Oxidation of Alloy 690TT samples either manually ground to 400 and 1500 grit, mechanically polished, or electropolished was performed in a solution of 1500 × 10-6 B and 2.3 × 10-6 Li with 2.5 × 10-6 dissolved H2, at 325 °C and 15.6 MPa for 60 days. The oxide films grown on samples with different surface states were analyzed using various techniques. Results show that a triple-layered structure was formed after immersion: an outermost layer with large scattered oxide particles rich in Fe and Ni, an intermediate layer with small compact oxide particles rich in Cr and Fe for the ground surfaces and loose needle-like oxides rich in Ni for the polished surfaces, and an inner layer with continuous Cr-rich oxides. The surface state was found to affect not only the surface morphology, but also the corrosion rate. Grinding accelerated the growth of protective oxide films such that the ground samples showed a lower oxidation rate than the polished ones. Samples of ground Alloy 690TT showed superior resistance to intergranular attack (IGA).
Surface oxide films have been thought to play an important role in stress corrosion cracking of materials used in high temperature and high pressure water of pressurized water reactors (PWRs)[1]. It has been found that many factors can affect the growth of oxide films in high temperature and high pressurized water, such as the surface states, dissolved hydrogen, dissolved oxygen, conductivity and so on, of which the surface state is considered to be particularly important[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17] and [18]. Scenini et al.[2] found that mechanically polished (MP) Alloy 600 presented outward oxidation but found inward diffusion for electro-polished (EP) Alloy 600. This change in the oxidation mechanism was ascribed to the cold-worked layers formed on MP sample surfaces that could affect the diffusion of metallic atoms in the substrate. Ziemniak et al.[3] found that EP treatment could remove the superficially deformed layer of stainless steel thereby benefiting the homogeneous nucleation of surface oxides. Robertson[4] concluded that the influence of MP or EP treatments on the oxidation behavior of stainless steels and nickel-based materials in high temperature and high pressure water were dependent on the test temperature and water chemistry.
The nickel-based alloy 690TT has been widely used for steam generator tubing in PWRs. The effect of surface finish on the near skin microstructure of Alloy 690TT samples has been studied comprehensively in our previous work[5]. A superficial cold-worked layer with refined nano-sized grains formed on ground and MP Alloy 690TT samples and no obviously deformed layer was found on the EP surfaces. The results of short-term oxidation tests in hydrogenated primary water showed that the surface state could affect not only the surface morphology of the outmost oxides, but also the growth rate of surface oxide films. Huang et al.[19] also investigated the short-term oxidation of Alloy 690 in high temperature and high pressure water and found that abrading treatment promoted corrosion. However, for new-generation nuclear power plants designed to last 60 years, the long-term service safety of the materials employed is a critical issue. During the service of the materials, near-surface deformed layers might persist for a long time, even for the entire lifetime. In this context, the long-term effects of surface finish on the oxidation behavior of Alloy 690TT should be evaluated. Furthermore, it was found that after extended immersion in a high temperature lead-containing caustic solution[20], the accelerated dissolution of Cr from surface oxide films prepared by a similar grinding treatment could increase the stress corrosion cracking sensitivity of Alloy 690TT. In the two papers mentioned above[5] and [19], the chemical composition of the surface oxide films was simply analyzed by X-ray photoelectron spectroscopy (XPS) while the cross-sections of the oxide films, which could reveal the corrosion mechanism, were not observed. Considering that the XPS signals were collected from a relatively large area of about 0.5 mm × 0.5 mm, the surface roughness may have affected the accuracy of the XPS results and the cross-sectional microstructures of the surface oxide films deduced from the XPS results might have been incomplete. Discussing the influence of surface deformation on oxidation only as an accelerating factor is insufficient where the protection of the surface oxide films should also be considered. So far, in the literature, few papers have compared the oxidation behaviors of ground and polished Alloy 690TT by transmission electron microscopy (TEM) after extended exposure in simulated primary water.
The purpose of this study is to analyze the microstructure and chemical composition of surface oxide films formed on Alloy 690TT with different surface states after immersion in hydrogenated primary water for 60 days. The emphasis will be placed on the effects of the surface state on the oxidation behaviors of Alloy 690TT.
Samples used in the present study were cut from thermally treated (TT) Alloy 690 tubing 19.07 mm in diameter with a 1.09 mm thick wall. Its chemical composition is given in Table 1. Sample preparations have been described elsewhere[5]. Samples were prepared with four kinds of surface state, viz. ground to 400 and 1500 grit with silicon carbide paper, mechanical polished (MP) to 1.5 μ m with diamond paste, and electro-polished (EP).
| Table 1 Chemical composition of Alloy 690TT provided by EPRI (wt%) |
The immersion test was conducted in a dynamic 7L Type 316 autoclave with a circulating loop. 1500 × 10-6 B as boric acid (H3BO3) and 2.3 × 10-6 Li as lithium hydroxide monohydrate (LiOH· H2O) were added into deionized water to simulate primary water. For this experiment, the dissolved hydrogen in the water tank was controlled to be 2.5 × 10-6 and the dissolved oxygen was below 8 × 10-9. The immersion test was performed at 325 ° C and 15.6 MPa for 60 days.
After immersion, the surface morphology and microstructure of the oxide films grown on samples of Alloy 690TT with different surface states were analyzed by scanning electron microscopy (SEM), grazing incidence X-ray diffraction (GIXRD), XPS and TEM.
The SEM machine used was a field emission gun scanning electron microscope (Phillip XL30), equipped with an energy dispersive X-ray spectroscopy system (EDS). The identification of oxide phases in the surface oxide films by GIXRD was performed at the National Synchrotron Radiation Laboratory of the University of Science and Technology of China (NSRL). The photon energy was set to 10 keV to reduce sample adsorption. The X-ray incidence angle was adjusted from 0.1° to 0.5° so that the X-rays received by the detector came mainly from the oxide film[21]. The scanning range of the X-ray detectors was from 20° to 55° . The counting time was 2 s and the step size was 0.05° .
The samples were weighed using an electronic balance with an accuracy of 0.01 mg before and after immersion without descaling the oxide films.
The experimental details for the chemical composition analysis by XPS ESCALAB250 and the microstructure characterization by TEM of the oxide films have also been given in a previous report[22].
After 60 days' immersion in hydrogenated primary water, the surface morphology of the formed oxide films was found to clearly depend upon the surface sate, as shown in Fig. 1(a-d). The ground surfaces are covered with fine compact oxide particles about 100-200 nm in size with a small amount of large polyhedral oxide particles about 1-2 μ m in size scattered on top (Fig. 1(a, b)). On the other hand, the MP and EP surfaces are mainly covered with loose needle-like oxides. Some polyhedral oxide particles larger than those on the ground surfaces are also scattered on the outmost layer of the MP and EP surfaces (Fig. 1(c, d)).
'> | Fig. 1 SEM images of oxide films grown on Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, with surfaces (a) ground to 400 grit, (b) ground to 1500 grit, (c) MP, and (d) EP. |
| Table 2 Chemical composition of the large polyhedral oxides particles shown in Fig. 1 as measured by SEM-EDS (at.%) |
Table 2 lists the chemical compositions of the large outmost oxide particles by SEM-EDS on the different surfaces shown in Fig. 1. All the large particles are rich in Ni and Fe but depleted in Cr, while the Fe content is higher than that of Ni.
The weight changes per unit area of samples with different surface states are given in Fig. 2. In the present study, most of the samples presented weight loss after immersion but the average weight loss was similar for all the samples.
'>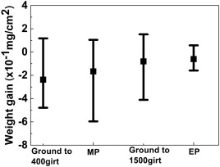 | Fig. 2 Weight changes for Alloy 690TT samples with different surface states after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa. |
Fig. 3 (a-c) show the GIXRD results obtained for the surface oxide films using synchrotron radiation. For the oxide film grown on the surface ground to 1500 grit (Fig. 3(a)), the diffraction peaks obtained with an incident angle of 0.1° correspond mainly to spinel oxides (such as NiFe2O4, PDF 89-4927; NiCr2O4, PDF 77-0008; FeCr2O4, PDF 89-3855; NiCrFeO4, PDF52-0068). Increasing the incident angle to 0.2° , the diffraction peaks corresponding to the base metal (2θ , 35° ) and metallic nickel (2θ , 35.6° ) appear in addition to those of the spinel oxides. The area marked at an incident angle of 0.2° in Fig. 3(a) is enlarged in Fig. 3(b) for detailed observation of the diffraction peaks. Fig. 3(b) therefore presents the 2θ diffraction profiles of the spinel oxides, Alloy 690TT (matrix) and metallic nickel. These are similar to the oxide phases formed on Alloy 600 and 625 in hydrogenated primary water, while the detected nickel seems to be recrystallized metallic nickel [23] and [24]. The low intensity of the diffraction peaks from metallic nickel demonstrates its low concentration in the surface films. The presence of diffraction peaks from the base metal at an incident angle of 0.2° indicates that the oxide films formed in the presence of this hydrogenated primary water are very thin. When the incident angle is increased to 0.5° , the intensity of the diffraction peaks increase markedly but the phases identified in the surface oxide films are similar. All the locations of the diffraction peaks obtained from the EP surfaces after immersion, as shown in Fig. 3(c), are identical to those found for the ground surfaces, indicating that the species formed on the EP surfaces are also mainly spinel oxides and metallic Ni.
'>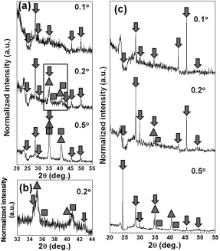 | Fig. 3 X-ray diffraction profiles obtained from films formed on Alloy 690TT samples with (a) a surface ground to 1500 grit (with (b) an enlarged view of the area marked by a rectangle in (a)) and (c) an EP surface, after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa. Three different markers indicate spinel oxide (arrow), Ni (square), and Alloy 690TT (triangle) diffraction peaks. |
Consequently, the GIXRD results verify that the oxide films formed on Alloy 690TT in hydrogenated primary water contain small inhomogeneities of recrystallized metallic nickel in addition to the dominant spinel phase, irrespective of the surface state or oxide morphology.
The XPS results obtained for the different surface films are given in Fig. 4 and Fig. 5, with Fig. 4 showing the corresponding Ni, Cr, Fe, and O depth profiles. The top layers of the ground surfaces are rich in Cr (Fig. 4(a, b)). The Fe and Ni contents are similar for depths between 0 and 50 nm but the Ni content then increases gradually. The Ni content is higher than that of Cr in the surface films on reaching the matrix. However, the MP and EP surfaces are rich in Ni and the Cr content is even lower than that of Fe for the top layer of the EP surface (Fig. 4(c, d)). Table 2 shows that the large outmost oxide particles are rich in Fe and Ni and that their Fe content is higher than that of Ni. It is thereby inferred that the chemical composition shown in Fig. 4(c, d) is mainly accounted for by the underlying needle-like oxides.
'> | Fig. 4 Composition depth profiles obtained by XPS for oxide films formed on Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, for samples (a) ground to 400 grit, (b) ground to 1500 grit, (c) MP, and (d) EP. |
'> | Fig. 5 Thickness of the oxide films grown on Alloy 690TT samples with different surface states after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa. |
In this study, the thickness limit of the surface oxide films was defined as the position where the O content in the films dropped below 5%. Fig. 5 shows the film thicknesses calculated from the XPS depth profiles in Fig. 4, with in terms order of oxide film thickness: EP > MP > ground to 1500 grit > ground to 400 grit.
The results of the valence state analysis of the O1s, Ni2p3/2, and Cr2p3/2 regions, obtained by XPS peak decomposition after different sputtering times, are summarized in Table 3 and Table 4. As the Fe content in the surface oxide films is the lowest, peak decomposition was not performed in the Fe2p3/2 region.
| Table 3 Valence states in the O1s, Ni2p3/2 and Cr2p3/2 regions measured by XPS for oxide films grown on Alloy 690TT ground to 1500 grit after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa |
| Table 4 Valence states in the O1s, Ni2p3/2 and Cr2p3/2 regions measured by XPS for oxide films grown on EP Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa |
For the oxide film grown on the surface ground to 1500 grit (Table 3), Ni(OH)2, Ni2+, Cr(OH)3 and Cr3+ were identified after 0 s sputtering by comparing the band energy with published data[8], [13], [25], [26], [27], [28] and [29]. Considering the GIXRD results in Fig. 3, the Ni2+ and Cr3+ can be ascribed to spinel oxides containing Ni and Cr. Also, the Cr hydroxide seems to be present as CrOOH[8], [30] and [31]. Ni0 signal appears after sputtering for 10 s. After 310 s sputtering, the O signal from OH- disappears. The Ni2+ signal disappears after 610 s sputtering. However, the signal from Cr0 appears after 460 s sputtering and the signal from Cr3+ is still present beyond 1210 s sputtering. This suggests that the Cr3+ signal observed for sputtering times between 610 s and 1210 s is associated with the Cr3+ in Cr2O3. The discovery of Cr2O3 at the interface between the base metal and oxide films is consistent with many previous studies[8], [9], [29], [32], [33] and [34]. The XPS peak decomposition therefore indicates that the oxide film is made up of an outer layer composed of hydroxides and oxides and an inner layer containing only oxides.
The peak decompositions obtained for the oxide film grown on the EP surface are similar to those for the ground surfaces, as shown in Table 4.
3.4.1. Plane-view observation of oxide films
Fig. 6 (a, b) shows TEM images of the large polyhedral oxide particles and small oxide particles grown on the surface ground to 1500 grit. The large oxide particles are several microns in diameter whereas the small oxide particles are about 50-200 nm in size. Supplemental TEM-EDS microanalyses in Table 5 indicate that the large oxide particles are rich in Ni and Fe but depleted in Cr, in agreement with the SEM-EDS results in Table 2. However, the small oxide particles are rich in Cr and contain more Fe than Ni. Electron diffraction patterns measured on these two kinds of oxide particles (insets in Fig. 6(a, b)) demonstrate that both types of oxide particle have a spinel-type crystal structure (NixCryFezO4), which is consistent with the results of GIXRD that the outer layer of the oxide films is composed of spinel oxides[21], [22], [23], [24] and [35]. A diffraction ring is observed in the diffraction pattern of the small oxide particles as these particles are less than 200 nm in size.
'> | Fig. 6 TEM images and electron diffraction patterns of the oxides formed on Alloy 690TT samples after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa: (a) large oxide particles formed on surfaces ground to 1500 grit, (b) small oxide particles formed on surfaces ground to 1500 grit, (c) large oxide particles formed on MP surfaces, and (d) needle-like oxide particles formed on EP surfaces. |
| Table 5 Chemical composition of the oxides shown in Fig. 6 as measured by TEM-EDS (at.%) |
Fig. 6(c, d) shows the morphologies viewed by TEM of the large oxide particles on the MP surface and the needle-like oxides on the EP surface, while Table 5 provides supplemental TEM-EDS microanalyses. The size and chemical composition of the large oxide particles are similar to those formed on the ground surface. The needle-like oxides are about 50 nm in diameter and rich in Ni but depleted in Cr. Furthermore, the electron diffraction patterns of the large oxide particles and needle-like oxides (insets in Fig. 6(c, d)) verify the spinel-type structure (NixCryFezO4) identified by GIXRD[21].
3.4.2. Cross-sectional observation
It should be noted that the large outmost oxide particles on the ground surfaces are eliminated by gallium ions during sample preparation, such that cross-sections of the oxide films grown on the EP surface (Fig. 7(a, b)) and on the surface ground to 400 grit (Fig. 8(a, b)) both show a three-layered structure. The first layer consists of large polyhedral oxide particles scattered on top of the films. The intermediate layer is made up of loose needle-like oxides for the polished surfaces, and of compact small oxide particles about 100-200 nm in size for the ground surface, while the third (inner) layer is composed of compact and continuous oxides The large topmost oxides particles seem to be embedded in the needle-like oxides. The third layer is about 100 nm thick for the EP surface (Fig. 7(b)) and about 50 nm thick for the surface ground to 400 grit (Fig. 8(b)). As shown in Fig. 7 and Fig. 8, there is a clear and straight interface between the intermediate layer and the inner layer, from which the needle-like oxide, small oxides particles, and inner layer oxides originate.
'> | Fig. 7 (a) Cross-sectional TEM images of the oxide film grown on EP Alloy 690TT after 60 days' immersion in 1500 × 10-6 B + 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, (b) a magnified view of the area marked by a rectangle in (a). |
'> | Fig. 8 (a) Cross-sectional TEM image of the oxide film grown on Alloy 690TT ground to 400 grit after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, (b) a magnified view of the area marked by a rectangle in (a). |
Fig. 9 shows TEM-EDS area maps of the chemical composition of oxide films grown on the surface ground to 400 grit and the EP surface. The small rectangle (labeled 1) in Fig. 9(a, b) is used for drift correction during scanning while the large rectangle (labeled 2) indicates the mapping area. The results indicate that the outmost layer with large oxide particles is rich in Fe and Ni and that the intermediate layer of the EP surface is rich in Ni, but that for the ground surface it is rich in Fe and Cr, in agreement with the results presented in Table 2 and Table 5. The inner layers of both the ground and EP surfaces have clearly increased Cr concentrations but decreased Fe and Ni contents compared with the matrix, verifying the suggestion from the XPS results that the inner layer oxides might be Cr2O3. The results of TEM-EDS point analysis in Table 5 and the cross-sectional area mapping in Fig. 9(b), in which the needle-like oxides are Ni-rich, verify the chemical composition obtained by XPS shown in Fig. 4(c, d) as mainly corresponding to the needle-like oxides. The loose character of the needle-like oxides results in thicker surface oxide films.
'> | Fig. 9 TEM images from surfaces of oxide films (a) ground to 400 grit and (b) EP; and (1) O, (2) Cr, (3) Ni, and (4) Fe EDS mapping of the same oxide films grown on Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa |
In conclusion, all the results obtained corroborate well and indicate that after immersion in hydrogenated primary water, the oxide films formed on Alloy 690TT with different surface states are composed of an outer layer with spinel oxides and hydroxides, and an inner layer containing Cr-rich oxides.
The triple-layered structure of the oxide films observed in this study, as shown in Fig. 7 and Fig. 8, are similar to the surface oxide films formed on Alloy 690TT in oxygenated primary water[22]. The corrosion mechanisms affecting the different layers have been discussed extensively with the conclusion that the large outmost oxide particles, the intermediate layer with small oxide particles in ground surfaces and needle-like oxides in polished surfaces, and the inner layer with Cr-rich oxides, are formed by re-deposition from the solution, external oxidation, and internal oxidation, respectively[23], [24], [32], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48] and [49]. Fig. 10 shows a schematic of the triple-layer structure of oxide films on the ground and polished surfaces, where the suggested corrosion mechanisms affecting the different layers are also indicated.
 | Fig. 10 Schematic of the growth of oxide films on Alloy 690TT with different surface states in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa |
After immersion in the same hydrogenated primary water for 60 days, small oxide particles formed in the intermediate layer of the ground surfaces, whereas for the polished surfaces needle-like structures appear. This suggests that the differences in oxide morphologies are related to the near-surface microstructure of the Alloy 690TT samples, comprehensively characterized by XRD and TEM in our previous study[5]. Grinding was found to severely deform the surface layer creating high strain and nano-sized grains. Near-surface dislocations were present in the form of parallel dislocation lines. By contrast, EP treatment avoided surface deformations and microstrain and resulted in very low dislocation densities.
For polished strain-free surfaces offering fewer nucleation sites and diffusion paths, it has been suggested that the growth of needle-like oxides is related to the fast surface diffusion of cations along the tunnel at the core of a screw dislocation or of a bundle of screw dislocations[22], just as oxide whiskers grow on polished surfaces[50], [51], [52], [53], [54], [55] and [56]. Indeed, the TEM bright field image of one needle-like oxide in Fig. 11(a) and the cross-sectional chemical composition measured by TEM-EDS line scanning in Fig. 11(b) demonstrate the existence of a hollow tunnel about 15 nm wide, which could allow metallic atoms to diffuse, in good agreement with the proposed growth mechanism.
'> | Fig. 11 (a) TEM comparison of the boundary and central regions of the needle-like oxides grown on EP Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, (b) cross-sectional EDS line-scan profile of a needle-like oxide. |
Oxide nodules were observed on EP Alloy 600 and 690 by Scenini et al.[57] after exposure to a mixture of steam and hydrogen at 480 ° C. They suggested that the oxide nodules formed were very likely to be composed of metallic Ni and found that a very high diffusivity was required to form a feature 200 nm high in 65 h. The morphology of the EP Alloy 600 surfaces with oxide nodules stemmed mostly from the fast surface and interfacial diffusion of Ni along dislocation pipes, with the internal compressive stress further enhancing the diffusivity[57] and [58]. The needle-like oxide particles formed here on the MP and EP Alloy 690TT are also rich in Ni, supporting the mechanism proposed for the growth of the needle-like oxides.
Compared to the EP surface, the ground surface has a deformed layer with a high density of dislocations and more grain boundaries due to grain refinement, leading atoms to diffuse through short-circuit paths, thereby facilitating oxide nucleation at high energy sites. However, in a reducing environment with hydrogen, these metal cations extracted from base metal cannot immediately associate with the adsorbed oxidants. Thus, the lateral diffusion of redundant cations becomes predominant[50]. This leads to almost identical lateral and vertical growth rates of the spinel oxides around the original nucleation sites and the formation of more spherical oxide particles[50], polyhedral or exactly octahedral as determined by its spinel structure.
Owing to their special conductive, optical, magnetic, and dielectric properties, a number of spinel-structured complex oxides, such as CuBi2O4, Co3O4, CoxNi1-xFe2O4, and CuFe2O4 have been prepared by sol-gel processing, hydrothermal synthesis, co-precipitation, sonochemical synthesis, and so on[59], [60], [61] and [62]. The morphologies of the synthesized oxides and the size of the oxide particles were found to be strongly dependent on the weight ratio of the chemical reagents used, and the reaction time and temperature. Indeed, increasing the NaOH concentration from 0.6 to 1.2 and 2.4 mol/L resulted in the morphologies of the synthesized CuBi2O4 oxides changing from nanosheets to hierarchical microspheres and nanoflowers[60]. The fact that most of the samples studied here presented weight loss after exposure indicates that metallic cations are released from the base metal during immersion (Fig. 2). This therefore suggests that the growth of intermediate-layer oxides is also affected by the local water chemistry near the sample surface.
According to previous results, the inner corrosion layer (50-100 nm) formed here is much thinner than the superficial deformed layer (285-470 nm)[5]. Fig. 12(a) shows a cross section of a Pt-marked Alloy 690TT sample whose surface was ground to 400 grit after exposure to hydrogenated primary water for 30 days. Several black strips are found in the surface deformed layer and the TEM-EDS line-scan profile of one strip (at line 1 in Fig. 12(a)) indicates that this strip is mainly composed of Cr oxides (Fig. 12(b)). The preferential oxidation in the cold-worked layer can thereby be ascribed to shortcut diffusion of oxygen through grain boundaries occurring due to grain refinement.
'> | Fig. 12 (a) Locations of preferential oxidation on Pt-marked samples of Alloy 690TT after 30 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa, (b) EDS lines scan profiles at location (1) in (a). |
The diffusion rates of metallic atoms in oxides follow a well-defined order: Fe > Ni > > Cr[63], [64] and [65]. Robertson[4] suggested that the faster diffusing atoms likely penetrate the outer layer. For the ground surface, the high density of defects promotes the outward diffusion of Fe, Ni, and Cr from the base metal to form compact Cr-rich Ni-Cr-Fe spinel oxide particles on the original sample surface. Meanwhile, the accelerated inward diffusion of oxygen through shortcuts can associate with the Cr in the base metal to form Cr-rich oxides in the inner layer, after the preferential dissolution of Ni and Fe. As soon as protective oxide film is formed on the sample surface, this hinders the outward and inward diffusion of atoms. The oxidation rate of Alloy 690TT would then be slowed down. However, the outward diffusion of Ni, Fe, and Cr from the EP surface is constrained by the low density of defects such that needle-like oxides rich in Ni and Fe are formed in the suggested surface diffusion mechanism. The non-compact character of the oxide films composed of needle-like oxides is clear and its ability to play a significant role in terms of alloy protection is therefore questionable. The inner layer would keep on growing until a continuous layer rich in Cr were formed. As a result, after 60 days' immersion, the internal corrosion was about 0.61 μ m/year for the EP surface, greater than the 0.30 μ m/year measured for the ground surface.
Lister et al.[66] have suggested that the nucleation and growth of the large outmost oxide particles are suppressed once a protective layer of Cr-rich inner oxide is established. The density and size of these large oxide particles grown on different surfaces are compared in Fig. 13. Larger and more numerous scattered oxide particles are observed on the MP and EP surfaces, compared with those on the ground surfaces, verifying the fast growth of protective oxide films on the ground surfaces.
'>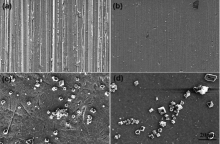 | Fig. 13 Comparison of the size and density of the large polyhedral oxide particles grown on Alloy 690TT after 60 days' immersion in 1500 × 10-6 B and 2.3 × 10-6 Li solution with 2.5 × 10-6 H2 at 325 ° C and 15.6 MPa for samples (a) ground to 400 grit, (b) ground to 1500 grit, (c) MP, and (d) EP. |
Therefore, the near-surface microstructure, including the dislocation density and grain boundaries, can affect not only the oxide morphologies but also the oxidation rate of Alloy 690TT. The oxide film formed on the ground surface of Alloy 690TT was more protective than the one formed on the EP surface after 60 days' immersion in simulated hydrogenated primary water.
After descaling the surface oxide films, a grain boundary was examined in the cross section of one of the ground samples after 60 days' immersion, as shown in Fig. 14(a). This grain boundary is about 5.6 μ m long and the V-shaped notch indicates that this is already preferentially corroded to a depth measured to be about 160 nm. The TEM-EDS line-scan profiles taken at lines 1 and 2 in Fig. 14(a) demonstrate that oxygen is not present on the inside of this grain boundary (Fig. 14(b, c)). Line 2 is a TiN strip. At the end of this grain boundary, chromium carbide was found by TEM and no obvious oxygen enrichment was found in the chemical composition measured by TEM-EDS (24.70 at.% C, 7.59 at.% O, 63.63 at.% Cr, 0.98 at.% Fe and 3.08 at.% Ni). The above results all verify that the attacked depth of this grain boundary remains within the near surface and that no Cr oxidation or depletion occurs on its inside. Ground Alloy 690TT thus shows good resistance to IGA after 60 days' immersion in this simulated hydrogenated primary water. An appropriate amount of near-surface cold working should increase the corrosion resistance when Alloy 690TT with a high Cr content is used for steam generator tubing in hydrogenated reducing high temperature water.
 | Fig. 14 (a) Cross-sectional TEM image of a grain boundary in a sample of Alloy 690TT ground to 1500 grit after descaling the surface oxide film. (b), (c) EDS line-scan profiles at lines (1) and (2) in (a) (from A to B). |
Oxide films grown on samples of Alloy 690TT with different surface states were characterized systematically in simulated hydrogenated primary water. Triple-layered oxide films were formed for all samples.
The surface states affected not only the oxide morphology but also the internal oxidation rate. The outmost layers contained some large polyhedral oxide particles rich in Ni and Fe. After immersion in hydrogenated primary water, the intermediate layer of the ground surfaces contained some small Cr-rich oxide particles with a spinel-type structure, while Ni-rich needle-like oxides with a spinel-type structure were found in the middle of the polished surfaces. The continuous inner layers for both the ground and polished surface were rich in Cr, likely present in the form of Cr2O3. The oxide film formed on the ground surface in the hydrogenated primary water used here was more protective and the internal corrosion rate for the ground surface was much smaller than for the polished surface.
Ground Alloy 690TT also showed superior IGA resistance after 60 days' immersion in simulated hydrogenated primary water.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|


