Corresponding authors:
Received: 2019-01-27
Revised: 2019-03-9
Accepted: 2019-03-13
Online: 2019-08-05
Copyright: 2019 Editorial board of Journal of Materials Science & Technology Copyright reserved, Editorial board of Journal of Materials Science & Technology
More
Abstract
Phase pure and well crystalline Cr2AlB2 powders are synthesized by heating the mixtures of CrB and Al powders at 900 °C. Cr2AlB2 exhibits nanolaminated morphology which transforms from flake-like crystallite to needle-like grain with the increase of holding time. The morphology-structure relationships of Cr2AlB2 are delicately discussed. Meanwhile, as the precursor for fabrication of Cr2AlB2, high purity CrB powders are also prepared by high-temperature reaction of B and Cr elemental powders at 1800 °C. CrB grains grow into regular plate-like morphology. Through Rietveld structure refinement, new sets of diffraction data are presented for both CrB and Cr2AlB2 and overlapped peak positions and intensities are revealed which make up for the deficiency of the existing data in ICDD PDF #32-0277 (CrB) and ICDD PDF #72-1847 (Cr2AlB2). Moreover, since MAB phases are precursors for preparing MBenes, 2D-CrB nanosheets are successfully prepared by completely etching out Al atomic layers from Cr2AlB2. 2D-CrB crystalizes in CrB structure with two-dimensional lamellar morphology. Simultaneously the formation mechanism of 2D-CrB is vividly depicted. A system of materials preparation from CrB to Cr2AlB2 and then to 2D-CrB is well established.
Keywords:
Binary transition metal borides are regarded as promising candidate materials for applications in hypersonic vehicles and scramjet engines due to their high melting point, good thermal and electrical conductivities, high hardness and strength as well as chemical inertness [[1], [2], [3], [4]]. Despite these unique properties, the intrinsic brittleness, poor oxidation and thermal shock resistance, difficulty of machinability and poor damage tolerance, are main obstacles that impede their applications in extreme environments [5]. In order to overcome the inherent brittleness and poor thermal shock resistance, an effective way is to intercalate layers of group IIIA or IVA elements, e.g. Al or Si, into the orthorhombic binary transition metal borides sublattices to form nanolaminated ternary transition metal borides called MAB phases which are closely analogous to the well-known nanolaminated MAX phases and MAX phase-like materials (herein M is a transition metal element, A is a group IIIA or IVA element, X is carbon or nitrogen, B is boron) in structures and properties [[6], [7], [8], [9], [10], [11], [12]].
MAB phases are a new family of layered materials, which were primitively discovered in 1960s and 1970s [[13], [14], [15], [16], [17]] and regained great interests since they were proposed and named as MAB phases by Ade and Hillebrecht [18]. To date, experimental and theoretical studies indicate that MAB phases possess nanolayered microstructure, good thermal and electrical conductivities, moderate modulus, good oxidation and thermal shock resistance and are tolerant to damage [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]]. These unique properties make them promising for applications as high and ultra-high temperature structural components, heating elements and damage tolerant ceramics.
From the structure point of view, typical MAB phases mainly include Cr2AlB2-type (space group Cmmm), Cr3AlB4-type (space group Pmmm), Cr4AlB6-type (space group Cmmm), MoAlB-type (space group Cmcm), and newly discovered Cr4AlB4-type (space group Immm) [18,[30], [31], [32], [33]]. For Cr2AlB2-type, it contains Cr2AlB2, Mn2AlB2, Fe2AlB2 and the corresponding solid solutions (Fe2-xMnx)AlB2 [31] and (Fe2-xCox)AlB2 [32]. For MoAlB-type, it involves MoAlB, WAlB and the corresponding solid solutions (MoxCr1-x)AlB and (MoxW1-x)AlB [33]. To the author's knowledge, preparation and properties of MoAlB [[22], [23], [24], [25]], Fe2AlB2 [28], Mn2AlB2 [29], have been widely investigated. However, experimental investigations on MAB phases in Cr-Al-B system, which play a representative role among MAB phases, are relative few except those on single crystal growth [18] and powder preparation [21,31,34], though theoretical studies were extensively conducted [19,35,36]. The only available experimental mechanical properties in Cr-Al-B system are values of Vickers hardness, viz. 6-7 GPa [18] or 10.4 GPa [20] for Cr2AlB2, 15-19.1 [18] GPa for Cr3AlB4.
Recently, Cr2AlB2 have also been demonstrated as the key precursor for the preparation of two dimensional MBene, i.e. 2D-CrB [37], by selectively etching out the Al element, which is expected to trigger enormous research activities in MBene field. MBenes (where M is a transition metal, B is boron) are a newly discovered family of two dimensional transition metal borides similar to the well-established graphene-like MXenes family [[37], [38], [39]]. Thereby, there is an urgent need to further explore the preparation methods and properties of MAB phases in Cr-Al-B system, especially the representative compound Cr2AlB2. In fact, the main obstacle is the difficulty in synthesis of high purity and well crystalline powders, which is the prerequisite for further study of its properties and preparation of corresponding bulk. A perusal of the literatures on MAB phases [18,20,21,30,31,34,37], one will find phase pure Cr2AlB2 powders have not been reported so far, due to the existence of appendant CrB impurities.
In our present work, phase pure and well crystalline Cr2AlB2 powders are synthesized using the reaction between CrB and Al. In addition, as the precursor for fabrication of Cr2AlB2, high purity CrB powders were also prepared using a synthesis method which is different from the existing reports [[40], [41], [42]]. Meanwhile, Rietveld structure refinement was conducted on pure CrB and Cr2AlB2 powders, and new sets of diffraction data are presented which make up for the deficiency of the original data in ICDD PDF #32-0277 (CrB) and ICDD PDF #72-1847 (Cr2AlB2). Moreover, since Cr2AlB2 is the precursor for preparing two dimensional CrB, 2D-CrB nanosheets are produced by completely etching out Al atomic layers from Cr2AlB2.
2.1.1. Synthesis of CrB
Commercially available B (99.2%, 10-20 μm), Cr (99.5%, -300 mesh) and Al (99.5%, -300 mesh) powders were used as the raw materials. Firstly, CrB was prepared through high-temperature reaction between B and Cr elemental powders. The mixtures in a molar ratio of Cr:B = 1:1 were wet ball-milled in polytetrafluoroethylene jars with agate balls and ethanol for 8 h. After fully dried in an oven at 60 °C, the mixtures were uniaxially cold-pressed at ca 150 MPa into pellets of 50 mm diameter in a cylindrical stainless steel die. Then, the cold-pressed pellets were heated to 1800 °C for 2 h at a rate of 5 °C/min under vacuum conditions in a graphite tube furnace, followed by furnace cooling. After cooling to ambient temperature, the reacted pellets were crushed into powders using a mortar-pestle for further analysis and usage.
2.1.2. Synthesis of Cr2AlB2
Using the as-prepared CrB powders and Al powders as reactants, phase pure Cr2AlB2 was synthesized. CrB and Al powders with a molar ratio of 2:1.5 were wet ball-milled for 6 h with agate balls and ethanol as medium in a polytetrafluoroethylene jar. Excess Al was employed to compensate its high temperature evaporation and facilitate the conversion from CrB to Cr2AlB2. After fully dried, the mixed powders were uniaxially cold-pressed into pellets of 30 mm diameter under a load of 100 MPa in a cylindrical stainless steel die. Synthesis of Cr2AlB2 was conducted by heating the pellets to 900 °C at a heating rate of 10 °C/min under flowing argon atmosphere and held at that temperature for 0.5 h, 1 h, 2 h, 4 h, respectively, in a horizontal tube furnace. After cooling, the reacted billets were ground into powders by a mortar-pestle. The unreacted Al was removed by soaking the powders in 20 wt% NaOH solution followed by washing in deionized water for several times until the pH approached 7 and then drying in an oven at room temperature.
2.1.3. Preparation of 2D-CrB
Two dimensional CrB nanosheets were produced by completely etching out Al atomic layers from Cr2AlB2 using dilute hydrochloric acid solution as etchant. Roughly 4 g of Cr2AlB2 powders (prepared at 900 °C for 4 h) were immersed in 200 ml dilute HCl (0.5 mol/L) solution for 7 days at room temperature. After immersing, the resulting suspension was centrifuged to separate the powders from the supernatant and subsequently washed several times using deionized water until the pH reached a value of approximately 7, and then air-dried at ambient temperature.
Phase identification was conducted on an X-ray diffractometer (Bruker D8 Advance, Karlsruhe, Germany) with incident Cu Kα radiation (λ = 1.54178 Å). The data used for phase identification was obtained at a scanning speed of 4°/min and a step of 0.02°. The data acquired for structure refinement was achieved at a scanning step size of 0.02° and 1 s per step. The microstructure was analyzed by a scanning electron microscope (SEM, Camscan Apollo300, UK) equipped with an energy dispersive X-ray spectroscopic system (EDS Inca X-Max 80 T, Oxford, UK), which allows analysis of light element like boron and carbon. Rietveld refinement [43,44] was conducted utilizing a TOPAS total pattern analysis solutions software (Bruker Corp., Karlsruhe, Germany). The intensity is calculated by:
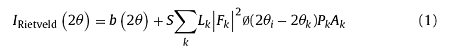
where b(2θ) is the background intensity, S is the scale factor, Lk contains the Lorentz polarization and multiplicity factors, φ is the profile function, Pk is the preferred orientation function, Ak is the absorption factor and Fk is the structure factor. The index k represents Miller indices for Bragg reflections. During refinement, lattice constants and atomic positions were variable, while the temperature factors were fixed. The pseudo-Voigt function was used to model the profile and a polynomial function was used to refine the background.
Theoretical X-ray diffraction pattern was simulated utilizing the Reflex Powder Diffraction code in Accelrys Materials Studio program (Accelrys Inc., San Diego, USA). BFDH (Bravais-Friedel Donnay-Harker) method was employed to simulate crystal morphology using the Morphology module of Accelrys Materials Studio program.
To prepare phase pure Cr2AlB2, synthesis of well-crystallized CrB powders is the first step. Fig. 1(a) shows the experimental XRD pattern of CrB powders prepared at 1800 °C for 2 h. Compared with theoretically simulated XRD pattern of CrB (Fig. 1(b)), good agreement can clearly be seen. As no other phase can be detected in Fig. 1(a), we can reasonably conclude that the as-prepared powders are phase-pure CrB. Nevertheless, the intensities of (0k0) reflections are much higher than those in theoretical XRD pattern and those in referenced XRD pattern of ICDD PDF #32-0277 (CrB, not shown), revealing the strong (0k0) orientation of CrB grains. To minutely understand it, the crystal structure of CrB is depicted in Fig. 2(a) and the simulated grain morphology (Fig. 2(b)) based on BFDH theory is compared to the experimental observed morphologies in Fig. 2(c) and (d). As can be seen in Fig. 2, the CrB grains are well faceted with regular plate-like morphology and the area of (020) is much larger than those of other faces. These morphology features facilitate the strong orientation of (0k0) reflections in XRD pattern, as shown in Fig. 1(a). In Fig. 2(c) and (d), the grain size is approximately 15-21 μm in length, 10-17 μm in width and 5-7 μm in height.
Fig. 1. X-ray diffraction patterns of experimental (a) and simulated (b) CrB powders.
Fig. 2. Crystal structure (a), simulated morphology (b) and experimental observed morphologies ((c) and (d)) of CrB grains.
To further confirm the synthesized powders are CrB and the intensity difference between theoretical and experimental XRD patterns is caused by plate-like morphology, Rietveld refinement was performed. As shown in Fig. 3, good agreement between the calculated and experimental patterns is found, demonstrating the reliability of the refinement. Meanwhile, the reliability factors Rp = 2.72%, Rwp = 3.50% and the goodness-of-fit χ2 = 1.23, again confirm the agreement. Table 1 summarizes the refined structure parameters of CrB. The obtained lattice constants and atomic positions are in excellent agreement with those reported for CrB single crystals by Okada and Atoda [45]. The calculated and experimental values of 2θ, d-spacings and intensities for (hkl) reflections of CrB from Rietveld refinement are listed in Table 2. In an elaborately view of Fig. 1 and Table 2, the diffraction peaks of (040) and (130), (041) and (131), (150) and (220), (151) and (221) reflections are overlapped actually. This characteristic of peak-overlapping is not shown in ICDD PDF #32-0277 (CrB). In fact, the (040), (041), (150) and (022) reflections are lacked in ICDD PDF #32-0277. So, this new set of XRD data not only provide detailed high resolution diffraction angle dependent intensities for structural analysis and phase identification but also make up for the deficiency of the original data in ICDD PDF #32-0277 (CrB).
Fig. 3. Experimental (black line) and calculated (red line) XRD patterns of CrB. The difference plot (blue line) is shown in the lower part of the figure. Green vertical marks indicate the Bragg reflection positions of CrB.
Table 1 Refined structure parameters for CrB.
| Formula | CrB | |
|---|---|---|
| Crystal system | Orthorhombic | |
| Space group | Cmcm (63) | |
| Formula units | 4 | |
| Cell volume (Å3) | 68.380 | |
| Crystal density (g/cm3) | 6.101 | |
| Structure parameters | Rietveld refinement | Reported [45] |
| Lattice constants (Å) | a = 2.9681(3) | a = 2.9782(7) |
| b = 7.8629 (1) | b = 7.870 (1) | |
| c = 2.9299(7) | c = 2.9346(7) | |
| Atomic positions | Cr 4c (0, 0.14450(7), 0.25) | Cr 4c (0, 0.14525(3), 0.25) |
| B 4c (0, 0.43096(8), 0.25) | B 4c (0, 0.4360(2), 0.25) | |
Table 2 Calculated and experimental data of 2θ, d-spacings and intensities for (hkl) reflections of CrB.
| Reflections (hkl) | m | 2θCal. (°) | 2θExp. (°) | dCal. (Å) | dExp. (Å) | I/I0Cal. (%) | I/I0Exp. (%) |
|---|---|---|---|---|---|---|---|
| 0 2 0 | 2 | 22.598 | 22.642 | 3.9315 | 3.9239 | 4.1 | 7.3 |
| 1 1 0 | 4 | 32.210 | 32.242 | 2.7769 | 2.7740 | 42.9 | 54.5 |
| 0 2 1 | 4 | 38.281 | 38.328 | 2.3493 | 2.3465 | 85.0 | 67.1 |
| 1 1 1 | 8 | 44.939 | 44.977 | 2.0155 | 2.0138 | 100.0 | 100.0 |
| 0 4 0 | 2 | 46.141 | 46.188 | 1.9657 | 1.9638 | 26.3 | 42.0 |
| 1 3 0 | 4 | 46.168 | 46.205 | 1.9646 | 1.9631 | 57.9 | 71.4 |
| 0 4 1 | 4 | 56.313 | 56.352 | 1.6324 | 1.6313 | 11.9 | 11.8 |
| 1 3 1 | 8 | 56.337 | 56.378 | 1.6318 | 1.6306 | 18.7 | 19.5 |
| 2 0 0 | 2 | 62.537 | 62.573 | 1.4841 | 1.4832 | 14.8 | 17.9 |
| 0 0 2 | 2 | 63.445 | 63.484 | 1.4650 | 1.4641 | 14.1 | 16.9 |
| 1 5 0 | 4 | 67.330 | - | 1.3896 | - | 0.2 | - |
| 2 2 0 | 4 | 67.393 | - | 1.3884 | - | 0.4 | - |
| 0 2 2 | 4 | 68.267 | - | 1.3728 | - | 0.4 | - |
| 0 6 0 | 2 | 72.002 | 72.059 | 1.3105 | 1.3096 | 2.2 | 3.7 |
| 1 1 2 | 8 | 72.953 | 72.985 | 1.2957 | 1.2952 | 6.6 | 7.0 |
| 1 5 1 | 8 | 75.689 | 75.729 | 1.2555 | 1.2550 | 18.6 | 20.6 |
| 2 2 1 | 8 | 75.749 | 75.812 | 1.2547 | 1.2544 | 18.2 | 19.5 |
Fig. 4 shows the XRD patterns of Cr2AlB2 powders prepared at 900 °C for 0.5 h, 1 h, 2 h, and 4 h respectively after treating in NaOH solution to remove excess Al. One can see that there are no other phases except Cr2AlB2 so that the powders synthesized at 900 °C using CrB and Al are phase-pure Cr2AlB2. A perusal of the relevant synthesis method of MAB phase [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]], we can rationally put forward that binary borides (e.g. CrB, FeB, MoB, etc.) are the better reagents for preparing high purity MAB phases, as they can restrain the formation of undesirable competing phases, i.e. M-A and M-B phases. Thus, the diffraction diagrams obtained from different holding times ranging from 0.5 h to 4 h are quite similar. This indicates that the chemical reaction kinetics for forming Cr2AlB2 is very fast although relatively low temperature is used and Cr2AlB2 is thermally stable and the grains grow up with the increase of holding time. To understand the grain growth and microstructure of Cr2AlB2, scanning electron microscopy was employed. Fig. 5 shows the morphologies of Cr2AlB2 grains prepared at different holding times. Obviously, the grain size, roughly in a range from hundreds of nanometers to more than a dozen of micrometers, generally increases with holding time and simultaneously nanolaminated microstructure and layer growth mechanism can be seen. The morphology transforms from small size flake-like crystallites to large size needle-like grains with the extension of holding time (Fig. 5(a)-(d)). To better understand the structure-morphology relationships, Fig. 6 presents the crystal structure, simulated morphology (BFDH method) and experimental observed morphology of Cr2AlB2. The structure of Cr2AlB2 (Fig. 6(a)) is comprised of Cr2B2 sublattices interleaved with single layer of Al atoms. And the morphology in Fig. 6(c) clearly reveals the layered structure characteristics. As marked in Fig. 6(b) and (c), the top and bottom faces are parallel to (020) plane and hence the crystal growth direction can be determined as [010] direction (Table 3).
Fig. 4. X-ray diffraction patterns of Cr2AlB2 powders prepared at 900 °C for 0.5 h, 1 h, 2 h and 4 h respectively, after treating in NaOH solution to remove excess Al. Cr2AlB2 is identified by ICDD PDF #72-1847.
Fig. 5. Morphologies of Cr2AlB2 powders prepared at 900 °C for 0.5 h (a), 1 h (b), 2 h (c) and 4 h (d) respectively.
Fig. 6. Crystal structure (a), simulated morphology (b) and experimental observed morphology (c) of Cr2AlB2 grains.
To further confirm the synthesized powders are Cr2AlB2, Rietveld refinement was performed on a sample prepared at 900 °C for 2 h, as shown in Fig. 7. Apparently, good agreement between the calculated and experimental patterns is found, demonstrating the reliability of the refinement. The reliability factors Rp = 2.35%, Rwp = 3.02% and the goodness-of-fit χ2 = 1.11 also confirm the agreement. Table 4 summarizes the refined structure parameters for Cr2AlB2. The obtained lattice constants and atomic positions are in good agreement with those reported for single crystal Cr2AlB2 by Ade and Hillebrecht [18]. A set of calculated and experimental values of 2θ, d-spacings and intensities for (hkl) reflections of Cr2AlB2 from Rietveld refinement are listed in Table 3. A careful check of Fig. 7 and Table 4, one will find the diffraction peaks of (201) and (112) are overlapped actually. This peak-overlapping feature is not shown in ICDD PDF #72-1847 (Cr2AlB2). So, this new set of XRD data is useful for structural analysis and phase identification and make up for the deficiency in ICDD PDF #72-1847 (Cr2AlB2) at the same time.
Fig. 7. Experimental (black line) and calculated (red line) XRD patterns of Cr2AlB2. The difference plot (blue line) is shown in the lower part of the figure. Green vertical marks indicate the Bragg reflection positions of Cr2AlB2.
Table 3 Refined structural parameters for Cr2AlB2.
| Formula | Cr2AlB2 | |
|---|---|---|
| Crystal system | Orthorhombic | |
| Space group | Cmmm (65) | |
| Formula units | 2 | |
| Cell volume (Å3) | 96.512 | |
| Crystal density (g/cm3) | 5.251 | |
| Structure parameters | Rietveld refinement | Reported [18] |
| Lattice constants (Å) | a = 2.9392(5) | a = 2.9373(3) |
| b = 11.0551 (1) | b = 11.0513(12) | |
| c = 2.9701(6) | c = 2.9675(3) | |
| Atomic positions | Cr 4i (0, 0.14663(7), 0) | Cr 4i (0, 0.14686(1), 0) |
| Al 2c (0, 0.5, 0.5) | Al 2c (0, 0.5, 0.5) | |
| B 4 j (0, 0.29403(6), 0.5) | B 4 j (0, 0.29401(8), 0.5) | |
Table 4 Calculated and experimental data of 2θ, d-spacings and intensities for (hkl) reflections of Cr2AlB2.
| Reflections (hkl) | m | 2θCal. (°) | 2θExp. (°) | dCal. (Å) | dExp. (Å) | I/I0Cal. (%) | I/I0Exp. (%) |
|---|---|---|---|---|---|---|---|
| 0 2 0 | 2 | 16.021 | 16.024 | 5.5275 | 5.5265 | 13.1 | 16.3 |
| 0 0 1 | 2 | 30.062 | 30.069 | 2.9702 | 2.9695 | 32.3 | 28.6 |
| 1 1 0 | 4 | 31.469 | 31.481 | 2.8406 | 2.8394 | 15.9 | 15.1 |
| 0 4 0 | 2 | 32.367 | 32.374 | 2.7638 | 2.7631 | 21.7 | 22.1 |
| 0 2 1 | 4 | 34.245 | 34.260 | 2.6164 | 2.6152 | 19.6 | 18.9 |
| 1 3 0 | 4 | 39.173 | 39.188 | 2.2978 | 2.2969 | 100.0 | 100.0 |
| 1 1 1 | 8 | 44.077 | 44.087 | 2.0529 | 2.0524 | 96.4 | 95.9 |
| 0 4 1 | 4 | 44.755 | 44.769 | 2.0233 | 2.0227 | 78.4 | 86.2 |
| 0 6 0 | 2 | 49.425 | 49.441 | 1.8425 | 1.8419 | 20.6 | 20.7 |
| 1 3 1 | 8 | 50.154 | 50.168 | 1.8174 | 1.8169 | 46.6 | 44.0 |
| 1 5 0 | 4 | 51.692 | 51.701 | 1.7669 | 1.7666 | 8.8 | 9.3 |
| 0 6 1 | 4 | 58.941 | 58.940 | 1.5657 | 1.5657 | 4.6 | 4.7 |
| 1 5 1 | 8 | 60.964 | 60.989 | 1.5185 | 1.5179 | 3.5 | 4.1 |
| 0 0 2 | 2 | 62.489 | 62.492 | 1.4851 | 1.4850 | 18.3 | 18.4 |
| 2 0 0 | 2 | 63.222 | 63.226 | 1.4696 | 1.4695 | 17.6 | 19.4 |
| 0 2 2 | 4 | 64.971 | - | 1.4342 | - | 0.2 | - |
| 2 2 0 | 4 | 65.688 | - | 1.4203 | - | 0.2 | - |
| 1 7 0 | 4 | 67.242 | 67.243 | 1.3912 | 1.3911 | 10.2 | 11.0 |
| 0 8 0 | 2 | 67.756 | 67.764 | 1.3819 | 1.3817 | 3.0 | 4.6 |
| 2 0 1 | 4 | 71.577 | 71.582 | 1.3172 | 1.3171 | 4.1 | 5.5 |
| 1 1 2 | 8 | 71.649 | 71.653 | 1.3161 | 1.3160 | 2.0 | 1.6 |
| 0 4 2 | 4 | 72.148 | 72.154 | 1.3082 | 1.3081 | 3.1 | 3.7 |
| 2 4 0 | 4 | 72.832 | 72.857 | 1.2976 | 1.2972 | 3.0 | 3.2 |
| 2 2 1 | 8 | 73.908 | 73.897 | 1.2813 | 1.2815 | 3.9 | 3.6 |
| 1 7 1 | 8 | 75.385 | 75.389 | 1.2599 | 1.2598 | 25.6 | 29.5 |
| 0 8 1 | 4 | 75.875 | - | 1.2529 | - | 0.7 | - |
| 1 3 2 | 8 | 76.281 | 76.282 | 1.2473 | 1.2472 | 23.4 | 25.5 |
Fig. 8(a) shows the XRD pattern of the sample produced by immersing Cr2AlB2 powders in dilute HCl (0.5 mol/L) solution for 7 days. Compared with the XRD pattern of CrB powders (Fig. 8(b)), one can find that the peak positions match with the powder XRD pattern but the peak intensities are distinct. So, we can reasonably conclude the as-etched samples crystalize in CrB structure, but possess strong (0k0) orientation due to differences in morphologies. To deeply understand the formation mechanism, SEM and EDS were employed to analyze the microstructure and chemical compositions. Fig. 9 presents the morphology of the as-etched samples. It demonstrates that the samples were etched into nanosheets with thickness range from several nanometers to tens of nanometers. This microscopic morphological feature is similar neither to that of Cr2AlB2 particles (Fig. 5) nor to that of CrB particles (Fig. 2(c) and (d)), but very much like that of the well-known 2D materials, such as graphene [46] and MXenes [47]. The morphology feature is also helpful in explaining the strong orientation of (0k0) reflections in XRD pattern which is attributed to the assembly of 2D-CrB nanosheets. Table 5 gives the representative chemical compositions obtained from different grains. Obviously, the main composition is Cr and B with less than one percent of Al, i.e. Al was completely etched out from Cr2AlB2. Thus the as-prepared samples possess CrB structure and the main composition is B and Cr. Based on the above results, we can rationally believe that the as-prepared samples are CrB nanocrystals with two-dimensional lamellar morphology, which are 2D-CrB in order to distinguish them from CrB particles [37]. To further explore the difference between 2D-CrB nanosheets and CrB particles, a precise identification and distinction on XRD data was performed. As a result, experimental 2θ, d-spacings for (hkl) reflections of CrB powders and 2D-CrB nanosheets are listed in Table 6. As can be seen, the 2θ of 2D-CrB shift to high angles and d-spacings shrink slightly compared to that of CrB particles. This indicates the shrink of lattice constants during the formation of 2D-CrB from Cr2AlB2. It also gives an implication on the formation mechanism of 2D-CrB. When Al was etched out from the structure of Cr2AlB2, the adjacent Cr2B2 units condensed and re-bonded to each other, finally leading to the formation of 2D-CrB. To detailedly illustrate the formation mechanism of 2D-CrB, a schematic diagram is depicted in Fig. 10. In the schematic diagram, Al atomic layers are removed by HCl and adjacent Cr2B2 layers slip relatively 1/2 <a+c> along [101] direction, re-bond and shrink in order to form CrB. At last, 2D-CrB forms in CrB structure with smaller lattice constants. Another consideration for the synthesis mechanism is the possible chemical reaction. We observed bubbles forming and escaping in the dilute HCl solution during the etching process, and the collected gas could be deflagrated, which presumed to be hydrogen (H2). As a consequence, the occurred chemical reaction can probably be described as:
Cr2AlB2 + 3HCl = 2CrB + AlCl3 + 3/2H2(g) (2)
Fig. 8. XRD patterns of 2D-CrB nanosheets produced by etching out Al from Cr2AlB2 in HCl (0.5 mol/L) solution for 7 days (a) and CrB particles prepared at 1800 °C for 2 h (b).
Fig. 9. SEM images of 2D-CrB nanosheets in different magnifications.
Table 5 Typical chemical compositions obtained from EDS analysis of 2D-CrB nanosheets.
| Chemical compositions | Cr (at.%) | Al (at.%) | B (at.%) |
|---|---|---|---|
| 2D-CrB | 44.47 | 1.21 | 54.31 |
| 49.59 | 0.95 | 49.46 | |
| 48.22 | 0.90 | 50.88 | |
| 42.71 | 2.19 | 55.11 | |
| 57.55 | 2.36 | 40.09 | |
| 48.42 | 1.67 | 48.91 | |
| 47.79 | 0.42 | 51.80 | |
| 48.31 | 1.55 | 50.14 | |
| 45.33 | 1.75 | 52.91 | |
| 54.43 | 0 | 45.57 | |
| 47.46 | 0.60 | 51.94 | |
| 56.33 | 0.47 | 43.19 | |
| 56.56 | 0.80 | 42.64 | |
| 58.94 | 0.68 | 40.38 |
Table 6 Comparison of experimental 2θ, d-spacings for (hkl) reflections between CrB particles and 2D-CrB nanosheets.
| Reflections | Experimental CrB particles | Experimental 2D-CrB nanocrystals | Difference | |||
|---|---|---|---|---|---|---|
| (hkl) | 2θCrB (°) | dCrB (Å) | 2θ2D-CrB (°) | d2D-CrB (Å) | 2θ2D-CrB - 2θCrB | d2D-CrB - dCrB |
| 020 | 22.642 | 3.9259 | 22.691 | 3.9175 | 0.049 | -0.0084 |
| 110 | 32.242 | 2.7755 | 32.290 | 2.772 | 0.048 | -0.004 |
| 021 | 38.328 | 2.3476 | 38.359 | 2.3458 | 0.031 | -0.0018 |
| 111 | 44.977 | 2.0148 | 45.002 | 2.0137 | 0.025 | -0.0011 |
Fig. 10. Schematic diagram illustrating the transformation from Cr2AlB2 to 2D-CrB. (a) Projection of Cr2AlB2 on (001) plane; (b) Al atomic layers; (c) crystal structure of 2D-CrB.
In short, 2D-CrB nanosheets are successfully prepared by completely etching out Al atomic layers from Cr2AlB2 at room temperature. It belongs to the family of MBenes. To the author's knowledge, this is the first reported fully etched MBene material.
In summary, firstly, high purity CrB powders have been prepared by heating the mixtures of B and Cr powders at 1800 °C for 2 h. The as-prepared CrB grains are well faceted with regular plate-like morphology which facilitates the strong orientation of (0k0) reflections in XRD pattern. A new set of diffraction data has been obtained which make up for the deficiency of the original data in ICDD PDF #32-0277 (CrB). Secondly, phase-pure and well crystalline Cr2AlB2 powders are synthesized at 900 °C using CrB and Al powders as reactants. Cr2AlB2 exhibits nanolaminated morphology which transforms from flake-like crystallites to needle-like grains with the extension of dwelling time. The grain size, roughly in a range from hundreds of nanometers to more than a dozen of micrometers, generally increases with holding time. The structure-morphology relationships of Cr2AlB2 are delicately discussed revealing that layered crystal structure leads to layered morphology. Thirdly, as MAB phases are precursors for preparing MBenes, 2D-CrB nanosheets are prepared by completely etching out Al atomic layers from Cr2AlB2 through immersing Cr2AlB2 powders in dilute HCl (0.5 mol/L) solution for 7 days. The thickness of 2D-CrB nanosheets range from several nanometers to tens of nanometers. 2D-CrB crystalizes in CrB structure with two-dimensional lamellar morphology and the formation mechanism of 2D-CrB is vividly depicted. The success in preparation of 2D-CrB gives us a hint to produce other fully etched MBene materials. Last but not least, a system of materials preparation from CrB (binary borides) to Cr2AlB2 (ternary borides) and then to 2D-CrB (two-dimensional borides) is well established.
We gratefully acknowledge financial supports from the National Natural Science Foundation of China under grant No. 51672064, No. U1435206 and No. 61271049.
The authors have declared that no competing interests exist.

/
| 〈 |
|
〉 |