Gelatin/Alginate hydrogels were engineered for bioplotting in tissue engineering. One major drawback of hydrogel scaffolds is the lack of adequate mechanical properties. In this study, using a bioplotter, we constructed the scaffolds with different pore architectures by deposition of gelatin/alginate hydrogels layer-by-layer. The scaffolds with different crosslinking degree were obtained by post-crosslinking methods. Their physicochemical properties, as well as cell viability, were assessed. Different crosslinking methods had little influence on scaffold architecture, porosity, pore size and distribution. By contrast, the water absorption ability, degradation rate and mechanical properties of the scaffolds were dramatically affected by treatment with various concentrations of crosslinking agent (glutaraldehyde). The crosslinking process using glutaraldehyde markedly improved the stability and mechanical strength of the hydrogel scaffolds. Besides the post-processing methods, the pore architecture can also evidently affect the mechanical properties of the scaffolds. The crosslinked gelatin/alginate scaffolds showed a good potential to encapsulate cells or drugs.
An implant of mimicking natural tissue structure is crucial for tissue engineering and regenerative medicine[1]. The traditional strategy of an artificial implant production is cell-seeded polymeric scaffolds, which is the most widely used[2] and [3]. However, scaffold manufacturing technologies face a challenge that achieves a high level of control over three-dimensional (3D) structure of scaffolds with incorporation of cells during the fabrication process[2] and [4]. In the late 1980s, rapid prototyping (RP) technology was born, which could quickly fabricate a scale model of a physical part or assembly on the basis of a pre-designed computer-assisted design (CAD) model[5]. Bioplotting is one of the RP technology for cell/organ printing, which can print live cells encapsulated in hydrogels with controlled architecture and defined cell placement in the mild condition[6]. This emerging technique can solve many problems that solid scaffold-based strategy has to face and become a powerful tool to mimic many natural tissue structures[7].
Selecting a suitable scaffold material to encapsulate and print live cells is a key of bioplotting[8]. Hydrogels are polymeric networks with a highly hydrated microenvironment, which makes it possible to permit easy transport of nutrients and oxygen, removal of waste products, and present bioactive stimuli that can influence cellular process[9] and [10]. Consequently, hydrogels become the most suitable materials for bioplotting, which can encapsulate cells in a 3D environment similar to that of many natural tissues[11] and [12]. Up to now, many nature derived or synthetic hydrogels, including alginate[13], collagen[14], gelatin[13] and [15] and Lutrol F127 Alal[16] have been used as scaffold materials. Sodium alginate, the seaweed-derived carbohydrate, is widely used to form hydrogels after ionotropic cross-linking with Ca2+ for drug delivery, cell encapsulation, and tissue regeneration, because Ca2+ crosslinked alginate is biocompatible and does not damage cells during the gelling process[17]. Gelatin, the denatured collagen, is widely used for drug delivery, gene delivery and tissue engineering[18], [19] and [20]. Since gelatin is sensitive to temperature changes, it can be suitable for depositing layer by layer through a sol-gel transformation. Recently, the combination of gelatin and alginate for bioplotting was widely attempted because of chemical similarity to the extra cellular matrix (ECM)[13].
However, one major drawback of gelatin/alginate hydrogels is the lack of adequate mechanical strength[21]. Therefore, many attempts have been carried out to improve the stability and mechanical properties of the hydrogel scaffolds. Chemical crosslinking is a popular and convenient approach to enhance the mechanical strength of the bioplotted hydrogel scaffolds. It is generally accepted that glutaraldehyde (GTA) is a good choice for cross-linking collagen-based biomaterials, because GTA is inexpensive, easily available and can effectively crosslink collagenous tissues quite rapidly[22]. Bigi et al.[23] studied that increasing the concentrations of GTA solutions can affect the mechanical, thermal, swelling and release properties of GTA crosslinked gelatin films. Most researches have been investigated previously using gelatin films and gels. However, as far as we know, the effect of GTA crosslinking on gelatin/alginate hydrogels has not previously been demonstrated on 3D porous scaffolds.
Herein, this paper aims to make a preliminary attempt to develop a gelatin/alginate system to generate 3D scaffolds with desired structure by bioplotting technique. The influence of crosslinking process and pore architecture on properties of scaffolds were studied, and the most appropriate post-processing methods and structure was discussed. Crosslinking process using GTA highly improved the stability and mechanical stiffness of the gelatin/alginate scaffolds. This kind of scaffold may have a potential to encapsulate cells or drugs.
High-viscosity biochemical-grade sodium alginate (biochemical grade,
The 3D scaffolds were manufactured from gelatin/alginate gel with a Bioplotter device (Envisiontec GmbH, Germany). First, a 10 mm × 10 mm × 2 mm rectangular block model was designed by a software package (Magics X, Materialise Software) and loaded on the Bioplotter computer-aided manufacturing (CAM) software. Then, the gel was loaded into a dispensing unit consisting of a cartridge and a nozzle (400 µm) and heated at 37 °C through a thermostatic cartridge unit. When the sol phase was attained, a nitrogen gas pressure of 2 × 104 Pa (0.2 bar) was applied to the syringe through a pressurized cap, and the rectangular blocks were plotted in a layer by layer manner for 7 layers. The plotting speed was set as 2 mm/s, and the spacing between two deposited fibers was 400 µm. To maintain the shape of the deposited fibers and the structure of the whole scaffolds, the fibers should solidify quickly after dispensing. The platform temperature was set as 5 °C, thus the gelling speed of the thermally reversible gelatin hydrogel could be quick enough to maintain the structure. To study the influence of pore architecture on the final performance of scaffolds, we designed two different manners of fiber deposition. The architecture was changed by plotting fibers with 60° and 90° angle steps between two successive layers (called 0-60 and 0-90 configurations, respectively).
To improve the stability and mechanical stiffness of the bioplotted scaffolds, the alginate was crosslinked with CaCl2, and the gelatin was crosslinked with glutaraldehyde (GTA, 25 wt%, Chengdu Kelong Chemical, China). First, all scaffolds were soaked in 5 wt% CaCl2 for 10 min, and then in GTA solution for 30 min. To study the influence of post-processing on scaffolds, GTA cross linker with different concentrations (0, 0.25, 1 and 2.5 wt%) was applied. After crosslinking, scaffolds were washed with ddH2O three times, and then soaked in glutamic acid to remove the remaining GTA. Finally, scaffolds were lyophilized at -20 °C for 48 h.
A Fourier-transform infrared (FTIR) spectrometer (Nexus Por Euro, Bruker, Germany) was used for measuring the cross-linkage of sodium alginate and gelatin. FTIR spectra represent the average of 30 scans between 400 cm-1 and 4000 cm-1 at a resolution of 8 cm-1.
To illustrate the structure and architecture of wet scaffolds, hydrogels were labeled with fluorescein isothiocyanate (FITC, Dojindo Laboratories, Japan). The bioprinted FITC-hydrogel scaffolds were observed under an inverse fluorescent microscope (40FL Aiokop, Zeiss, Germany) without lyophilization. We also used a low vacuum environment scanning electron microscope (ESEM, Nova Nano SEM 430, FEI, The Netherlands) to study the surface morphology and structure of the wet scaffolds.
The geometry and architecture of dried scaffolds after lyophilization were characterized by both three-dimensional rotational optical microscopy (HiroX7700, HiroX, Japan) and ESEM analysis.
The porosity of 3D plotted scaffolds was calculated following the theoretical approach by Landers et al.[24]:

where
Porosity, pore size and distribution were also experimentally measured by micro-CT (XTV160H, X-TEK, Britain). Reconstruction of 3D images of dried scaffolds was carried out with the micro-CT scanning.
The pre-weighed dry scaffolds were immersed in distilled water for 24 h at 37 °C in a shaking table. The weight change of each scaffold was monitored at different time intervals. The percent increase in water absorption was calculated as

where
For each group, five samples were used for measurement and average data were used for calculations.
The pre-weighed dry scaffolds were sterilized with UV-light, soaked in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, and incubated in an atmosphere of 5% CO2 at 37 °C. At different time intervals, the scaffolds were taken out and weighted after lyophilization. The medium was changed every second day, and scaffolds were incubated for 15 days. The degradation rate was calculated as
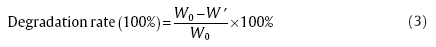
where
The compressive stress-strain measurements were performed on the water swollen printed hydrogels using a dynamic mechanical analysis (DMA, Q800DE, TA Instruments, USA). The rectangular block hydrogel scaffolds (10 mm × 10 mm × 2 mm) were precisely measured using a digital caliper micrometer. The stress-strain curves of the scaffolds were recorded at a compressing speed of 1 mm/min, and the Young's modulus was derived from the regression of the linear portion of stress-strain curves. Each measurement was performed in quintuplicate. Results are reported as the mean standard deviation.
Mouse bone mesenchymal stem cells (mBMSCs, ATCC CRL12424) were used to observe the cell adhesion on the scaffolds. mBMSCs at passage 7-8 were used in the following experiments. Cells were cultured in Dulbecco's Modified Eagles's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C in a moist atmosphere of 5% CO2, and collected by trypsinization by 0.25% trypsin-EDTA solution. To trace the cellular behavior, we used CellTracker (CellTracker Green CMFDA, Invitrogen) to label mBMSCs before seeding. The scaffolds were sterilized with UV-light and placed into a 24-well plate. Cell suspension (4 × 104 cells per scaffold, 500 µL) was then seeded on the scaffolds. After culturing for 3 days, the cell adhesion on the scaffolds was observed with an inverse fluorescent microscope.
All experiments were independently repeated at least three times, and the data are presented as the mean standard error (SE). The data were analyzed by one-way ANOVA.
Scaffolds were fabricated by Bioplotter as previously described by Landers et al.[24]. Briefly, this device involves a moving dispense head (
The FTIR spectra (
Because the hydrogel fibers were transparent in water and inconvenient to observe, FITC was used to label the hydrogel for better observation. The fibers deposited layer by layer for seven layers with the deposition angle of either 60° (
Fig. 2. Fluorescent images of wet scaffolds treated with different crosslinking processes: (a) CaCl2; (b) CaCl2+0.25% GTA; (c) CaCl2+1% GTA; (d) CaCl2+2.5% GTA; (e) CaCl2+0.25% GTA -90°.
Fig. 3. Low vacuum ESEM images of wet scaffolds of different magnifications: (a) CaCl2; (b) CaCl2+0.25% GTA; (c) CaCl2+1% GTA; (d) CaCl2+2.5% GTA; (e) CaCl2+0.25% GTA -90°.
Given that the wet scaffolds were easy to degrade by bacteria and difficult to operate, all scaffolds were lyophilized. To study the effect of lyophilization, scaffolds were observed by 3D rotational microscopy and scanning electron microscopy (SEM). From the optical microscopy images, we can see that CaCl2 crosslinked scaffold is transparent (
Fig. 4. 3D rotational microscopy images of magnified surface and cross section of the dried scaffold: (a) CaCl2; (b) CaCl2+0.25% GTA; (c) CaCl2+1% GTA; (d) CaCl2+2.5% GTA; (e) CaCl2+0.25% GTA-90°. 1, 2 indicate the horizontal sections with different magnifications and 3 represents the vertical cross-sections of scaffolds.
Fig. 5. SEM images of magnified surface and cross section of the dried scaffold: (a) CaCl2; (b) CaCl2+0.25% GTA; (c) CaCl2+1% GTA; (d) CaCl2+2.5% GTA; (e) CaCl2+0.25% GTA-90°. 1, 2 indicate the horizontal sections with different magnifications and 3 represents the vertical cross-sections of scaffolds.
The porous architecture design plays a vital role in tissue regeneration by providing temporary mechanical function, preserving tissue volume and circulating of nutrients and waste products[27]. The porosity calculated from theoretical formula was 50%, while the experimental results from micro-CT indicated that the porosity of wet and dried scaffolds were 40% and 75%, respectively (
Fig. 6.
(a) Porosity of both wet and dried scaffolds treated with different crosslinking processes, (b-f) pore size distribution of dried scaffolds. * indicated that there was significant difference (
For bone tissue engineering, studies demonstrated that a pore size above 300 µm benefited to increase bone formation through vascularization, and pore sizes of 100-400 µm may be good for osteoconduction[28] and [29].
Fig. 7. Reconstruction of 3D images of dried scaffolds by micro-CT: (a) CaCl2; (b) CaCl2+0.25% GTA; (c) CaCl2+1% GTA; (d) CaCl2+2.5% GTA; (e) CaCl2+0.25% GTA-90°.
The characterization of scaffold structure demonstrated that the GTA crosslinked hydrogel scaffolds maintained satisfactory outer and inner structure after the crosslinking and lyophilization process, while only CaCl2 treated scaffolds were not strong enough, leading to a little damage to the inner structure and a decrease in scaffoldporosity. The fiber diameter as well as porosity of both wet and dried scaffolds had minor differences among groups treated with different concentrations of GTA (0.25, 1, and 2.5%). These two kinds of scaffolds had distinct pore size and distribution, which may affect the mechanical properties of scaffolds.
The water absorption ability has been demonstrated to be an important feature for tissue engineering scaffolds, because it concerns the absorption of body fluid as well as permeation and transportation of nutrients and waste products[30].
Bigi et al.[31] reported that the crosslinking process provided a significant reduction of the swelling. With the GTA concentration increased, the percentage of water uptake decreased, and the time of reaching the swelling equilibrium was prolonged, which was supposed to be caused by the different degrees of crosslinking. Osmotic pressure forces, electrostatic forces and viscoelastic restoring forces are the three main forces governing the swelling behavior of hydrogels[32]. The increase in the concentration of GTA lead to the higher degree of crosslinking of the hydrogel network; thus viscoelastic restoring forces attenuated and the water uptake ability decreased. The gelatin of only CaCl2-treated scaffolds was not crosslinked, so the scaffolds quite easily collapsed in solutions. After soaking for 5 h, the water absorption ratio began to decline. The swelling percentage of 0-60 scaffolds was a little higher than that of 0-90 scaffolds. This could be explained by the more complex deposition form of 0-60 scaffolds, leading to higher specific surface area. We can conclude that the water absorption ability was mainly determined by the composition of scaffold materials.
The degradation rate is another important factor for tissue engineering scaffolds. An ideal scaffold should be able to degrade with time
The attainment of appropriate mechanical strength is a vital requirement for tissue engineering scaffolds[33]. It has been generally accepted that adjusting the mechanical properties to the desired tissue is essential. For example, soft tissue strengths are typically located between 0.4 and 350 MPa, while hard tissue regeneration requires strengths of 10-1500 MPa[34]. Compressive stress-strain curves were recorded using a DMA in the dried state (
Fig. 10.
(a) Stress-strain curves with a compressing rate of 1 mm/min, (b) and (c) comparison of final deformation rate and Young's modulus. * indicated that there was significant difference (
The Young's modulus of each group was 20 ± 0.88, 21.13 ± 1.15, 27.36 ± 1.25, 34.33 ± 1.05 MPa, and 27.36 ± 1.26 MPa, respectively (
In this study, we have engineered the gelatin/alginate 3D scaffolds by bioplotting technique and studied the effects of pore architecture and post-processing methods on physicochemical properties and biocompatibility of each scaffold. According to the experiment results, 1% GTA-crosslinked scaffolds may have the most appropriate properties for tissue regeneration. Our next plan is to encapsulate cells or drugs in this kind of scaffolds and apply for bone or cartilage tissue engineering and regeneration.
This work was supported by the National Basic Research Program of China (“973 Program”, No. 2012CB619100), the National Natural Science Foundation of China (Grant No. 51372085), the Guangdong-Hongkong Common Technology Bidding Project (No. 2013B010136003), and the Postdoctoral Science Foundation of China (No. 2013M542172).
The authors have declared that no competing interests exist.
| [1] |
Bone disorders are of significant concern due to increase in the median age of our population. Traditionally, bone grafts have been used to restore damaged bone. Synthetic biomaterials are now being used as bone graft substitutes. These biomaterials were initially selected for structural restoration based on their biomechanical properties. Later scaffolds were engineered to be bioactive or bioresorbable to enhance tissue growth. Now scaffolds are designed to induce bone formation and vascularization. These scaffolds are often porous, made of biodegradable materials that harbor different growth factors, drugs, genes, or stem cells. In this review, we highlight recent advances in bone scaffolds and discuss aspects that still need to be improved.
|
| [2] |
[Cited within:2]
|
| [3] |
<h2 class="secHeading" id="section_abstract">Abstract</h2><p id="">Culture of osteogenic cells on a porous scaffold could offer a new solution to bone grafting using autologous human mesenchymal stem cells (hMSC) from the patient. We compared coralline hydroxyapatite scaffolds with pore sizes of 200 and 500 μm for expansion and differentiation of hMSCs. We cultivated the hMSC statically or in spinner flasks for 1, 7, 14 and 21 days and found that the 200-μm pore scaffolds exhibited a faster rate of osteogenic differentiation than did the 500-μm pore scaffolds as shown by an alkaline phosphatase activity assay and real-time reverse transcriptase polymerase chain reaction for 10 osteogenic markers. The 500-μm scaffolds had increased proliferation rates and accommodated a higher number of cells (shown by DNA content, scanning electron microscopy and fluorescence microscopy). Thus the porosity of a 3D microporous biomaterial may be used to steer hMSC in a particular direction. We found that dynamic spinner flask cultivation of hMSC/scaffold constructs resulted in increased proliferation, differentiation and distribution of cells in scaffolds. Therefore, spinner flask cultivation is an easy-to-use inexpensive system for cultivating hMSCs on small to intermediate size 3D scaffolds.</p>
|
| [4] |
Here we developed a composite scaffold of pearl/poly(lactic-co-) (pearl/PLGA) utilizing the low-temperature deposition manufacturing (). makes it possible to fabricate scaffolds with designed microstructure and macrostructure, while keeping the bioactivity of biomaterials by working at a low temperature. Process optimization was carried out to fabricate a mixture of pearl powder, PLGA and with the designed hierarchical structures, and freeze-dried at a temperature of -40 degrees C. Scaffolds with square and designated bone shape were fabricated by following the 3D model. Marrow stem (MSCs) were seeded on the pearl/PLGA scaffold and then cultured in a rotating culture system. The adhesion, proliferation and differentiation of MSCs into osteoblasts were determined using scanning electronic microscopy, WST-1 assay, activity assay, immunofluorescence staining and real-time polymerase chain reaction. The results showed that the composite scaffold had high porosity (81.98 +/- 3.75%), proper size (micropores: <10 microm; macropore: 495 +/- 54 microm) and mechanical property (compressive strength: 0.81 +/- 0.04 MPa; elastic modulus: 23.14 +/- 0.75 MPa). The pearl/PLGA scaffolds exhibited better biocompatibility and osteoconductivity compared with the /PLGA scaffold. All these results indicate that the pearl/PLGA scaffolds fulfill the basic requirements of bone tissue engineering scaffold.
|
| [5] |
[Cited within:2]
|
| [6] |
Abstract Additive manufacturing, otherwise known as three-dimensional (3D) printing, is driving major innovations in many areas, such as engineering, manufacturing, art, education and medicine. Recent advances have enabled 3D printing of biocompatible materials, cells and supporting components into complex 3D functional living tissues. 3D bioprinting is being applied to regenerative medicine to address the need for tissues and organs suitable for transplantation. Compared with non-biological printing, 3D bioprinting involves additional complexities, such as the choice of materials, cell types, growth and differentiation factors, and technical challenges related to the sensitivities of living cells and the construction of tissues. Addressing these complexities requires the integration of technologies from the fields of engineering, biomaterials science, cell biology, physics and medicine. 3D bioprinting has already been used for the generation and transplantation of several tissues, including multilayered skin, bone, vascular grafts, tracheal splints, heart tissue and cartilaginous structures. Other applications include developing high-throughput 3D-bioprinted tissue models for research, drug discovery and toxicology.
|
| [7] |
[Cited within:1]
|
| [8] |
Abstract Current in vitro models for tumor growth and metastasis are poor facsimiles of in vivo cancer physiology and thus, are not optimal for anti-cancer drug development. Three dimensional (3D) tissue organoid systems, which utilize human cells in a tailored microenvironment, have the potential to recapitulate in vivo conditions and address the drawbacks of current tissue culture dish 2D models. In this study, we created liver-based cell organoids in a rotating wall vessel bioreactor. The organoids were further inoculated with colon carcinoma cells in order to create liver-tumor organoids for in vitro modeling of liver metastasis. Immunofluorescent staining revealed notable phenotypic differences between tumor cells in 2D and inside the organoids. In 2D they displayed an epithelial phenotype, and only after transition to the organoids did the cells present with a mesenchymal phenotype. The cell surface marker expression results suggested that WNT pathway might be involved in the phenotypic changes observed between cells in 2D and organoid conditions, and may lead to changes in cell proliferation. Manipulating the WNT pathway with an agonist and antagonist showed significant changes in sensitivity to the anti-proliferative drug 5-fluoruracil. Collectively, the results show the potential of in vitro 3D liver-tumor organoids to serve as a model for metastasis growth and for testing the response of tumor cells to current and newly discovered drugs.
|
| [9] |
URL
[Cited within:1]
|
| [10] |
The Brookville terrane in southern New Brunswick contains some of the oldest known sedimentary rocks of the Ganderian microcontinent of the northern Appalachian orogen. It includes the stromatolitic metacarbonate- and quartzite-dominated Ashburn Formation and metasiltstone-dominated Martinon Formation, in mylonitic contact with the Brookville Gneiss, an assemblage of low-pressure/high-temperature paragneiss and tonalitic orthogneiss. All of the metasedimentary units were intruded by the ca. 550鈥528Ma subduction-related Golden Grove Plutonic Suite. Analysis by LA-ICP-MS and TIMS of zircon grains from the Ashburn Formation quartzite yielded no <10% discordant ages younger than ca. 1200Ma, a predominance of Meso- and Paleoproterozoic ages back to ca. 2100Ma, and a few Neoarchaean ages. A sample from the metasiltstone matrix of a carbonate olistostrome in the Martinon Formation contains rounded grains with ages mainly between ca. 1000Ma and 2200Ma, indicating provenance similar to that of the Ashburn Formation. However, the sample also contains euhedral grains with ages averaging around 650Ma, indicating the maximum depositional age. A quartzite/calc-silicate sample from the Brookville paragneiss yielded detrital zircon grains with most ages between 1150Ma and 2000Ma, indicating provenance similar to that of the Ashburn and Martinon formations. An orthogneiss sample yielded euhedral (igneous) zircon grains with a concordia age of 615卤4Ma, indicating an interval of continental margin subduction at least 60Ma older than that represented by the Golden Grove Plutonic Suite. The Ashburn and Martinon formations and Brookville paragneiss are evidence for development of a Late Cryogenian passive margin on the Proto-Andean-Caribbean edge of Amazonia after it had separated from Laurentia by ca. 650Ma to open the Puncoviscana Ocean. By 615Ma at least part of this margin had become a subduction zone which remained active during the Late Neoproterozoic-Early Cambrian, part of the Pampean arc system. Brookville and related terranes rifted away in the mid-Cambrian to form the foundation of Early Paleozoic Ganderia, which ultimately collided with composite Laurentia during the Salinic orogeny.
|
| [11] |
[Cited within:1]
|
| [12] |
[Cited within:1]
|
| [13] |
<h2 class="secHeading" id="section_abstract">Abstract</h2><p id="">One of the major obstacles in drug discovery is the lack of <em>in vitro</em> three-dimensional (3D) models that can capture more complex features of a disease.Here we established a <em>in vitro</em> physiological model of the metabolic syndrome (MS) using cell-assembly technique (CAT), which can assemble cells into designated places to form complex 3D structures. Adipose-derived stromal (ADS) cells were assembled with gelatin/alginate/fibrinogen. Fibrin was employed as an effective material to regulate ADS cell differentiation and self-organization along with other methods. ADS cells differentiated into adipocytes and endothelial cells, meanwhile, the cells were induced to self-organize into an analogous tissue structure. Pancreatic islets were then deposited at designated locations and constituted the adipoinsular axis with adipocytes. Analysis of the factors involved in energy metabolism showed that this system could capture more pathological features of MS. Drugs known to have effects on MS showed accordant effects in this system, indicating that the model has potential in MS drug discovery. Overall, this study demonstrated that cell differentiation and self-organization can be regulated by techniques combined with CAT. The model presented could result in a better understanding of the pathogenesis of MS and the development of new technologies for drug discovery.</p>
|
| [14] |
Bioprinting is a promising technique for engineering composite tissues, such as osteochondral tissues. In this study, as a first step toward bioprinting-based osteochondral tissue regeneration, we systematically examined the behavior of chondrocytes and osteoblasts to hyaluronic acid (HA) and type I collagen (Col-1) hydrogels. First, we demonstrated that cells on hydrogels that were comprised of major native tissue extracellular matrix (ECM) components (i.e. chondrocytes on HA hydrogels and osteoblasts on Col-1 hydrogels) exhibited better proliferation and cell function than cells on non-native ECM hydrogels (i.e., chondrocytes on Col-1 hydrogels and osteoblasts on HA hydrogels). In addition, cells located near their native ECM hydrogels migrated towards them. Finally, we bioprinted three-dimensional (3D) osteochondral tissue-mimetic structures composed of two compartments, osteoblast-encapsulated Col-1 hydrogels and chondrocyte-encapsulated HA hydrogels, and found viability and functions of each cell type were well maintained within the 3D structures up to 14聽days . These results suggest that with proper choice of hydrogel materials, bioprinting-based approaches can be successfully applied for osteochondral tissue regeneration.
|
| [15] |
ABSTRACT Brain tissue engineering has now emerged as one of the most promising treatments for the traumatic brain injury. In this article, two groups of three-dimensional (3D) hydrogel structures composed of gelatin and gelatin/hyaluronan have been formed using our 3D cell assembly technique for in vivo study in rats, in order to investigate their effects in reparation of injury in the central nervous system (CNS). The structures were implanted into cortical defects created in rat brains, and their abilities to improve the brain tissue reconstruction were then evaluated. After 4, 8, 10, and 13 weeks of implantation, sections of brains were processed with NISSL staining for observing the immigration of host neural cells into the implanted materials and the degradation property of the materials. The results showed that simplex gelatin and gelatin/hyaluronan (20:1) with 3D structures both have good biocompatibility with brain tissue while gelatin/hyaluronan has a better contiguity with the surrounding tissue. Through our primary study, it seems that 3D gelatin/hyaluronan structures may be useful in brain tissue repair.
|
| [16] |
Application of hydrogels in tissue engineering and innovative strategies such as organ printing, which is based on layered 3D deposition of cell-laden hydrogels, requires design of novel hydrogel matrices. Hydrogel demands for 3D printing include: 1) preservation of the printed shape after the deposition; 2) maintaining cell viability and cell function and 3) easy handling of the printed construct. In this study we analyze the applicability of a novel, photosensitive hydrogel (Lutrol) for printing of 3D structured bone grafts. We benefit from the fast temperature-responsive gelation ability of thermosensitive Lutrol-F127, ensuring organized 3D extrusion, and the additional stability provided by covalent photocrosslinking allows handling of the printed scaffolds. We studied the cytotoxicity of the hydrogel and osteogenic differentiation of embedded osteogenic progenitor cells. After photopolymerization of the modified Lutrol hydrogel, cells remain viable for up to three weeks and retain the ability to differentiate. Encapsulation of cells does not compromise the mechanical properties of the formed gels and multilayered porous Lutrol structures were successfully printed.
|
| [17] |
To test the hypothesis that a prototype LED light curing unit, (LCU), a commercial LED LCU and a halogen LCU achieve similar cure depths, using two shades of a camphorquinone photoinitiated dental composite. To measure the LCUs' outputs and the frequency of the LED LCU's pulsed light, using a blue LED array as a photodetector.Cure depth and light output characterisation to compare the LCUs.An in vitro laboratory study conducted in the UK.The LCUs cured A2 and A4 composite shades. A penetrometer measured the depth of cure. Analysis was by one-way ANOVA, two-way univariate ANOVA and Fisher's LSD test with a 95% confidence interval. A power meter and spectrograph characterised the LCUs' emissions. A blue LED array measured the pulsed light frequency from an LED LCU.Statistically significant different LCU irradiances (119 mW/cm2 to 851 mW/cm2) and cure depths (3.90 mm SD +/- 0.08 to 6.68 mm SD +/- 0.07) were achieved. Composite shade affected cure depth. A blue LED array detected pulsed light at 12 Hz from the commercial LED LCU.The prototype LED LCU achieved a greater or equal depth of cure when compared with the commercial LCUs. LEDs may have a potential in dentistry for light detection as well as emission.
|
| [18] |
There is a need for rapid fabrication of tissue or organs with well-defined structures and functions in regenerative medicine. Two patterns of cell/matrix constructs containing hepatic cells, gelatin and fibrinogen were successfully created by automated rapid prototyping techniques and stabilized with thrombin. No apparent cell damage was found during the process. Mechanical characterization demonstrated that a 1:1 ratio gelatin/fibrin mixture had the greatest elasticity modulus and compressive strength. Microscopic and histological observations showed that hepatic cells were embedded in the gelatin/fibrinogen matrix and were proliferating. Immunostaining and biochemical analysis indicated that the embedded hepatocytes secreted albumin. Fibrin appears to be a favorable component for a gelatin based cell assembly matrix in that it is bioresorbable, easily manipulated, and supports in vitro cell functions.
|
| [19] |
In this study, a novel therapeutic cell delivery methodology in the form of hydrogel encapsulating cell-laden microspheres was developed and investigated. As a model cell for cartilage tissue engineering, chondrocytes were successfully encapsulated in gelatin-based microspheres (mostly of diameter 50–10002μm, centred at 75–10002μm) with high cell viability during the formation of microspheres via a water-in-oil single emulsion process under a low temperature without any chemical treatment. These cell-laden microspheres were then encapsulated in alginate-based hydrogel constructs. By elevating the temperature to 3702°C, the cell-laden microspheres were completely dissolved within 202days, resulting in the same number of same-sized spherical cavities in hydrogel bulk, along with which the encapsulated cells were released from the microspheres and suspended inside the cavities to be cultivated for further development. In this cell delivery system, the microspheres played a dual role as both removable cell vehicles and porogens for creation of the intra-hydrogel cavities, in which the delivered cells were provided with both free living spaces and a better permeable environment. This temperature-cured dissolvable gelatin microsphere-based cell carrier (tDGMC) associating with cell-laden hydrogel scaffold was attempted and evaluated through WST-1, quantitative polymerase chain reaction, biochemical assays and various immunohistochemistry and histology stains. The results indicate that tDGMC technology can facilitate the delivery of chondrocytes, as a non-anchorage-dependent therapeutic cell, with significantly greater efficiency.
|
| [20] |
Fluorofenidone (FD) is a novel pyridone agent with significant antifibrotic effects in vitro. The purpose of this study is to investigate the effects of FD on renal interstitial fibrosis in rats with obstructive nephropathy caused by unilateral ureteral obstruction (UUO). With pirfenidone (PD, 500mg/kg/day) and enalapril (10mg/kg/day) as the positive treatment controls, the rats in different experimental groups were administered with FD (500mg/kg/day) from day 4 to day 14 after UUO. The tubulointerstitial injury, interstitial collagen deposition, and expression of type I and type III collagen, transforming growth factor-β 1 (TGF-β 1 ), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), α-smooth muscle actin (α-SMA), and tissue inhibitor of metalloproteinase-1 (TIMP-1) were assessed. FD treatment significantly attenuated the prominently increased scores of tubulointerstitial injury, interstitial collagen deposition, and protein expression of type I and type III collagen in ureter-obstructed kidneys, respectively. As compared with untreated rats, FD also significantly reduced the expression of α-SMA, TGF-β 1 , CTGF, PDGF, and inhibitor of TIMP-1 in the obstructed kidneys. Fluorofenidone attenuates renal interstitial fibrosis in the rat model of obstructive nephropathy through its regulation on fibrogenic growth factors, tubular cell transdifferentiation, and extracellular matrix.
|
| [21] |
The combined potential of hydrogels and rapid prototyping technologies has been an exciting route in developing tissue engineering scaffolds for the past decade. Hydrogels represent to be an interesting starting material for soft, and lately also for hard tissue regeneration. Their application enables the encapsulation of cells and therefore an increase of the seeding efficiency of the fabricated structures. Rapid prototyping techniques on the other hand, have become an elegant tool for the production of scaffolds with the purpose of cell seeding and/or cell encapsulation. By means of rapid prototyping, one can design a fully interconnected 3-dimensional structure with pre-determined dimensions and porosity. Despite this benefit, some of the rapid prototyping techniques are not or less suitable for the generation of hydrogel scaffolds. In this review, we therefore give an overview on the different rapid prototyping techniques suitable for the processing of hydrogel materials. A primary distinction will be made between (i) laser-based, (ii) nozzle-based, and (iii) printer-based systems. Special attention will be addressed to current trends and limitations regarding the respective techniques. Each of these techniques will be further discussed in terms of the different hydrogel materials used so far. One major drawback when working with hydrogels is the lack of mechanical strength. Therefore, maintaining and improving the mechanical integrity of the processed scaffolds has become a key issue regarding 3-dimensional hydrogel structures. This limitation can either be overcome during or after processing the scaffolds, depending on the applied technology and materials. (C) 2012 Elsevier Ltd. All rights reserved.
|
| [22] |
A non-invasive, luminescence quenching technique is developed for continuous monitoring of oxygen spatial-temporal concentration distribution in fully hydrated gelatine gels, intended for use as scaffolds in tissue engineering. Two mass transfer-diffusion models were used to simulate the unsteady-state oxygen mass transport in the system. Oxygen diffusion coefficient and mass transfer coefficient at the water-gel interface were determined for un-crosslinked gelatine, as well as gelatine crosslinked with 1 and 1.5% w/v glutaraldehyde. While crosslinking and increased concentration of the crosslinking agent reduced oxygen mass transfer across the gel surface, both factors increased the diffusion coefficient of oxygen in the bulk of the gel. Voids in the gelatine's microstructure, which were generated during the crosslinking process due to shrinkage and associated internal stresses, were associated with both increasing the diffusion coefficient within the gel, as well as inhomogeneous diffusion of oxygen within the gel.
|
| [23] |
<h2 class="secHeading" id="section_abstract">Abstract</h2><p id="">The mechanical, thermal, swelling and release properties of glutaraldehyde (GTA) crosslinked gelatin films have been investigated in order to verify the influence of GTA concentration on the stability of the films. Air-dried films were submitted to treatment with GTA solutions at concentrations ranging from 0.05 to 2.5 wt%. At the smallest GTA concentration, the crosslinking degree, determined by trinitrobenzensulfonic acid assay, amounts to about 60% and increases up to values near 100%, obtained with GTA concentrations ?1 wt%. Simultaneously, the deformability of the films decreases, whereas the stress at break, <em>σ</em><sub>b</sub>, and the Young's modulus, <em>E</em>, increase. A crosslinking degree of about 85%, obtained using 0.25% GTA, is enough to prevent gelatin release in buffer solution and to provoke a significant reduction of the swelling in physiological solution. Furthermore, crosslinking greatly affects the thermal stability of the samples, as indicated by the results of differential scanning calorimetry (d.s.c.) investigation carried out on wet and air-dried films. The data suggest that the use of GTA at low concentration, which is desiderable to prevent toxicity, allows to modulate the physico-chemical properties of gelatin films, in order to obtain stable materials with a wide range of possible biomedical applications.</p>
|
| [24] |
Scaffolds are of great importance for tissue engineering because they enable the production of functional living implants out of cells obtained from cell culture. These scaffolds require individual external shape and well defined internal structure with interconnected porosity. The problem of the fabrication of prototypes from computer assisted design (CAD) data is well known in automotive industry. Rapid prototyping (RP) techniques are able to produce such parts. Some RP techniques exist for hard tissue implants. Soft tissue scaffolds need a hydrogel material. No biofunctional and cell compatible processing for hydrogels exists in the area of RP. Therefore, a new rapid prototyping (RP) technology was developed at the Freiburg Materials Research Center to meet the demands for desktop fabrication of hydrogels. A key feature of this RP technology is the three-dimensional dispensing of liquids and pastes in liquid media. The porosity of the scaffold is calculated and an example of the data conversion from a volume model to the plotting path control is demonstrated. The versatile applications of the new hydrogel scaffolds are discussed, including especially its potential for tissue engineering.
|
| [25] |
This study focuses on determining the effect of varying the composition and crosslinking of collagen-based films on their physical properties and interaction with myoblasts. Films composed of collagen or gelatin and crosslinked with a carbodiimide were assessed for their surface roughness and stiffness. These samples are significant because they allow variation of physical properties as well as offering different recognition motifs for cell binding. Cell reactivity was determined by the ability of myoblastic C2C12 and C2C12-alpha 2+ cell lines (with different integrin expression) to adhere to and spread on the films. Significantly, crosslinking reduced the cell reactivity of all films, irrespective of their initial composition, stiffness or roughness. Crosslinking resulted in a dramatic increase in the stiffness of the collagen film and also tended to reduce the roughness of the films (R-q = 0.417 +/- 0.035 mu m, E = 31 +/- 4.4 MPa). Gelatin films were generally smoother and more compliant than comparable collagen films (R-q = 7.9 +/- 1.5 nm, E = 15 +/- 3.1 MPa). The adhesion of alpha 2-positive cells was enhanced relative to the parental C2C12 cells on collagen compared with gelatin films. These results indicate that the detrimental effect of crosslinking on cell response may be due to the altered physical properties of the films as well as a reduction in the number of available cell binding sites. Hence, although crosslinking can be used to enhance the mechanical stiffness and reduce the roughness of films, it reduces their capacity to support cell activity and could potentially limit the effectiveness of the collagen-based films and scaffolds. (c) 2012 Acts Materialia Inc. Published by Elsevier Ltd. All rights reserved.
|
| [26] |
[Cited within:1]
|
| [27] |
[Cited within:1]
|
| [28] |
<h2 class="secHeading" id="section_abstract">Abstract</h2><p id="">Porosity and pore size of biomaterial scaffolds play a critical role in bone formation in vitro and in vivo. This review explores the state of knowledge regarding the relationship between porosity and pore size of biomaterials used for bone regeneration. The effect of these morphological features on osteogenesis in vitro and in vivo, as well as relationships to mechanical properties of the scaffolds, are addressed. In vitro, lower porosity stimulates osteogenesis by suppressing cell proliferation and forcing cell aggregation. In contrast, in vivo, higher porosity and pore size result in greater bone ingrowth, a conclusion that is supported by the absence of reports that show enhanced osteogenic outcomes for scaffolds with low void volumes. However, this trend results in diminished mechanical properties, thereby setting an upper functional limit for pore size and porosity. Thus, a balance must be reached depending on the repair, rate of remodeling and rate of degradation of the scaffold material. Based on early studies, the minimum requirement for pore size is considered to be ∼100 μm due to cell size, migration requirements and transport. However, pore sizes >300 μm are recommended, due to enhanced new bone formation and the formation of capillaries. Because of vasculariziation, pore size has been shown to affect the progression of osteogenesis. Small pores favored hypoxic conditions and induced osteochondral formation before osteogenesis, while large pores, that are well-vascularized, lead to direct osteogenesis (without preceding cartilage formation). Gradients in pore sizes are recommended for future studies focused on the formation of multiple tissues and tissue interfaces. New fabrication techniques, such as solid-free form fabrication, can potentially be used to generate scaffolds with morphological and mechanical properties more selectively designed to meet the specificity of bone-repair needs.</p>
|
| [29] |
In our previous work, a novel microcavitary hydrogel was proven to be effective for proliferation of chondrocytes and maintenance of chondrocytic phenotype. In present work, we further investigated whether the size of microcavity would affect the growth and the function of chondrocytes. By changing the stirring rate, gelatin microspheres in different sizes including small size (80–120μm), middle size (150–200μm) and large size (250–300μm) were prepared. And then porcine chondrocytes were encapsulated into alginate hydrogel with various sizes of gelatin microspheres. Cell Counting Kit-8 (CCK-8), Live/dead staining and real-time PCR were used to analyze the effect of the pore size on cell proliferation and expression of specific chondrocytic genes. According to all the data, cells cultivated in microcavitary hydrogel, especially in small size, had preferable abilities of proliferation and higher expression of cartilaginous markers including type II collagen, aggrecan and cartilage oligomeric matrix protein (COMP). Furthermore, it was shown by western blot assay that the culture of chondrocytes in microcavitary hydrogel could improve the proliferation of cells potentially by inducing the Erk1/2-MAPK pathway. Taken together, this study demonstrated that chondrocytes favored microcavitary alginate hydrogel with pore size within the range of 80–120μm for better growth and ECM synthesis, in which Erk1/2 pathway was involved. This culture system would be promising for cartilage tissue engineering.
|
| [30] |
[Cited within:1]
|
| [31] |
<h2 class="secHeading" id="section_abstract">Abstract</h2><p id="">The possibility to stabilize gelatin films by crosslinking with genipin was investigated through a mechanical, chemical and thermal characterization of samples treated with genipin solutions at different concentrations. The extent of crosslinking, evaluated as difference between the number of free <em>ε</em>-amino groups before and after crosslinking, increases as a function of genipin concentration up to about 85%. Simultaneously, the deformability of the films decreases whereas the Young's modulus <em>E</em>, increases. Furthermore, crosslinking provokes a significant reduction of the swelling in physiological solution, and enhances the thermal stability of the samples, as indicated by the results of the d.s.c. investigation. The data obtained from the films treated with genipin at concentrations higher than 0.67% are quite similar, and indicative of a good stabilizing effect of genipin. In spite of the small gelatin release (2%) observed after 1 month of storage in buffer solution, the mechanical, thermal and swelling properties of the films are very close to those previously obtained for glutaraldehyde crosslinked gelatin, and suggest that genipin, which is by far less cytotoxic, can be considered a valid alternative for crosslinking gelatin biomaterials.</p>
|
| [32] |
During the past decades, hydrogels have been introduced suitable as novel materials for a variety of applications such as biomedical engineering, sanitary products, agriculture, bioseparation, enhanced oil recovery, etc. They have been successfully used as superabsorbent materials and in drug delivery, cell encapsulation and tissue repair due to their high water content and consequent biocompatibility. Considering the fact that water retention in the hydrogels provides a suitable drug diffusion pathway; many hydrogel-based networks have been designed and fabricated as intelligent carriers of drugs. The rate and degree of hydrogel swelling are the most important parameters which control the release patterns of solvents and drugs from these polymeric networks. Therefore, the precise account of hydrogel behaviour as well as mathematical description of equilibrium swelling, dimensional changes due to solvent uptake, desorption and drug release profiles were the main objectives in many investigations. The objective of this manuscript is to give a brief review on existing mathematical models and theories in the field of hydrogel swelling as well as the description of the drug release mechanism from swelling-controlled networks. The most important properties of hydrogels relevant to their swelling behaviour as well as kinetics and thermodynamic of swelling are also presented.
|
| [33] |
Magnesium has been recently recognized as a biodegradation metal for bone substitute application. In the present work, porous magnesium foams were prepared by powder metallurgical process. The structural characteristics, mechanical properties and the in vitro biodegradation of the porous Mg specimens were investigated. These open-cellular specimens (porosities: 36–55%; pores: 200–400μm) have the appropriate mechanical properties and the changeable in vitro degradation rates. These results suggest that the porous magnesium metals have the potential to serve as degradable implants for bone substitute applications.
|
| [34] |
A paradigm shift is taking place in medicine from using synthetic implants and tissue grafts to a tissue engineering approach that uses degradable porous material scaffolds integrated with biological cells or molecules to regenerate tissues. This new paradigm requires scaffolds that balance temporary mechanical function with mass transport to aid biological delivery and tissue regeneration. Little is known quantitatively about this balance as early scaffolds were not fabricated with precise porous architecture. Recent advances in both computational topology design (CTD) and solid free-form fabrication (SFF) have made it possible to create scaffolds with controlled architecture. This paper reviews the integration of CTD with SFF to build designer tissue-engineering scaffolds. It also details the mechanical properties and tissue regeneration achieved using designer scaffolds. Finally, future directions are suggested for using designer scaffolds with in vivo experimentation to optimize tissue-engineering treatments, and coupling designer scaffolds with cell printing to create designer material/biofactor hybrids.
|
| [35] |
One of the main issues in tissue engineering is the fabrication of scaffolds that closely mimic the biomechanical properties of the tissues to be regenerated. Conventional fabrication techniques are not sufficiently suitable to control scaffold structure to modulate mechanical properties. Within novel scaffold fabrication processes 3D fiber deposition (3DF) showed great potential for tissue engineering applications because of the precision in making reproducible 3D scaffolds, characterized by 100% interconnected with different shapes and sizes. Evidently, these features also affect mechanical properties. Therefore, in this study we considered the influence of different structures on dynamic mechanical properties of 3DF scaffolds. were varied in size and shape, by changing fibre diameter, spacing and orientation, and layer thickness. With increasing porosity, dynamic mechanical analysis () revealed a decrease in elastic properties such as dynamic stiffness and equilibrium modulus, and an increase of the viscous parameters like damping factor and creep unrecovered strain. Furthermore, the Poisson's ratio was measured, and the shear modulus computed from it. Scaffolds showed an adaptable degree of compressibility between and incompressible materials. As comparison, cartilage was tested and its properties fell in the fabricated scaffolds range. This investigation showed that viscoelastic properties of 3DF scaffolds could be modulated to accomplish mechanical requirements for tailored tissue engineered applications.
|