1. Introduction
To meet the increasing demands of high turbine efficiency, the gas turbine inlet temperatures have reached or even exceeded the maximum service temperature of superalloy components in recent decades [[1], [2], [3], [4], [5]]. To protect superalloy parts from such harsh operating environments, MCrAlY coatings (M = Co and/or Ni) are widely used due to their excellent oxidation resistance [[6], [7], [8], [9], [10]]. Generally, MCrAlY coatings exhibit multi-phase structures, such as γ + β or γ+γʹ+β, and some minor phases have also been reported [[11], [12], [13], [14]]. Among the available phases in MCrAlYs, the β-NiAl phase is the oxidation-resistant phase by acting as an effective Al reservoir for the oxide growth [[15], [16], [17], [18]]. The growth of surface oxides, mainly alumina, during exposure to high temperatures causes the continuous loss of Al. Since the β-NiAl phase is the Al reservoir, the continuous consumption of Al due to the oxide growth leads to the subsequent β-phase depletion [[19], [20], [21], [22], [23], [24], [25], [26], [27]]. The progressive depletion of the β phase results in the degradation of mechanical and chemical integrity of MCrAlY coatings and, eventually, the coating loses its protective effects when insufficient β phase reserves are left.
The mechanisms of oxidation and β-phase depletion have been widely reported [[28], [29], [30], [31], [32], [33]]. Different types of oxides can form, depending on the oxidation conditions [34]. It is reported that the spinel oxides are likely to form during the early stages of oxidation and an initial application of heat treatment can facilitate the formation of alumina rather than spinel oxides during oxidation [[35], [36], [37], [38], [39], [40]]. It is also generally recognised that oxide growth follows nearly parabolic growth kinetics [41], though non-parabolic oxide growth behaviour has also been reported [29]. The kinetics of β-phase depletion largely relies on the oxidational and microstructural characteristics. It is suggested that the β-phase depletion kinetics generally exhibit parabolic behaviour [42]. It is further reported that the minor element, Y, can form oxide pegs at the oxide/coating interface to prevent the oxide spallation [43,44]. This phenomenon becomes more predominant in coatings that are prepared by different deposition techniques [[45], [46], [47]], e.g. Electron-Beam Physical Vapour Deposition (EB-PVD) [[48], [49], [50]] and Vacuum Plasma Spraying (VPS) [[51], [52], [53]]. More recently, one of the cost-effective deposition methods, High Velocity Oxy-Fuel (HVOF) thermal spraying, has been shown to exhibit good process capabilities in obtaining MCrAlY coatings with comparable mechanical and chemical performances [[54], [55], [56], [57], [58]]. Hitherto, work concerned with the process parameters to manufacture MCrAlY coatings has been widely reported [59]. It is possible to achieve defect-free MCrAlY coatings when the process parameters are carefully controlled. But even with carefully controlled deposition parameters, discrepancies in the oxidation kinetics can still be found [41,53]. Comparative studies of MCrAlY coatings that have been prepared by different deposition methods have been previously conducted [[60], [61], [62]]. These research works are mainly focused on oxide formation, growth and spallation. Studies that focus on the β-phase depletion kinetics in MCrAlY coatings are quite limited in number. Distinct microstructural variations such as the oxide stringers in HVOF coatings can strongly influence the subsequent β-phase depletion. These oxide stringers usually appear in HVOF coatings due to the nature of the spraying process. They can act as non-diffusional sites to inhibit the outward diffusion of Al along the grain boundaries and, as a result, affect the growth of the β-phase depletion zone. This indicates that the β-phase depletion behaviour in the HVOF coatings could be different than that in the oxide-free coatings, such as VPS coatings. However, work concerned with this aspect does not appear to have been detailed yet.
Therefore, the aim of this paper is to investigate the effects of oxide stringers on the kinetics of β-phase depletion in MCrAlYs. The same CoNiCrAlY powder is used to minimise the effects of the coating composition on the β-phase depletion. Isothermal oxidation experiments concerned with as-sprayed and heat-treated HVOF and VPS CoNiCrAlY coatings are conducted at 1100 °C. Since the β-phase depletion is driven by oxidation, the oxide growth kinetics is measured and discussed. Subsequently, the evolution of the β-phase depletion in the HVOF and VPS CoNiCrAlY coatings is examined to elucidate the effects of oxide stringers on the β-phase depletion kinetics through quantitative measurements, analytical analysis and microstructural investigations.
2. Materials and experimental procedure
The coatings were prepared by High-Velocity Oxy-Fuel (HVOF) thermal spraying and Vacuum Plasma Spraying (VPS) using Praxair CO-210-24 CoNiCrAlY powder with a nominal composition as presented in Table 1. The powders were sprayed onto pre-ground mild steel substrates by HVOF and VPS. Different spray parameters were previously examined [63] and a set of spray parameters were selected to minimise the coating porosity, as summarised in Table 2, Table 3 for HVOF and VPS respectively. Coatings (~0.5 mm in thickness) were detached from the substrates to obtain free-standing coatings. The surface roughness, Ra, of the exposed surface is measured as 6.1 ± 0.8 μm and 5.8 ± 0.6 μm for HVOF and VPS coatings respectively by a surface profilometer (SURFTEST SV-600, Mitutoyo). Prior to oxidation, a batch of free-standing coatings was initially heat-treated in a vacuum with a nominal pressure of 6.0 × 10-3 mbar at 1100 °C for 2 h followed by natural air cooling. Subsequently, as-sprayed and heat-treated HVOF and VPS coatings were isothermally exposed to laboratory air at 1100 °C for time periods up to 250 h. The detailed powder microstructure was analysed via a scanning Transmission Electron Microscope (STEM) equipped with a High Angle Annular Dark-Field (HAADF) detector. The microstructures of the coatings before and after isothermal oxidation were examined in a Field Emission Scanning Electron Microscope (FESEM) equipped with an Energy-Dispersive X-ray Spectroscopy (EDS) system operated at 20 kV. Coating cross-sections were ground and polished to a 1 μm surface finish and were characterised using Backscattered Electron (BSE) imaging. The phase fractions and the evolution of the β-phase depletion zone were measured and quantified by the Image analysis software, ImageJ [64].
Table 1 The nominal composition (wt%) of the as-received CoNiCrAlY powder.
| Co | Ni | Cr | Al | Y |
|---|---|---|---|---|
| Bal. | 31.7 | 20.8 | 8.1 | 0.5 |
Table 2 The HVOF spraying parameters.
| N2 gas flow rate (l/min) | 4.3 |
|---|---|
| O2 gas flow rate (l/min) | 890 |
| Kerosene flow rate (ml/min) | 470 |
| Stoichiometry | 98% |
| Powder feed rate (g/min) | 64 |
| Traverse speed, mm/s | 1000 |
| Number of passes | 30 |
Table 3 The VPS spraying parameters.
| Power (kW) | 40 |
|---|---|
| Current (A) | 600 |
| Voltage (V) | 63 |
| Ar flow (l/min) | 50 |
| Powder feed rate (g/min) | 60 |
| Traverse speed, mm/s | 84 |
| Number of passes | 5 |
3. Results and discussion
3.1. Microstructural characterisation
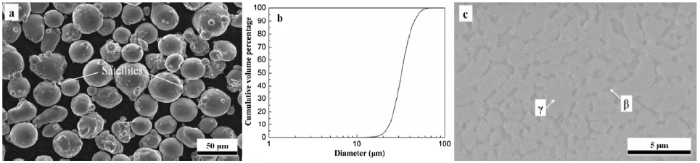
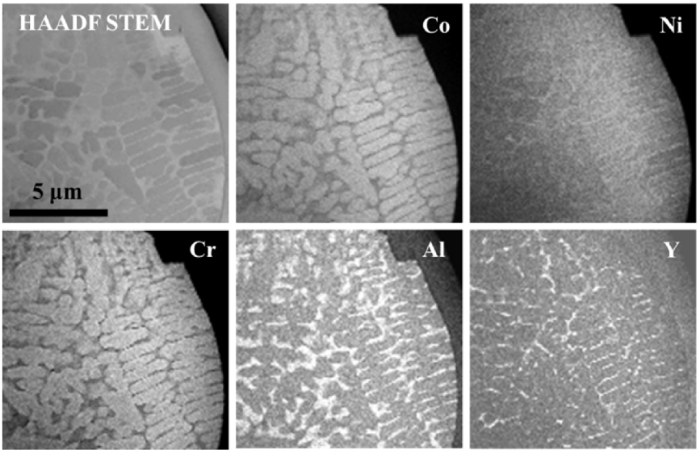
Fig. 1(a) shows the morphology of the as-received CoNiCrAlY powder. Most particles are spherical shapes whilst small satellites are also visible in Fig. 1(a). Such satellites are likely a result of the gas atomisation process, due to particle collision. The size distribution of the as-received powder particles is illustrated in Fig. 1(b). The mean particle diameter is found to be about 34μm. Fig. 1(c) depicts the microstructure of the as-received powder, showing a two-phase structure with the dark contrast β-NiAl phase and the bright contrast γ phase. A STEM elemental mapping of the powder cross-section is presented in Fig. 2. The two-phase structure with Al-rich β phase and Co-rich γ phase are clearly identified. Y-rich regions, which are distributed along the γ/β interphase boundaries, are also visible. This agrees well with previously reported work that states that Y is likely to form Y-aluminates along the phase boundaries in MCrAlY coatings [65].
Fig. 1.
Fig. 1.
CoNiCrAlY (Praxair CO-210-24) powder characterisation: (a) morphology of the powder particles, (b) cumulative particle size distribution and (c) powder cross-section.
Fig. 2.
Fig. 2.
STEM elemental mapping of the powder particle cross-section showing the element distribution.
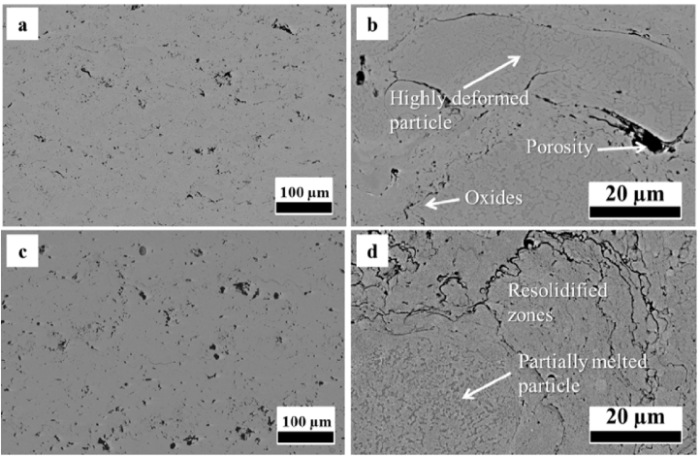
Fig. 3 shows the microstructure of as-sprayed HVOF and VPS CoNiCrAlY coatings. The initial two-phase structure of the powder is retained in the as-sprayed coatings. The oxygen content of the HVOF and VPS coatings are chemically analysed to be 0.442 wt% and 0.255 wt% respectively. Evidence of Y in the as-sprayed coatings is limited. Partially melted particles are shown in the HVOF coating in Fig. 3(b). These particles have been seriously deformed due to the high impact velocity in HVOF spraying. Unlike the deformed particles in the HVOF coating, Fig. 3(d) shows that the partially melted particles in the VPS coating generally retain the original spherical shape. Re-solidification zones can be seen around the particles, where only the γ phase exists due to rapid cooling. The two-phase structure is still visible within the powder particles in Fig. 3(b) and (d). The porosity of the as-sprayed HVOF and VPS CoNiCrAlY coatings was about 4% and 5% respectively, as measured by image analysis. Since the porosity in the as-sprayed HVOF and VPS CoNiCrAlY coatings is similar, it is believed that the effects of porosity on the subsequent β-phase depletion are limited.
Fig. 3.
Fig. 3.
Microstructure of as-sprayed HVOF (a and b) and VPS (c and d).
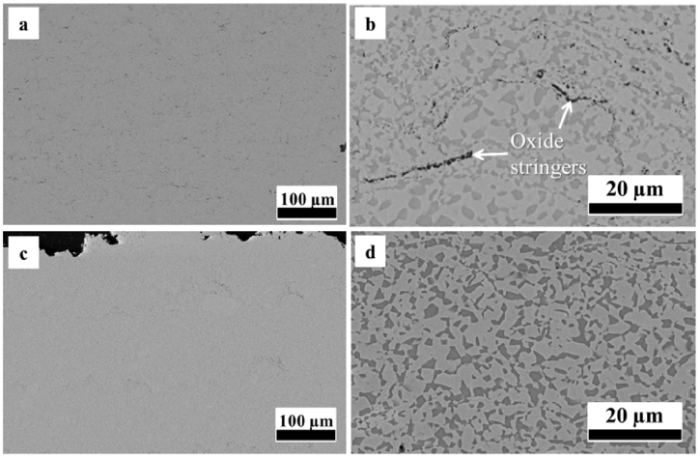
Fig. 4 shows the microstructure of the heat-treated HVOF and VPS CoNiCrAlY coatings. The heat-treated coatings exhibit a dense structure with a minimum level of porosity, indicating that the heat treatment has a sintering effect, healing the pores from the as-sprayed conditions. The β-phase can be seen clearly after the initial heat treatment in Fig. 4(b and d). Since the pores have been healed during the heat treatment, the micron-sized and dark contrast particles in Fig. 4(b) are believed to be the internal oxides, which is due to the in-flight oxidation of powder particles during HVOF spraying. This is supported by an EDS mapping in Fig. 5, demonstrating that these dark contrast particles are oxides. They are mostly distributed along the powder particle boundaries and are found to be Al-rich oxides. The volume fraction of the oxides in the heat-treated HVOF coating is measured to be around 1.5%. By contrast, the VPS coating in Fig. 4(d) exhibits an oxide-free microstructure, which agrees well with the nature of the process - minimum particle oxidation. Furthermore, the splat boundaries in the VPS coating are not easily observed, suggesting that the heat treatment process allows a more homogeneous distribution of the β phase in the VPS coating. The volume fractions of the β phase in the HVOF and VPS coatings are measured to be 30 ± 2% by image analysis.
Fig. 4.
Fig. 4.
Microstructure of heat-treated HVOF (a and b) and VPS (c and d).
Fig. 5.
Fig. 5.
EDS mapping of heat-treated HVOF coating to show the dark-contrast particles are Al-rich oxides.
3.2. Microstructure after oxidation
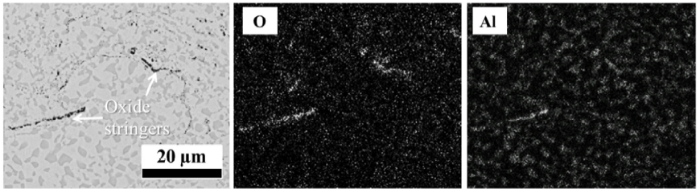
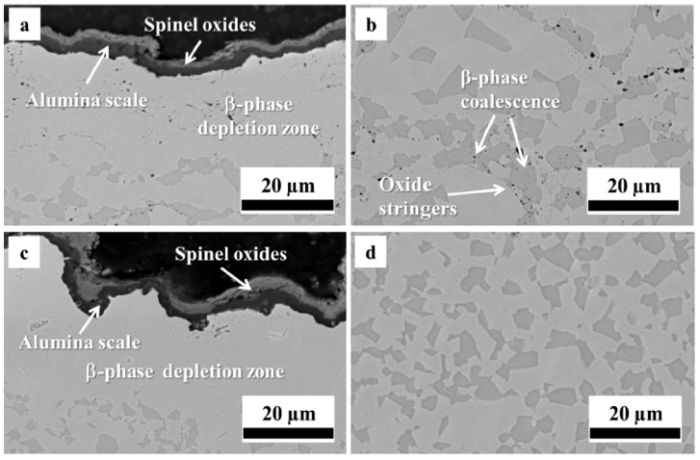
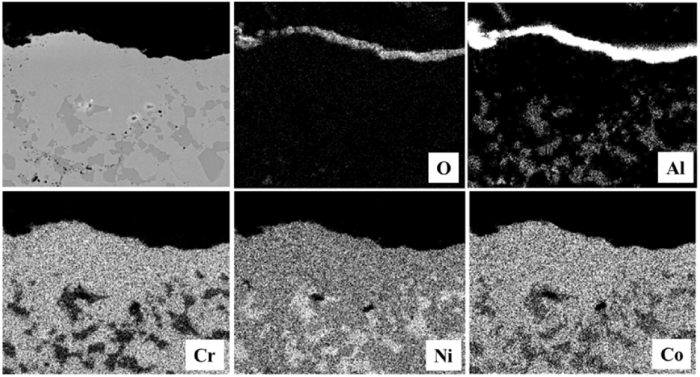
Fig. 6 shows the microstructure of as-sprayed HVOF and VPS coatings after oxidation at 1100 °C for 100 h. A dual layer of oxides has formed at the coating surface, with an outer layer of spinel oxides and an inner layer of alumina. EDS mapping analysis in Fig. 7 shows that the spinel oxides are most likely (Ni,Co)(Al,Cr)2O4. The β-phase depletion zone, which only consists of a single γ phase, is formed in contact with the oxide layers. The coatings still exhibit the γ + β structure in the two-phase region, in which the β-phase fraction is found to be similar to the initial fraction. It can be further noted from Fig. 6(b) that the β phases tend to coalesce along the oxide stringers. However, this phenomenon is not widely observed in the VPS coating since few or no oxide stringers exist. In addition, the Y-rich pegs are not prevalent in either HVOF or VPS coating due to its low content.
Fig. 6.
Fig. 6.
Microstructure of as-sprayed HVOF (a and b) and VPS (c and d) coatings after 100 h oxidation at 1100 °C, showing the spinel and alumina scale formation and the remaining two-phase structure.
Fig. 7.
Fig. 7.
EDS mapping analysis of the oxides formed in the as-sprayed CoNiCrAlY coating.
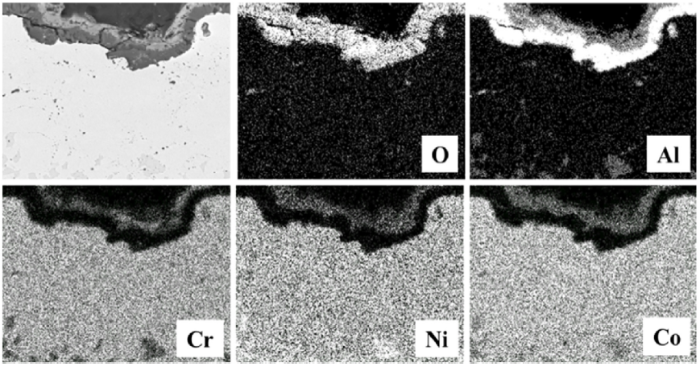
The microstructure of heat-treated HVOF and VPS coatings after oxidation at 1100 °C for 100 h is shown in Fig. 8. Only a single oxide layer is formed at the coating surfaces. EDS element mapping analysis in Fig. 9 shows that the oxide layer is rich in Al and O. This single oxide layer has been widely recognised as the alumina scale, especially forming at the surface of heat-treated CoNiCrAlY coatings. Like the as-sprayed coatings, the β-phase depletion here occurs in response to the oxide formation. Localised coalescence of the β phase at the oxide stringers is still visible in the HVOF coating from Fig. 8(b). Such β-phase coalescence at the internal oxide stringers is a distinct feature in the HVOF coating.
Fig. 8.
Fig. 8.
Microstructure of heat-treated HVOF (a and b) and VPS (c and d) coatings after 100 h oxidation at 1100 °C, showing the alumina scale formation and the remaining two-phase structure.
Fig. 9.
Fig. 9.
EDS mapping analysis of the oxides formed in the heat-treated CoNiCrAlY coating.
3.3. Oxide growth behaviour
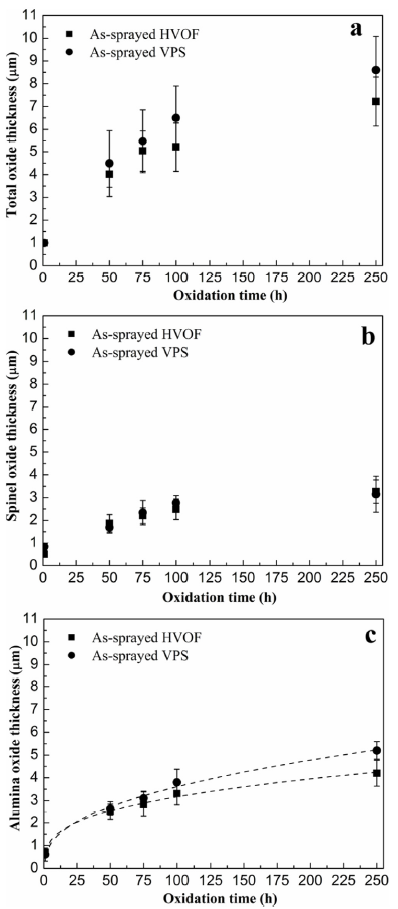
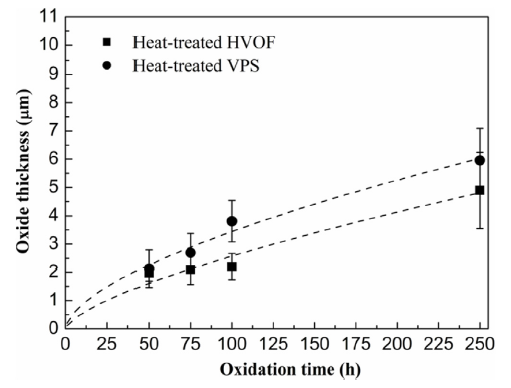
Oxide growth measurements against oxidation time of the as-sprayed HVOF and VPS coatings are presented in Fig. 10(a). Due to the curved nature of the exposed surfaces, the oxide thickness was measured using the area of oxides divided by the image width. It can be seen the oxide growth is similar in both coatings at short exposure times, i.e. 50 h and 75 h. But the VPS coating exhibits larger oxide thickness at 100 h and above. Since spinel oxides and alumina have formed in the as-sprayed CoNiCrAlY coatings after oxidation in Fig. 6, the thicknesses of the spinel oxides and alumina are plotted separately for HVOF and VPS coating in Fig. 10(b) and (c). It can be seen in Fig. 10(b) that the spinel oxides grow at the early stage of oxidation and have a limited growth rate after the alumina scale has formed. This indicates that the growth of spinel oxides is restricted when the alumina starts to form. It is shown in Fig. 10(c) that the alumina scale grows with time and the thickness of the alumina scale becomes larger than the spinel oxides after 100 h. No significant difference in the spinel oxide thickness is found between the as-sprayed HVOF and VPS coatings but a slightly larger alumina scale is noticed in the VPS coating. Fig. 11 shows the alumina growth behaviour in the heat-treated HVOF and VPS coatings. It is seen that both coatings exhibit small oxide growth rates, which agrees well with the protective nature of the alumina scale. The oxide growth kinetics exhibit a nearly parabolic behaviour, though some small discrepancies exist in terms of fitting the growth data. For longer oxidation times, i.e. 250 h, it is found that the oxide thickness in the VPS coating is slightly larger than the HVOF coating.
Fig. 10.
Fig. 10.
Oxide thickness measurements against oxidation time at 1100 °C for as-sprayed HVOF and VPS coatings: (a) total oxide thickness, (b) spinel oxide thickness and (c) alumina scale thickness.
Fig. 11.
Fig. 11.
Oxide thickness measurements against oxidation time at 1100 °C for heat-treated HVOF and VPS coatings. Only alumina is considered in the heat-treated conditions since very little spinel oxide is found.
When comparing the oxide growth between the as-sprayed coatings and heat-treated coatings in Fig. 10, Fig. 11, it can be seen that the oxide growth in the as-sprayed coatings is larger than that in the heat-treated coatings. Since spinel oxides are formed in the as-sprayed coatings, the oxide growth rate can be high in the early stage of oxidation. Hence, thicker oxide layers are obtained in the as-sprayed coatings. When comparing the HVOF coatings with VPS coatings in either as-sprayed conditions or heat-treated conditions, it is shown that the VPS coatings generally exhibit larger oxide growth behaviour than the HVOF coatings. In this study, the surface roughness of the exposed surfaces in the HVOF and VPS CoNiCrAlY coatings are similar, 6.1 ± 0.8 μm and 5.8 ± 0.6 μm respectively, indicating no significant difference in the oxygen contact area. Given both coatings were produced using the same powder and underwent the same heat treatment history, the oxygen activity at the surface is likely to be close in both coatings. As a result, the inward diffusional flux of oxygen is similar while the outward diffusion of oxide-forming elements like Al can be different due to the microstructural difference. The outward diffusional flux of Al is investigated and discussed below, in an attempt to understand the difference of the β-phase depletion kinetics in the HVOF and VPS CoNiCrAlY coatings.
3.4. β-Phase depletion kinetics
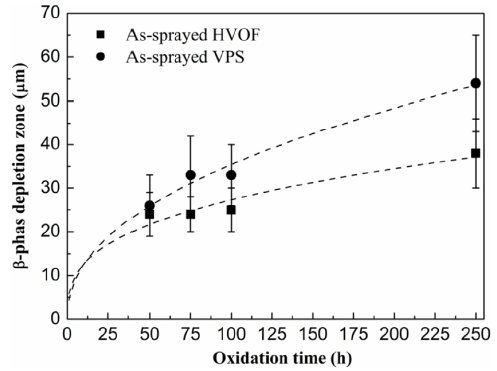
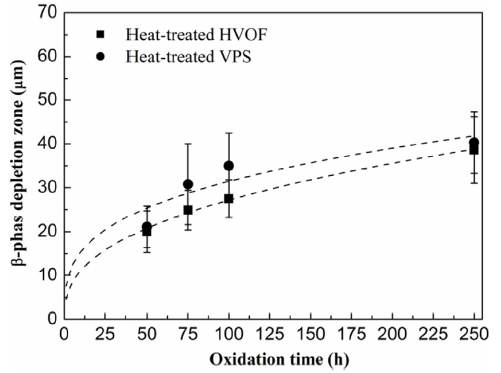
Fig. 12 depicts the evolution of the β-phase depletion against oxidation time in the as-sprayed HVOF and VPS coatings. It can be seen that the β phases deplete quite rapidly in the first 50 h and a large β-phase depletion zone, around 25 μm, is formed. At the beginning of oxidation, the oxide growth rate is very high and a large amount of β phase is consumed to form the spinel oxides. Thus, a large depletion zone is formed at periods below 50 h. Subsequently, no significant increase in the β-phase depletion is noticed between 50-100 h. This is because the oxidation rate is reduced due to the formation of inner alumina scale, which shows good agreement with Fig. 10(b) and (c). For longer oxidation periods, the β-phase depletion increases with time and larger β-phase depletion is found in the VPS coating. Fig. 13 shows the β -phase depletion versus oxidation time in the heat-treated HVOF and VPS coatings. It is noted that the VPS coating exhibits slightly larger β-phase depletion zones compared to the HVOF coating, in agreement with the as-sprayed conditions.
Fig. 12.
Fig. 12.
β-phase depletion against oxidation time in the as-sprayed HVOF and VPS coatings at 1100 °C.
Fig. 13.
Fig. 13.
β-phase depletion against oxidation time in the heat-treated HVOF and VPS coatings at 1100 °C.
When looking at the β-phase depletion in as-sprayed and heat-treated conditions for HVOF and VPS coatings respectively from Fig. 12, Fig. 13, it can be found that the β-phase depletion is larger in the as-sprayed conditions compared to the heat-treated conditions. The flux of Al required to form the oxides in the as-sprayed conditions can be given in Eq. (1) [42],
where $J_{\text{Al}}^{\text{as}-\text{sprayed}}$ is the flux of Al in the as-sprayed coatings, $J_{\text{Al}}^{\text{spinel}}$ is the flux of Al in forming the spinel (Co,Ni)Al2O4 oxides, and $J_{\text{Al}}^{\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}}$ is the flux of Al to form the alumina. $J_{\text{Al}}^{\text{spinel}}$ and $J_{\text{Al}}^{\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}}$ can be related to the oxide growth behaviour in Fig. 10(b) and (c) and are described in Eqs. (2) and (3) respectively,
where: ${{\rho }_{(\text{Co},\text{Ni})\text{A}{{\text{l}}_{2}}{{\text{O}}_{4}}}}$ and ${{\rho }_{\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}}}$ are the densities of spinel oxides and alumina, taken as 4.51 g/cm3 and 3.95 g/cm3 respectively [66]; ${{\overset{\mathop{{\dot{\delta }}}}\,}_{(\text{Co},\text{Ni})\text{A}{{\text{l}}_{2}}{{\text{O}}_{4}}}}$ and ${{\overset{\mathop{{\dot{\delta }}}}\,}_{\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}}}$ are the growth rates of spinel oxides and alumina derived from Fig. 10(b) and (c); ${{r}_{(\text{Co},\text{Ni})\text{A}{{\text{l}}_{2}}{{\text{O}}_{4}}}}$ and ${{r}_{\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}}}$ are the ratios for converting the required mass of Al to moles. Since only alumina forms in the heat-treated conditions, the flux of Al in the heat-treated coatings can be written as.
By deriving the oxide growth rate from Fig. 11, the Al flux in the heat-treated conditions can then be obtained.
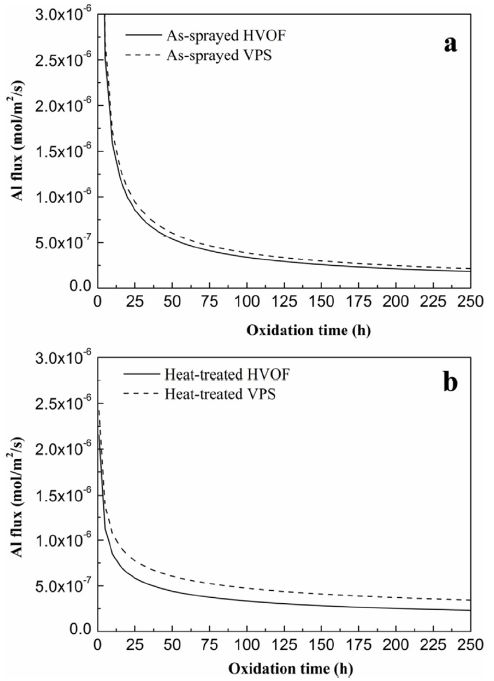
Fig. 14 illustrates the Al flux in the as-sprayed and heat-treated conditions of HVOF and VPS coatings respectively. The total area underneath the Al flux curves represents the loss of Al due to oxidation. It can be seen that the HVOF coatings exhibit less Al flux compared to the VPS coatings. Since the same powder was used and the coatings were subjected to the same heat treatment history, and the surface roughness and porosity are almost identical in both coatings, it is believed that the difference in the Al loss and β-phase depletion in the HVOF and VPS coatings are mainly attributed to microstructural differences. It has been previously reported that both coatings exhibit similar grain sizes (1~2 μm) [67,68]. When the β-phase depletion occurs, elements like Al diffuse from the coating to the surface. In such a fine-grained structure, element diffusion is dominated by grain boundary diffusion [16]. However, it can be seen that the internal oxide stingers mostly distribute along the γ/ β grain boundaries in Fig. 6, Fig. 8. These internal oxide stringers can inhibit the outward diffusion of Al, causing the coalescence of β phase to occur along the oxide stringers. This results in a reduction in the β-phase depletion rate in the HVOF coating.
Fig. 14.
Fig. 14.
The derived Al flux from oxide growth behaviour for HVOF and VPS coatings, (a) HVOF coating and (b) VPS coating.
Although the internal oxide stringers exist in the HVOF coatings, no significant difference in the β-phase depletion between HVOF and VPS coatings is found in Fig. 12, Fig. 13. This is possibly because the volume fraction of the internal oxides is only around 1.5% and the size of internal oxides is in the sub-micron scale; their effectiveness as a diffusion barrier is thus limited. Other types of diffusion barriers have also been reported in the literature, such as ReNi [69], YSZ [70], RuNiAl [71], NiW [72], etc. These diffusion barriers which are deposited as continuous layers in the coating/substrate system exhibit strong effects on inhibiting the element diffusion. However, unlike these continuous diffusion barriers, the internal oxide stringers are distributed discretely throughout the HVOF coating. Similarly, Peng et al. have also reported that the oxide dispersed NiCoCrAlY coatings can effectively prevent the outward diffusion of elements, which shows good consistency with this study [73]. It has to be mentioned that these short oxide stringers can act as diffusion barriers in the CoNiCrAlY coatings, but their level of effectiveness on the element diffusion and β-phase depletion also depends on their volume fraction. Future work will be necessary to address how the distribution of the oxide stringers would affect the β-phase depletion.
4. Summary
A CoNiCrAlY powder (Praxair CO-210-24) has been deposited by HVOF and VPS. The oxide growth behaviour and the β-phase depletion kinetics during oxidation of the free-standing HVOF and VPS CoNiCrAlY coatings have been investigated. Internal oxides were found to exist in the HVOF coatings due to the oxidation of in-flight powder particles during the spraying process, while the VPS coatings exhibited an oxide-free structure. It is shown that the HVOF coating exhibits smaller oxide growth compared to the VPS coating, both in as-sprayed and heat-treated conditions. Meanwhile, less β-phase depletion occurred in the HVOF coatings. It is proposed that the oxide stringers along the grain boundaries can cause the coalescence of the β phase and inhibit the grain boundary diffusion. The combination of the above results in a lower β phase depletion in the HVOF coatings.
Acknowledgements
The authors would like to thank Prof. J.R. Nicholls, Prof. D.G. McCartney and Dr K.T. Voisey for helpful discussions. The authors would also like to acknowledge the Faculty of Engineering, University of Nottingham for provision of laboratory facilities and Dr G. West from Loughborough University for the STEM analysis. This research was supported by Zhejiang Provincial Natural Science Foundation under Grant No. LQ18E010002, Natural Science Foundation of China under Grant No. 51901107, Ningbo Natural Science Foundation under Grant No. 2018A610168, 2019A610176 and Zhejiang Qianjiang Talent Scheme under Grant No. QJD1803012.
Reference
High velocity oxygen fuel (HVOF) spraying method was used in order to obtain very dense and good adhesive CoNiCrAlY-coatings deposited onto nickel-based alloy. The coatings were differently treated (preoxidized, vacuum treated or electron beam irradiated) before their exposure to cyclic oxidation tests in air at 1000 degrees C for periods up to 5 h. Changes of the coatings morphology and structure were analysed by scanning electron microscopy (SEM) and X-ray diffraction technique (XRD). The surface temperature of the samples was measured during cooling, between the oxidation cycles, and finally was associated with the thickness of the grown protective oxide scale on the CoNiCrAlY-surface. The experimental results demonstrated that depending on the thickness respectively on the different structures of the grown oxide scale, the cooling rate of the sample surface will be different as well. (C) 2012 Elsevier B. V.






 WeChat
WeChat