** Corresponding author. Prof., Ph.D.; Tel.: t86 24 23971682; E-mail address:kyang@imr.ac.cn (K. Yang).
Biocompatible and biodegradable magnesium (Mg) based metals have attracted great interest for use in orthopedic implants and devices. Based on our previous study that Mg with and without micro-arc oxidation (MAO) coating showed obvious cytotoxic effect on tumor cells due to the increase of pH value during the degradation of Mg, this study further evaluated the cytotoxic effect of Mg and MAO coated Mg on osteosarcoma (MG-63) cells by analyzing the cell adhesion, morphology and number through observation of scanning electron microscope, as well as live/dead staining, lactate dehydrogenase (LDH) activity and 4, 6-diamidino-2-phenylindole (DAPI) assay. The results indicated that, compared to titanium, Mg could strongly inhibit the cell adherence, morphology and number of MG-63 on the surface of the naked Mg, whereas the MAO coated Mg showed relative weak cytotoxic effect on MG-63 cells, expecting that magnesium based metals with suitable coating can be designed to be applied as tumor prosthesis in the clinical practice.
Osteosarcoma (OS) is the most common primary solid tumor in bones for children and young adults[ 1],[ 2], comprising about 20% of primary bone sarcomas[ 3], and represents the second highest cause of cancer-related death in this age group[ 4]. Currently, the primary treatments to this disease include chemotherapy and surgery. However, most chemotherapeutics bring about the risk of both short-term and long-term toxic effects. In addition, despite improved aggressive treatment comprising multi-agent chemotherapy and surgical resection, approximately 30%-40% of patients still were dead from this disease[ 4],[ 5].
In order to treat the OS, it is usually excised thoroughly during the surgery operation, and the treatment of bone defects, especially the large bone defects resulting from tumors represent a major challenge for clinicians[ 1]. To solve these issues, bioactive porous scaffolds have been widely studied to regenerate the lost bones[ 2]. However, the currently used biomaterials for these bone repairs have not possessed the function of inhibiting the recurrence of tumor by their own, and hence often failed to eradicate the OS. Therefore, the reoccurrence and systematically spread of OS are common in clinical.
Magnesium (Mg) based metals including pure Mg and its alloys are receiving increasing attention as a new class of biodegradable implant materials for orthopedic applications[ 6], [ 7], [ 8],[ 9]. Mg is a natural ionic presence with significant functional roles in biological systems, and has the ability to stimulate the growth of new bone tissue, which is important for both osteoconductivity (for guidance of new bone growth) and osteogenesis (for promoting new bone formation)[ 10],[ 11]. Moreover, Mg and its alloys are lightweight, with mechanical properties similar to those of natural bone. The elastic modulus and compressive strength of Mg alloys are closer to those of natural bone than that of the other commonly used metallic biomaterials[ 12],[ 13]. In particular, Mg and its alloys are biodegradable in the human body, where biodegradation of the Mg implants involves the formation of a soluble and non-toxic oxide that can be safely excreted in the urine. In addition, our previous works have also well proved that Mg and its alloys possessed the antibacterial ability[ 14], and hence could inhibit the happening of osteomyelitis[ 15], an ever-present and serious complication caused by bacterial infection. Therefore, Mg and its alloys with such impressive versatile bio-functions have significant potentials to be a new class of bioactive porous scaffolds for bone repair resulting from the OS.
What's more interesting and striking is that a pure Mg showed cytotoxic effect on tumor cells in our recently published work[ 16]. Our results pointed out that the reason of such cytotoxic effect on tumor cells came from the high alkalinity, i.e., a great increase of pH value caused by the degradations of Mg in the culture medium, whereas the increase of Mg2+ concentration did not have such cytotoxic effect. Thus, a porous bone scaffold consisting of Mg or its alloy should not only have the above mentioned performances but also may have the ability of inhibiting recurrence of the OS, which will be a more perfect and potential scaffold for bone repair in the treatment of OS.
Accordingly, in order to further investigate the phenomenon of cytotoxic effect of Mg based metals on tumor cells, a pure Mg with and without micro-arc oxidation (MAO) that is a successful surface coating technique was examined by scanning electron microscopy (SEM), and in vitro adhesion and proliferation of human osteosarcoma MG-63 cells on both surfaces of Mg and MAO-Mg were evaluated by several ways including live/dead staining, lactate dehydrogenase (LDH) activity and 4, 6-diamidino-2-phenylindole (DAPI) assay in this study. The information from present study may provide an expectation for future application of Mg based metals as a new type of bone tumor prosthesis.
For all the experiments, samples were obtained by machining high-purity Mg (99.9%) rod into discs of Φ1 mm × 1 mm in dimension. In order to reduce the corrosion rate, a Si-containing coating on Mg was conducted by MAO method. Mg samples were passivized by soaking in an electrolyte at 1000-1100 Hz with a voltage of 440-450 V and an oxidation time of 10 min to obtain the Si-containing coating on the surface. Pure titanium (Ti) was chosen as the control in this study.
After the above surface treatment, the surface morphology and chemical composition of the samples were examined by using a scanning electron microscope (HITACHI, S-3700) equipped with an energy dispersive spectrometer (EDS) attachment.
The human osteosarcoma MG-63 cells were maintained in a Dulbecco's Modified Eagle's Medium (Gibco, Life technologies, Carlsbad, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Thermo, Fremont, USA) in a humidified 5% CO2 atmosphere at 37 °C, and the medium was changed every two days. Cell suspension (500 μl) was seeded directly onto the Mg, MAO coated Mg and Ti samples, respectively, at a density of 2 × 104 cells/ml, and three samples were measured for each condition.
Cell viability was determined by the live/dead staining to determine the viable and non-viable MG-63 cells after culturing on samples for 24 h. The living and dead cells were stained with Calcein-AM and Ethidium homodimer (Sigma, USA) at 37 °C for 30 min, respectively, and then visualized with a fluorescence microscope (LEICA, DM IRB).
The LDH activity was used as an indicator of cell membrane integrity and served as a general index of the cytotoxicity in the culture media. Briefly, cells were cultured on the samples in 48-well plates for 3 h (the maximum period could be observed due to the serum deprivation before cell death), and 50 μl of the supernatant was collected and determined spectrophotometrically at 440 nm according to the manufacturer's instructions.
Cell morphology and adhesion were analyzed by using SEM and DAPI staining technology. For morphological analysis of the MG-63 cells after being cultured for 24 h, the samples were washed with phosphate buffered saline (PBS) for three times and fixed with 3% glutaraldehyde overnight at 4 °C. The cells were dehydrated through graded concentrations of ethanol (30%, 50%, 70%, 95%, 100%) and finally dried. Then samples were sputtering coated with gold in a Balzers SCD040 sputtering system and observed by SEM.
For the early stage adhesion analysis of the MG-63 cells, cells were cultured on samples for 30, 60 and 120 min, then the samples were washed with PBS and fixed by 4% paraformaldehyde and stained with 4, 6-diamidino-2-phenylindole (DAPI). The cell numbers in six random fields were counted under a fluorescence microscope.
After culture for 24 h, the cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Once fixed, the cells were washed twice with 1 × PBS. To permeate the cells, 0.2% Triton X-100 was added for 10 min. The cells were then washed twice with 1 × PBS and the samples were incubated for 1 h in a blocking solution (1% bovine serum albumin (BSA)/1 × PBS). After incubation, cells were washed three times for 5 min with PBS. TRITC-conjugated phalloidin (5 μg/ml, Sigma) was added and incubated for 1 h, and then the cells were washed three times for 5 min with PBS. Following this, DAPI (10 μg/ml, Sigma) was added and incubated for 5 min. Again the cells were washed three times, and then samples were visualized and photographed using a red (actin) and blue (DAPI) filter by a fluorescence microscope.
Statistical analysis was carried out by a one-way analysis of variance (one-way ANOVA) using statistics package for social science (SPSS 13.0) software. Experiments were performed three times with three replicates for each sample and all the graphs were expressed as mean ± standard error (SD), where n = 3. p-value < 0.05 was considered statistically significant and p < 0.01 was considered to be highly significant.
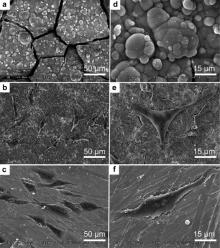
The microstructure and the EDS analysis of the MAO coated Mg are shown in Fig. 1. Rough and loose surface morphology with porous structure and small cracks could be observed clearly on the MAO-Mg. EDS analysis on a random area of the coating surface, as shown in Fig. 1(d), indicates that the Si-containing MAO coating consist of O, Mg and Si.
The cell viability was also evaluated using live/dead staining, after 24 h incubation on the different samples. Representative fluorescence micrographs were chosen to show the live (green) and dead (red) cells, as shown in Fig. 2. The photos revealed that there were almost no obvious live cells adhering on the pure Mg sample after 24 h incubation, meaning that there was obvious toxic effect on MG-63 cell for pure Mg. However, relatively large amount of live (green) MG-63 cells spreading well on the surface were visible on the MAO-Mg.
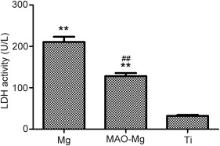
The cytotoxicity of adherent cells on different experimental materials after 3 h culture indicated by the LDH activity is shown in Fig. 3. The LDH level in MAO group was significantly lower ( p < 0.01) than that in Mg group, whereas higher than that in Ti group, indicating that the cytotoxic effect of MAO-Mg on MG-63 cells was weaker than that of Mg, but stronger than that of Ti.
The morphologies of the cells cultured for 24 h on the surfaces of different samples are shown in Fig. 4. Various responses of cells to different sample surfaces were obvious. For the naked Mg sample, the cells maintained round morphologies and no obvious live cells were observed on the surface during the whole incubation period. For the MAO group, many cells were observed, and the cells were sail-like, polygonal elongated and thicker in the central area of the nucleus and nucleolus. For the pure Ti, most of the cells were observed on the surface and the cells were spindle-like, spread out largely with numerous filamentous extensions at the leading edges and displayed a strong adhesion to the sample. Both the MAO group and the pure Ti group showed significantly better cell response than the naked Mg sample after cell culture for 24 h.
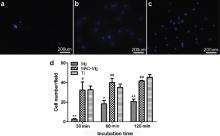
The DAPI method was used for early stage adhesion testing. The number of adhered cells on the three different samples after 30, 60 and 120 min of incubations were counted, respectively, and the photos of 60 min incubation are shown in Fig. 5. The results revealed significant differences, and that the adherent cell numbers on the MAO-Mg and Ti were dramatically larger than that on the naked Mg at each time interval. Cell numbers on the pure Ti surface was higher than that on the MAO surface after incubation for 30 and 120 min, but the cell number on the MAO group was significantly higher than that on the pure Mg group at 60 min ( p < 0.01).
The actin staining graphs (red) and DAPI staining graphs (blue) were merged using camera software in the same perspective. The mergence results are shown in Fig. 6. Fluorescent images of the cytoskeletal actin in cells cultured on the different surfaces of samples can be displayed with corresponding DAPI nuclear staining. It can be seen that there were no obvious intact dyeing cells on surface of the naked Mg; the endocellular matrix is leaky, and the cells have prominently been disrupted, whereas there were numerous visible cytoskeletons staining on surface of the pure Ti. The quantity of actin staining regions on the MAO surface was less than that on the pure Ti, but there was a trend of increasing cellular elongation on the MAO surface.
Various studies involving Mg-doped biomaterials have indicated that Mg based implants possessed higher biological activity than the conventional metals used for medical implants, showing the potential to serve as biodegradable metal scaffolds suitable for repair of load bearing defects in osseous tissue[ 17] and [ 18]. In the present work, both pure Mg and the surface modified Mg were studied for the cytotoxic effect on osteosarcoma cells, including observations of adhesion, morphology and proliferation.
In our previous study[ 16], we found that the high alkalinities, i.e., a great increase of pH value, caused by the degradations of Mg with and without MAO coating in the culture medium all showed strong cytotoxic effects on tumor cells, because cancer cells are easily survival in an acidic environment. By contrast, cancer cells are more susceptive to the alkaline environment, implying that a great increase of local alkalinity around cancer cells may be fatal to or inhibit the survival of cancer cells. A Mg based metal is such a case in point, which causes the increase in the pH value by its degradation in the physiological environment. However, the increase of Mg2+ concentration had no such cytotoxic effect. More rapid degradation of the naked Mg samples would give rise to higher pH value compared to the MAO-Mg samples. In our previous study[ 14], the pH value of the culture medium was changed after co-cultured with the Mg samples with and without MAO coating at different time points. After 4 h culture, an alkaline environment with pH of 8.5 was caused by the rapid degradation of Mg, and then the pH value increased to 9.5 and 10 after 12 h and 24 h culture, respectively. As for the MAO coated Mg samples, the pH value was 8.0, 8.5 and 8.8 after 4 h, 12 h and 24 h culture, respectively. But for the pure titanium samples, the pH value was maintained at 7.50-7.75 for different time of the culture. For this reason, there should be different cytotoxic effect for Mg, MAO-Mg and Ti on tumor cells according to the different level of pH value.
In this study, the LDH assay (Fig. 3) was used as an index of cytotoxicity, and significant increase of the LDH activity in the culture media was also detected for both the naked Mg group and the MAO-Mg group in comparison to the Ti sample. It means that the cells for the two groups were in damage or under stress due to the disruption of cytoplasmic membrane and cell necrosis. However, the cytotoxic effect of the naked Mg on tumor cells was more stronger than that of the MAO-Mg.
Cells adhesion, spreading and migration are the first sequential reactions when coming into contact with a material surface, which is crucial for cells survival. The observation on cell morphology (Fig. 4) and the number of cell adhesion (Fig. 5) reflected that the naked Mg had significant negative effect on the adhesion of osteosarcoma cells. The cytotoxicity and the inhibition effect on cell adhesion of Mg were caused by the great increase of pH during degradation. A porous structure at the micro-scale level was formed on the surface of the MAO coated Mg sample, which would have a protection on the naked Mg, showing a relatively lower cytotoxic effect on tumor cells due to the smaller change of pH during degradation[ 18]. Thus, there was still a small amount of tumor cells adhered on the surface with better morphology compared with the naked Mg.
It has been well known that the actin skeleton could mediate many important physiological functions of eukaryotic cells and provide necessary structure for cells, and thus the regulation of actin cytoskeleton reorganization is essential for cell adhesion, motility and morphological change[ 19]. Some studies indicated that the metastasis process of tumor cells was closely related to the change of the cytoskeleton structure, and the destruction of the cell microfilament bundles and the reorganization of the F-actin were significantly related with the tumor metastasis[ 20]. On the other hand, actin-modulating proteins and their upstream signaling molecules appear to play crucial roles in a variety of processes during the invasion and metastasis of tumor cells[ 21]. This study demonstrated that there was an obvious damage of osteosarcoma cell skeleton F-actin on the surface of Mg. This result was in agreement with the previous reports on changes in cytoskeleton, which is a key factor in regulating the neoplastic progression as well as tumor growth[ 22], and proved that Mg based metals are potential to inhibit the metastasis of tumor cells. Further studies including animal test are needed to deeply understand the biological reasons for these findings and to give a comprehensive interpretation.
Furthermore, in addition to the correlation with tumor development, some investigations also focused on the osteoconductive and osteoinductive of Mg, and the results showed that Mg based metals had stimulatory effects on the new bone formation in vivo[ 16],[ 23]. The corrosion product of Mg based metals, Mg(OH)2, is the major origin of the enhanced effect on bone growth in vivo[ 24].
For the role of tumor suppression, Mg based metals have their unique superiorities over the current materials used for orthopedics, and it is expected to be a viable prosthesis for application in tumor resection. Now the main limitation in the implementation of Mg as an orthopedic implant material is the fast degradation that occurs before the formation of the stable tissue around the implant. Therefore, alloying to Mg is necessary to improve the corrosion resistance, and surface modification is also an effective way to reduce the corrosion rate and improve the biocompatibility and bioactivity of Mg based metals. More efforts need to be made to select more suitable alloying elements, and more practical surface treatment is necessary for the success in clinical applications.
The present study indicated that the degradation of pure magnesium showed strong cytotoxic effect on osteosarcoma MG-63 cells in vitro, and this cytotoxicity was declined because of the existence of the protective MAO coating on Mg to reduce its degradation rate. This finding may remind us that magnesium based metals with suitable coating are potential to be applied as tumor prosthesis in the clinical practice.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (Grant No. 2012CB619100) and the Military Medical Research fund of “12th Five-Year Plan” (Grant No. cws11c268).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|