ceramic micro-structure at 1160 ℃ with different holding time. When the foaming temperature is the same and holding time is too short, foaming agent reaction is not sufficient. Thus, it will result in low porosity, uneven aperture or incomplete pore structure (Fig. 5(a)). If holding time is too long, it will result in overlarge hole, and unconsolidated hole wall (Fig. 5(c)). In this study, while the foaming temperature is 1160 ℃, the suitable holding time is 1 h (Fig. 5(b)).
Fig. 5.
Fig. 5.
SEM micrographs of foam ceramic with different holding time: (a) 0.5 h, (b) 1 h, (c) 2 h.
3.2. Effects of SiC content on porosity
SiC is used as a high-temperature foaming agent [17], which can be oxidized at high temperature and different oxidation condition. The following complex redox reactions will occur as:
SiC + 2O2 = SiO2 + CO2;
SiC + 3/2O2 = SiO2 + CO;
SiC + O2 = SiO + CO;
SiC + O2 = Si + CO2.
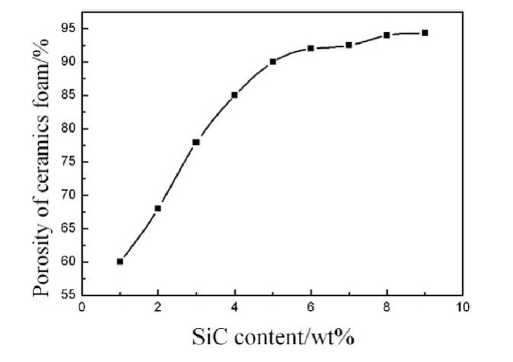
The content of SiC also has a great influence on the porosity of foam ceramic [18]. Fig. 6 shows the porosity of prepared foam ceramic varying with the content of SiC. With the increasing of foaming agent content, the porosity of prepared foam ceramic increases rapidly. But when it reaches to about 9.0%, further increasing SiC content brings about very slow porosity increasing. The porosity reaches to the highest value of 94.3%, when the content of SiC is 9.0%.
Fig. 6.
Fig. 6.
Porosity of CaO-Al2O3-SiO2 foam ceramic with different SiC content.
3.3. Effect of density on the flexural strength and porosity
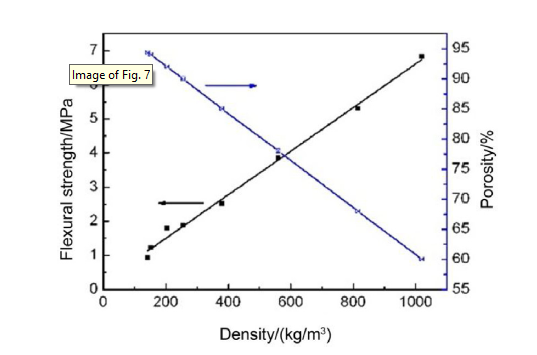
According to the curves of foam ceramic density, porosity and strength (Fig. 7), as the density decreases, the flexural strength of CaO-Al2O3-SiO2 foam ceramic is reduced. Based on the foam ceramic model given by Gibson and Ashby, the relationship between strength and density of brittle foam ceramic may be expressed by power function as follows [19]:
where σ is the strength of foam ceramic, C3 is a constant, and ρ is the density of foam ceramic. Fig. 7 shows a logarithmic curve, that is a result of linear fitting to density-strength according to Eq. (3), and n was 1.65, while n in Eq. (2) is 1.5. It indicates that in this study, density of foam ceramic has more influences on flexural strength than the results predicted by Gibson-Ashby equation. The difference is due to that the foam ceramic model of Gibson-Ashby assumed that aperture is a constant, while in the real research process, the aperture of foam ceramic changes with the size of density [22].
Fig. 7.
Fig. 7.
Flexural strength and porosity of CaO-Al2O3-SiO2 foam ceramic with different densities.
In this experiment, when the porosity reaches above 92%, because of less strength, it is difficult to meet actual needs. Finally the CaO-Al2O3-SiO2 foam ceramic has a porosity of 85%-92%, density of 204 kg/m3-560 kg/m3.
3.4. Thermal insulation property
Foam ceramic used in out-wall external insulation, is required to possess low density and high strength, and have better thermal insulation effect. The main ingredients were SiO2 and CaO in the foam ceramic, according to Kingery phase distribution model, the thermal conductivity of dense body is calculated by the following equation [23]:
where λm is the thermal conductivity of dense body, λo is thermal conductivity of SiO2, λd is thermal conductivity of CaO, and Vd is volume percentage of CaO. The foam ceramic prepared in this experiment contained a lot of holes, and it is required for amendment with Leisel equation [24].
where λp is thermal conductivity of foam ceramic, λm is the thermal conductivity of dense body, λa is thermal conductivity of air, P is foam ceramic porosity.
According to Eqs. (4) and (5), under different porosity, calculated thermal conductivity and tested thermal conductivity are shown in Table 1.
Table 1 Calculated thermal conductivity and tested thermal conductivity of CaO-Al2O3-SiO2 foam ceramic.
| Porosity (%) | Calculated thermal conductivity (w/m·K) | Tested thermal conductivity (w/m·K) |
|---|---|---|
| 60 | 0.52 | 0.67 |
| 68 | 0.41 | 0.45 |
| 78 | 0.27 | 0.26 |
| 85 | 0.18 | 0.14 |
| 90 | 0.12 | 0.08 |
| 92 | 0.10 | 0.07 |
| 94 | 0.07 | 0.05 |
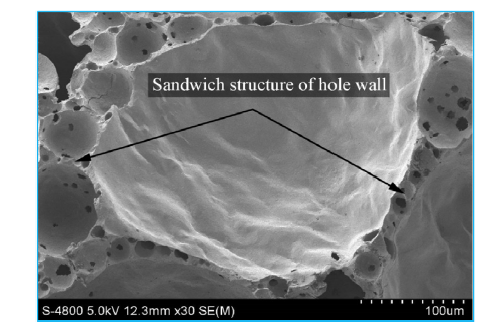
Table 1 shows that actual thermal conductivity is similar to theoretical calculation value, when porosity is low, due to uneven distribution of porosity and inconsistent size, thermal conductivity is partial high. When porosity reaches above 78%, the actual thermal conductivity is less than the calculation value. This is due to the fact that the hole wall structure is not a single-walled structure, but a sandwich structure (Fig. 8). Fig. 8 shows that hole-wall is composed by large micro-windows (Defined as a sandwich structure) which can effectively block heat transfer, and made the foam ceramic thermal conductivity decrease slightly compared with the theory calculation value. The experiments indicate that the existence of sandwich structure can effectively reduce the thermal conductivity of foam ceramic and increase its strength. Finally the CaO-Al2O3-SiO2 foam ceramic has a flexural strength of 1.79-3.86 MPa, and thermal conductivity of 0.07-0.26 w/m K.
Fig. 8.
Fig. 8.
Sandwich structure of hole wall of the foam ceramic.
4. Conclusion
In this study, CaO-Al2O3-SiO2 foam ceramic with a porosity of 85%-92%, density of 204 kg/m3-560 kg/m3, flexural strength of 1.79 MPa-3.86 MPa, and thermal conductivity of 0.07-0.26 w/m K, was prepared by melt-foaming and solid wastes as main raw materials. Foaming process has great effects on micro-structure, the best preparation process is holding for 1 h at 1160 ℃. The porosity increases with the increasing of SiC doping content. When the content of SiC reaches 9.0%, porosity reaches the maximum of 94.3%. The prepared foam ceramic has a uniform sandwich microstructure, which can effectively reduce the thermal conductivity of foam ceramic and increase its strength.
Acknowledgment
The authors gratefully acknowledge the financial support of the project from the National Natural Science Foundation of China (51172016).
Reference




 WeChat
WeChat