In the present study, morphology, size distribution, structure, biocompatibility and magnetic properties of potassium ferrite nanoparticles (KFeO2 NPs), synthesized by conventional sol–gel method have been reported. The formation of spherical nanoparticles with orthorhombic structure has been confirmed by scanning electron microscopy and X-ray diffraction. The particle size, as obtained by transmission electron microscopy has been found to be in the range of 4–7 nm. Further, the size distribution has been scrutinized using Analyse-it software, where a platykurtic feature in the size distribution was observed. Fourier transform-infrared spectroscopy and thermogravimetric analysis showed the formation of metal (Fe, K) bonds at Neel temperature of 337 °C. Vibrating sample magnetometer analysis revealed the superparamagnetic behaviour of the synthesized KFeO2 NPs, with saturation magnetization of 25.72 emu/g.
Nanocrystalline materials are gaining a lot of attention owing to their uniquely structural, optical, magnetic and electronic properties, which is observed when the size reduces from bulk to nano-scale. Different scientific communities, ranging from medical to engineering, are deeply investigating and developing novel materials as per their respective requirements. In the biomedical field, the foremost need is to develop biocompatible nanomaterials, as biocompatibility is an imperative parameter and needs to be ascertained before practical applications. Another essential requirement is the superparamagnetic behaviour of biocompatible nanomaterials. Superparamagnetic material remains to be magnetized as long as the external magnetic field is applied, which ensures their controlled behaviour. Other crucial requisites are nanometer size, narrow size distribution, spherical shape and crystallinity. The above-mentioned requirements have been extensively scrutinized in ferrites, where much research has been conducted on magnetite, nickel ferrite, cobalt ferrite and manganese ferrite nanoparticles [1], [2], [3], [4], [5], [6], [7], [8] and [9]. However, use of Ni, Co, Mn in ferrite nanoparticles for biomedical research is hampered by the high inherent toxicity of these transition metals. So, the need of the hour is to synthesize highly bio-favourable ferrite nanoparticles, which exhibit superparamagnetic behaviour with no toxicity threat. Instead of these metals (Ni, Co, Mn), potassium metal can be considered as a good candidate, as potassium ions are inherently non-toxic and are even necessary for functioning of the living cells. The combination of potassium and iron based compounds can be regarded as a potential biocompatible material. Though this compound is inherently non-toxic, no work has been reported to ascertain its biocompatibility so far. As reported in the literature, researchers have studied potassium and iron based compounds in bulk form, i.e. potassium ferrite, potassium ferrate, etc [10], [11], [12], [13], [14], [15], [16], [17] and [18]. Most of the studies have been performed on potassium ferrate, which are considered as an environmental friendly chemical reagent for dyeing, waste-water purification and disinfection [10], [11], [12], [13] and [14]. A few reports are available on the synthesis of bulk and nano-sized potassium ferrite particles [15], [16], [17], [18] and [19].
In the present study, the synthesis of potassium ferrite nanoparticles by sol–gel method has been reported; and their superparamagnetic behaviour and biocompatibility at nano-scale have also been studied.
All the chemicals used for synthesis were of analytical grade. Potassium nitrate (KNO3), ferric nitrate (Fe(NO3)3·9H2O), and citric acid (C6H8O7·H2O), were purchased from Loba Chemie, India. Ethylene glycol was purchased from SD Fine chemicals, India. All the chemicals were used without any further purifications.
The synthesis of potassium ferrite nanoparticles (KFeO2 NPs) was done by conventional sol–gel method [20]. This process is based on metallic citrate polymerization using ethylene glycol [20]. Briefly, 1 M (0.101 g/mol) solution of potassium nitrate and 2 M (0.808 g/mol) solution of ferric nitrate were mixed. To this mixture, 2 M (0.420 g/mol) citric acid solution was added along with 5–7 ml of ethylene glycol. Aqueous solution of citric acid was used in order to chelate cations; it also behaved as non-polymeric filler for size modification and porosity control of nano-powders and can be removed by either heat treatment or thermal decomposition, without affecting the properties of a material [21]. The solution was constantly magnetically stirred and was heated at 80–90 °C. Viscous gel began frothing, when finally all water molecules were removed from the mixture. Until the whole citrate complex was consumed, the decomposition process would not stop. Polymerization, promoted by heating, resulted in a homogeneous resin, in which metal ions were uniformly distributed throughout the organic matrix [20]. During heating, the solution became viscous and gradually formed a very viscous brown gel. On further heating dried gel was formed and it continued to burn in a self-propagating combustion manner until all the gel was completely converted to a brown-coloured powder, which also indicated the completion of auto-ignition process. The powder was thoroughly washed with ethanol and distilled water and was dried overnight in a vacuum oven at 60 °C. To obtain thorough crystallinity and to remove the undesired elements, the powder was calcined at 550 °C for 2 h, yielding KFeO2 NPs as the final product, as shown in Fig. 1.
Fig. 1 shows the accumulation of KFeO2 NPs dissolved in distilled water (by sonicating for 5 min) near a handheld magnet. Once the particles have been concentrated close to the magnet, they can be easily dragged by moving the magnet to a different position. This suggests that the synthesized KFeO2 NPs can be moved and influenced by an applied external magnetic field.
The thermal analysis of the synthesized KFeO2 NPs was done with differential thermal analyzer (DTA; Pyris 1 TGA, Perkin Elmer) and differential scanning calorimeter (DSC; LINSEIS L63).
The morphology and composition of synthesized KFeO2 NPs were studied by scanning electron microscopy (SEM; JSM-6510LV, JEOL, U.S.A) where the dried sample was first coated with gold using JEOL, JFC sputter coater. The KFeO2 NPs were also characterized by transmission electron microscopy (TEM; Hitachi (H-7500)), energy-dispersive X-ray analysis (EDAX, Oxford) and X-ray diffraction (XRD, PANalytical X’Pert PRO MRD).
The FT-IR spectrum of KFeO2 NPs, in the frequency range from 4000 cm-1 to 400 cm-1, was recorded on Perkin Elmer Spectrum BX (II) spectrophotometer.
The superparamagnetic properties and saturation magnetization of the synthesized KFeO2 NPs was determined using vibrating sample magnetometer (VSM; Princeton Applied Research Model 151/155).
It is a matter of crucial concern that the biocompatibility and non-toxicity of nanoparticles can be ensured, before they are applied for in vitro or in vivo applications [16] and [17]. The viability of T cell lines (Jurkat cells) incubated with synthesized KFeO2 NPs at different concentrations was investigated by MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, a tetrazole) colorimetric assay. Cells were cultured on tissue culture polystyrene falcon flasks in RPMI-1640 supplemented by 10% FCS (fetal calf serum) at 37 °C in 5% CO2. This solution was then centrifuged at 1200 r/min at 4 °C for 5 min to separate dead cells. The cells were trypsinized and counted on haemocytometer. After trypsinization, the cells in media were placed in 96-well tissue culture flat bottom plate at a density of 1.5 × 104 cells per well. Required concentration (5, 25, 50, 100, 250, 500 μg/ml) of nanoparticles ( n = 5) was added to the wells. It was then incubated at 37 °C for 48 h in CO2 incubator (5% CO2). Controls were carried out consisting of growing cells without any treatment. After 48 h, the cell suspension was removed from each well and 20 μl MTT solution was added; it was incubated for 4–5 h. Viable cells have the ability to reduce yellow MTT to insoluble purple formazan (This reduction takes place only when mitochondrial reductase enzymes are active, and therefore conversion can be directly related to the number of viable (living) cells). Reaction was stopped by addition of stop lysis solution (20% sodium dodecyl sulfate (SDS) in 50% dimethyl formamide + 50% distilled water). Plates were further incubated for 1 h and absorbance was recorded at 570 nm on ELISA Plate Reader. Mean and standard deviation (SD) was obtained from 5 replicates. Cell viability was calculated by the following formula:
The statistical analysis of the experimental data, as obtained for the cytotoxicity of potassium ferrite NPs, utilised the student's t-test and the results were presented as mean ± SD. Statistical significance was accepted at a level of p <0.05.
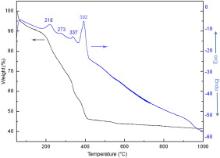
Fig. 2 shows the thermogravimetric analysis (TG) and corresponding differential thermal analysis (DTA) of the as prepared (uncalcined) KFeO2 NPs in nitrogen atmosphere. As can be observed, the entire thermogram consists of four exothermic peaks at 218, 273, 337, 392 °C and corresponding to all the above-mentioned peaks, weight loss regions can be well observed. Initially, a gradual and steady weight loss (∼10%) at 218 °C can be attributed to the loss of physical water from the surface of nanoparticles. The following small peak at 273 °C corresponds to reaction between the nitrate and citrate systems [23] and [24]. The weight loss related with this exothermic reaction is probably ascribed to the removal of water steam, H2, NO x ( x = 1, 2) and CO2 [24]. Therefore, this could be considered as a thermally induced anionic, redox reaction, where the citrate ions act as reductant and nitrate ions act as oxidant [24], leading to the decomposition of citric acid. The decomposition of citric acid favours the formation of small crystallite size in this synthesis. Since the nitrate ions provide an in situ oxidizing environment for the decomposition of the organic component, the rate of oxidation reaction increases slightly, resulting in a self-propagating combustion of nitrate–citrate gel [25]. The combination of the lowering of the reaction temperature and the increase in rate results in a self-propagating combustion of the nitrate–citrate gel [23]. The effect at 337 °C can be related to the Neel temperature of KFeO2 NPs, corresponding to the magnetic phase transition (see ). The prominent exothermic peak at about 392 °C with a weight loss of ∼10% depicts the onset of crystallization of the orthorhombic phase of KFeO2 NPs, as illustrated by XRD pattern.
On these bases, we proposed the following chemical reaction:
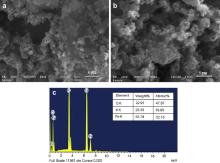
Fig. 3(a and b) displays the SEM micrograph of KFeO2 NPs. The micrographs were taken at different magnifications; in both the micrographs the agglomeration of KFeO2 NPs can be well observed. This agglomeration is attributed to the high surface energy and magnetic interaction between the nanocrystallites. Fig. 3(c) shows the EDAX of the synthesized KFeO2 NPs, which confirms the presence of all the required elements. EDAX confirms the stoichiometric formation of the synthesized KFeO2 NPs.
TEM micrograph in Fig. 4(a) displays the spherical formation of KFeO2 NPs, with diameter in the range of 4–7 nm. The agglomeration of the nanoparticles can be well observed, pertaining to the high surface energy and magnetic interactions between them. Fig. 4(b) shows the number (%), surface area (%) and volume (%) distribution of the synthesized NPs. As per the observed distribution, the maximum number of nanoparticles synthesized lies in the diameter range of 5.6–6.0 nm. The size distribution has been further studied by using Analyse-it software (a statistical analysis add-in) in Microsoft Excel [22]. The histogram, normal quartile and notched box plot of the size distribution of synthesized KFeO2 NPs were further plotted, as shown in Fig. 4(c–e), respectively. The histogram in Fig. 4(c) depicts that the particle size distribution with the maximum frequency (diameter of KFeO2 NPs) is in the diameter range of 5.5–6.0 nm, with mean value of 5.7 nm. Fig. 4(d) displays the normal quartile plot for the observations of the sample (diameter of synthesized KFeO2 NPs) against the expected normal fit. The kurtosis value indicates that it is a platykurtic distribution and is skewed to the left [26], [27] and [28]. This suggests that in the distribution more number of nanoparticles is formed in the range of 5.5–6.0 nm, lying close to the mean value 5.7 nm, towards the lower end of the distribution. This is also represented by the notched box plot, in Fig. 4(e), displaying the sample distribution [29]. The inter quartile range determines the spread; also the distance of the median from the upper and lower quartiles measures the symmetry of the distribution [28]. As shown in Fig. 4(e), the length of the whiskers far exceeds the length of the box; this is representative of a platykurtic distribution. As the lower whisker is longer than the upper whisker, this shows that the data is skewed to the left, or in other words more number of nanoparticles are formed close to the mean value and towards the lower extreme of the distribution.
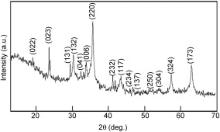
Fig. 5 shows the XRD diffractogram of KFeO2 NPs depicting their crystallinity, where all the peaks are indexed and well-matched to the pure orthorhombic structure (File No. 83-2152, space group: Pbca). The broadening of the diffraction peaks depicts the formation of nanocrystallites. XRD analysis reveals the formation of orthorhombic structure with lattice constants a = 0.5613 nm, b = 1.121 nm, c = 1.526 nm and volume V = 0.9602 nm3. These coincide well with the theoretical values, a = 0.5577 nm, b = 1.122 nm, c = 1.589 nm and volume V = 0.9943 nm3. shows the theoretical and calculated values of the unit cell parameters, as expected the unit cell parameters coincide well with the literature data.
| Table 1. Theoretical and calculated values of the unit cell parameters of KFeO2 |
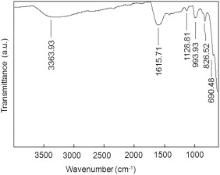
As shown in Fig. 6, the small band at 3363.93 cm-1 is assigned to the stretching hydroxyl group at the surface of KFeO2 NPs [30]. The absorption band at 1615.71 cm-1 is assigned to scissor bending vibration of absorbed water [31] and [32]. The peak at 1128.81 cm-1 is attributed to the stretching vibration of Fe–OH [33]. The band at 993.93 cm-1 is assigned to Fe–K (metal) alloy system [31]. The band at 826.52 cm-1 is assigned to Fe–O–H bending vibrations [34]. The band at 690.48 cm-1 corresponds to the stretching vibrations of the metal oxygen bonds, i.e. Fe–O bond [31] and [35].
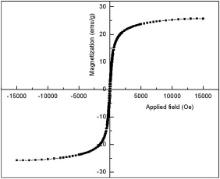
Fig. 7 shows the M– H curve as obtained for the synthesized KFeO2 NPs using VSM. It reveals that the synthesized KFeO2 NPs exhibit superparamagnetic behaviour with a high magnetic saturation, MS, value of 25.72 emu/g at room temperature. The superparamagnetic behaviour is reflected in the low squareness (remanent magnetization ( MR)/magnetic saturation, MS ratio) value [36] and [37]. The squareness value for non-interacting superparamagnetic particles is 0.5 [38]. In the case of interacting superparamagnetic particles, the dipolar interactions reduce both the magnetic squareness ( MR/ MS ratio) and coercivity ( HC) values due to demagnetizing effect [39] and [40]. Magnetic squareness value pertaining to superparamagnetic behaviour is 0.1, i.e. it loses greater than 90% of its magnetism when the applied magnetic field is removed [41]. The synthesized KFeO2 NPs possess a magnetic squareness of 0.1. Therefore, in the light of the above, it exhibits the characteristic feature of superparamagnetic behaviour.
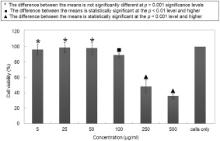
The concentration dependent cytotoxicity of the KFeO2 NPs was studied in detail. Fig. 8(a) shows the optical density (OD) values as obtained at all the concentrations for all the repetitions along with the standard deviation error values. Each data point in Fig. 8(b) was obtained by averaging that of five wells, and the untreated cells were used as control (marked as ‘cells only’), depicting a good cell viability at concentration below 250 μg/ml, but at higher concentrations, a significant loss of viability is observed, indicating that higher concentration of KFeO2 NPs shows more cytotoxicity. Fig. 8(c) shows the linearly fitted data for all the repetitions at all the concentrations, where the inset gives the linear fitting equations for all the values of concentrations and repetitions. The interaction of nanoparticles with cells begins with their adherence to the surface of the cell, which is followed by internalization by endocytosis, further leading to accumulation in digestive vacuoles. The nanoparticle–protein interaction is a key issue for defining and understanding the toxicity of the MNPs; the unfavourable changes in the protein configuration, the denaturation of the proteins on interaction could cause the exposure of new antigenic sites, which may commence a new immune response [17]. It can also be well assumed that the particle overload to the cells, at higher concentrations can be the cause of their cytotoxicity [20].
As the experiment was repeated at all the concentrations to ensure reproducibility, the repeatability of the experiment at all the concentrations was also studied in detail. displays the calculated values of coefficient of variation, repeatability (using analysis of variance) and repeatability error [42]. It can be well observed that all the repetitions are consistent and reproducible. Low values of coefficient of variation and repeatability error values imply high repeatability of the results obtained [43] and [44].
| Table 2. Coefficient of variation, repeatability, repeatability error at all concentrations |
Fig. 9 shows the statistical analysis using two-tail paired t-test (with SD error bars). The t-distribution has a wide number of applications in statistics [37]. The cell viabilities at 5, 25, 50, 100, 250, 500 μg/ml, calculated according to Eq. were 96.5%, 98.9%, 98.2%, 89.2%, 57.2%, 39.2%, respectively. The t-values were determined at each concentration with null hypothesis that the NPs did not affect the cells so that there must not be any significant difference in the means of untreated and NPs treated cells. The MTT results indicate that the KFeO2 NPs supported continuous growth of the cells on their surface. No significant differences were found in the cellular proliferation for the concentrations, i.e. 5, 25, 50, 100 μg/ml; significant difference was obtained at the higher concentration, i.e. 250 and 500 μg/ml, depicting that below 100 μg/ml the NPs were biocompatible and non-toxic. Even though at 250 and 500 μg/ml, the viability drops down to significant values, it might have been caused due to the shortage of culture media required for the growth of the cells, rather than any toxicity of the synthesized KFeO2 NPs.
On comparing these results with other reported results of NiFe2O4, ZnFe2O4, which had cell viability of 77.6% and 70.5% for 24 h, at the concentration of 100 μg/ml, on Neuro-2A cell lines [18], it is well evident that KFeO2 NPs exhibited enhanced cell viability. Bare MNPs (iron oxide) at low concentrations ranging from 16 to 31 μg/ml reduced the cell viability by less than 20%, whereas KFeO2 NPs exhibited around 89% cell viability even at 100 μg/ml [37].
With appropriate functionalization, these synthesized KFeO2 NPs find applications in areas such as, magnetic cell-separation of labelled cells and biological entities, therapeutic drug and gene delivery vehicles, contrast enhancement agents in magnetic resonance imaging (MRI), magnetic biosensing, hyperthermia treatment.
This paper reports the synthesis of KFeO2 NPs by sol–gel method. Their morphology, size distribution, structure, biocompatibility and magnetic studies have been investigated. SEM, XRD and TEM studies confirm the spherical formation of orthorhombic structure, with particle size in the range of 4–7 nm, respectively. The size distribution, as studied by Analyse-it software, suggests platykurtic feature. The synthesized KFeO2 NPs exhibit superparamagnetic characteristics with magnetic saturation of 25.72 emu/g and Neel temperature of 337 °C. In vitro cytotoxicity test on T cell lines (Jurkat cells) using MTT assay shows that the KFeO2 NPs are biocompatible at a particle concentration of 100 μg/ml. The superparamagnetic behaviour and biocompatibility of KFeO2 NPs establishes its suitability for biomedical applications and gives them an edge over other ferrites of transition metals (Ni, Co, Mn), where their inherent toxicity raises apprehensions and speculations on their use for biological applications.
The following is the supplementary data related to this article:
One of the authors, Lavanya Khanna, gratefully acknowledges Department of Science and Technology, Government of India, New Delhi for awarding her INSPIRE Fellowship to carry out this research work.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. | [Cited within: 1] |
| 42. |
|
| 43. |
|
| 44. |
|