1. Introduction
The rapid industrial growth greatly promotes the social advancement, yet it also brings the unprecedented pressure on ecological environments, such as the oily wastewater in ocean [[1], [2], [3]]. It is a huge challenge to effectively handle with this issue, and still needs researchers’ more efforts to develop the advanced technologies. Therein, the membrane technology, as a green and economic strategy, is considered to be effective to separate oil/water mixture, and has been made a great progress due to the continuous academic research and industrial development [[4], [5], [6], [7], [8], [9], [10], [11], [12]]. For instance, polymeric membrane can achieve hydrophobicity and antifouling properties by either blending with hydrophobic additives or altering its surface properties via chemical or physical modification [13,14].
At present, various methods have been used in research for the preparation of oil-water separation membranes [[15], [16], [17], [18]]. The core idea is to make the membrane materials to act as a semi-permeable layer and regulate the transportation between the two phases, i.e., oil and water. Generally, the semi-permeable layer will allow oil phase to flow through, and intercept the water phase above the membrane materials [[19], [20], [21]]. Actually, this is a competitive issue, where the separation ability is highly depended on the apparent surface energy (synergetic factor between membrane microstructures and surface energy). If the apparent surface energy closes to the value of oil phase, the oil will flow through the membrane materials. Oppositely, water will flow the membrane materials [22]. However, there are still many limitations in large-scale fabrication of such functional films and practical applications currently because of the expensive and complicated preparation process, poor physical conditions, low stability and flexibility, as well as poor selectivity and recyclability [23,24], etc. Consequently, the current task is to develop a simple strategy to prepare the excellent membrane materials and overcome the above-mentioned limitations.
Many researchers around the world are paying more attentions to the potential of superhydrophobic materials in the field of oil-water separation [[25], [26], [27], [28]]. Superhydrophobic coatings, as a popular enhancement technology, have been widely investigated for the modification of membranes. Many reports or literatures have shown the possibility of preparing superhydrophobic and superoleophobic functional materials by a combination of low surface energy and suitable rough morphology, as well as the potential of using these materials for the effective oil-water separation [[29], [30], [31], [32]]. Because the surface wettability of materials could be tuned by adjusting surface energy and morphology, various materials with simultaneously superhydrophobic and superoleophilic characters have been developed by modifying a micro/nanostructured surface with low surface-energy composition or constructing roughness morphology on low surface-energy materials [33]. Zhang et al. [34] fabricated the composite membranes composed of two inexpensive materials, porous PU and PS microspheres, which have the special non-wetting properties with both superhydrophobicity and superoleophilicity. With such an “intelligent” wettability property, the composite membranes can be utilized to efficiently separate oil and water systems. Furthermore, Feng et al. [35] developed a novel interfacial material of hard coatings, and coated it on the surface of mesh film to produce both superhydrophobic and superoleophilic properties. Also, this material was mainly fabricated via constructing the micro-nanostructured rough surfaces on a fluorine-containing material. The separation of diesel oil from water-mixture was very efficient in this study, which was suitable for many practical applications. In addition, these functional materials mainly focus on the coated-copper mesh made by solution-immersion process [36], low-energy polyurethane sponges [37], as well as the metal meshes made by the electrochemical deposition method [38], and the wet chemical approach [39], etc. However, there remain a lot of limitations for large-scale fabrication of such functional materials [40].
On this basis, many researchers have begun to think about giving superhydrophobicity to ordinary textiles directly. They consider that the superhydrophobic and superoleophilic textiles with nano-coatings can be effectively used for oil-water separation, which is also an easily-achieved method to prepare the large-scale membrane materials with the assistance of material flexibility [[41], [42], [43], [44], [45], [46]]. The composite coating can be obtained on the textile with particular non-wettability, repelling water completely while allowing the permeation of oil. Since water stays exclusively on the textile surface, oil could be successfully separated from the mixture. The as-prepared fabric maintains high separation efficiency and stable recyclability during the repeated separating experiments. Zhang et al. [47] prepared the smart textiles with switchable superoleophilicity and superoleophobicity in aqueous media by grafting a block copolymer comprising pH-responsive P2VP and oleophilic/hydrophobic PDMS blocks on the textiles, and these functionalized materials were confirmed with the great ability in oil-water separation field. Also, Li et al. fabricated superhydrophobic textiles by coating fibers with nanocrystals and modification of octadecyl thiol [48]. These hydrophobic/oleophilic filter membranes are frequently used in practice owing to their low-cost and availability, however they still face some problems of poor separation selectivity and efficiency [49]. Therein, there are less discussions on the mechanic relation between the geometric size of the function coated-mesh and surface tension of oil phase, yet the mechanic relation is a non-ignored factor to result in the poor separation selectivity and efficiency.
In this work, we proposed a two-step impregnation method for the fabrication of superhydrophobic polyethylene terephthalate (PET) textiles with PDMS and hydrophobic silica particles. The obtained functional textiles exhibited superhydrophobic property, which was confirmed with the effective oil-water separation ability towards various organic liquids. Also, under the assistance of physical model, it was confirmed that there was a mechanical relation between pore size and organic liquids, where the main connotation was the matching issue of surface tension of organic liquids with the geometrical mechanic (induced by the pore size of the superhydrophobic coated-mesh).
2. Experimental
2.1. Materials
PET textile fabrics with different meshes (60, 120, 200, 250, 300, and 400 meshes) were purchased from Peshanni Hardware Store, in Jiangsu, China. Silica nanoparticles (20 nm, 99.0%) were supplied by Nanjing XFNANO Materials Tech Co., Ltd. 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDTES, 96%) were provided by Shanghai Macklin Biochemical Co., Ltd (China). Polydimethyl-siloxane (PDMS) prepolymer (Sylgard 184 silicone elastomer kit with curing agent) was purchased from Dow Corning Co., Ltd (USA). Anhydrous ethanol (99.7%), ammonium hydroxide (25.0 wt.%-28.0 wt.%) and tetrahydrofuran (THF, 99.0%) were obtained from Sinopharm Chemical Reagent Co., Ltd (China). Trichloromethane, n-decane, kerosene, n-octane and n-hexane were acquired from Shanghai Aladdin Reagent Co., Ltd (China).
2.2. Preparation of hydrophobic F-SiO2 nanoparticles
3 g of SiO2 nanoparticles were weighed into mixed solution containing 4 mL of ammonia water and 16 mL of deionized water, followed by sonication for 10 min. Afterwards, 0.6 mL of PFDTES was added to 80 mL of ethanol and magnetically stirred at room temperature for 60 min. Subsequently, the SiO2 suspension was added dropwise into the above mixed fluorosilane mixed solution, and stirring was continued at 40 °C so that the hydrolysis condensation reaction was proceeded completely. After completion of the reaction, the mixture was cooled to room temperature, and the reaction product was centrifuged at a centrifugal speed of 10,000 rpm for 5 min, and washed with absolute ethanol and centrifuged twice. Thereafter, the product obtained by centrifugation was dried under vacuum environment at 60 °C for 12 h to finally obtain hydrophobic SiO2 nanoparticles, which were labelled as F-SiO2 nanoparticles.
2.3. Preparation of superhydrophobic F-SiO2/PDMS@PET mesh
The PET meshes were placed in deionized water and absolute ethanol for 15 min, dried for 10 min, respectively, to reserve for spare. According to the mass ratio of 10:1, 0.3 g PDMS and 0.03 g curing agent were weighed and added to 15 mL THF organic solvent, magnetically stirred for 5 min, and sonicated for 25 min to dissolve the two components, respectively, to obtain A and B solutions. Subsequently, the two solutions were mixed to obtain mixed PDMS solution. Thereafter, the PET meshes were placed in the mixed PDMS solution and sonicated for 30 min, and subsequently the PET meshes were pre-cured in the oven at 80 °C for 10 min to obtain hydrophobic PDMS PET meshes. At room temperature, 0.1 g of F-SiO2 nanoparticles were weighed into 15 mL of THF solution and ultrasonically dispersed for 30 min. The pre-cured PET meshes were placed in the F-SiO2 solution for 15 min, then allowed to hold for 5 min, and dried in an oven at 80 °C for 5 min. The PET meshes were taken out and placed in F-SiO2 solution for 15 min again, then kept for 5 min and placed in an oven at 80 °C for 1 h. Finally, the superhydrophobic F-SiO2/PDMS@PET meshes were successfully obtained for the application investigation of oil-water separation.
2.4. Characterizations
All scanning electron microscopy (SEM) images were carefully detected using a field-emission scanning electron microscope (FE-SEM, Hitachi S4800, Japan) at an acceleration voltage of 5 kV. Elemental analysis was performed using the energy dispersive spectroscopy (EDS) device attached to the FE-SEM. Surface chemical composition and high-resolution spectra were obtained from X-ray photoelectron spectroscopy (XPS, K-Alpha, USA) measurements. The surface roughness was measured using an atomic force microscope (AFM, ICON2-SYS, USA), and each sample was tested for 3 times with a scan area of 2 μm × 2 μm. The water contact angle (CA) and sliding angle (SA) were measured using an optical contact angle measuring instrument (JC2000D2, Shanghai Zhongchen Digital Technology Apparatus Co., Ltd, China) with a 6 mL water droplet. All averaged CA and SA values were determined by measuring the same surface at five different positions. Furthermore, the oil-water separation measurements were well performed by a home-made device in our laboratory.
3. Results and discussion
3.1. Modification of SiO2 nanoparticles for superhydrophobic microstructures
As shown in Fig. 1, it is clearly illustrated that a two-step impregnation strategy is used to prepare the superhydrophobic F-SiO2/PDMS@PET meshes. First, the low-energy and high-viscosity silicone resin PDMS was utilized to directly modify the surface of PET meshes with the great flexibility and robust bonding strength with the PET substrate materials [50]. Also, the introduction of PDMS will act as a binder to immobilize SiO2 nanoparticles for the controlled surface microscopic morphologies [51]. On this basis, the modified F-SiO2 nanoparticles were used for the second modification to produce the microscopic morphologies, and subsequently a curing process is needed to finally obtain the superhydrophobic F-SiO2/PDMS@PET meshes. Here, the entire modification processes mainly involve two basic chemical reactions. The PFDTES molecule is firstly hydrolyzed in an ethanol solution to produce a large amount of long-chain silanol, and then the hydroxyl groups on the surface of F-SiO2 nanoparticles are polycondensed with the hydroxyl groups from the above silanol. It should be noted that, despite the presence of a large amount of hydroxyl groups, no hydroxyl groups can take place the chemical reaction with the original SiO2 nanoparticles, and the successfully assembled fluorine-containing groups are necessary to make guarantee hydrophobic state of SiO2 nanoparticles.

Fig. 1.
Schematic illustration of basic procedures to prepare the superhydrophobic F-SiO2/PDMS@PET meshes via a two-step impregnation method.
Based on this requirement, the original SiO2 nanoparticles should be treated with the fluorine modification, and the detailed change of surface chemical compositions has been reported in our previous article [52]. Also, it has been confirmed that the modified F-SiO2 nanoparticles will facilitate the connection with the basic layer of PDMS materials. As shown in Fig. 2, the water droplets will wet the untreated SiO2 nanoparticles due to their high surface energy causing hydrophilicity. However, the modified F-SiO2 nanoparticles can well keep the water droplets spherical with the robust hydrophobicity. It indicates that the surface energy of SiO2 nanoparticles is reduced with the desired fluorine-containing groups.

Fig. 2.
Comparison of wetting properties between original SiO2 (a) and modified F-SiO2 nanoparticles (b).
Afterwards, the superhydrophobic F-SiO2/PDMS@PET meshes can be well obtained according to the above described two-step route, and the 3-Dimentional morphologies and 2-Dimentional rough curves (including those of the smooth and PDMS-coated PET meshes) are shown in Fig. 3. The PET meshes have a remarkable change trend from smooth to rough microscopic morphologies. The surface of the uncoated PET meshes is relatively smooth, and the root mean square (RMS) surface roughness can be calculated to be approximately 8.1 nm from the 2-Dimensional topography (Fig. 3(a)). Apparently, the RMS value of surface roughness of PDMS-coated PET meshes increased to 18.9 nm, showing a certain extent of change in roughness. However, the surface roughness of PDMS-coated PET meshes is still low, so that the superhydrophobic state cannot be achieved. After the further impregnation in the F-SiO2 suspension solution, the 3-Dimensional surface morphology changes significantly, and there were many undulating haw-like structures on the surface, which are also verified by the SEM results below. The RMS value of surface roughness of the superhydrophobic F-SiO2/PDMS@PET meshes further increases to 84.2 nm, indicating that the formed microstructures can well conform to the requirement of superhydrophobicity via capturing more air pockets inside.

Fig. 3.
(a-c) 3-Dimentional morphologies of smooth, PDMS-coated, and superhydrophobic F-SiO2/PDMS@PET meshes. (d-f) Corresponding 2-Dimentional surface morphologies. (g-i) Roughness curves obtained along the diagonal direction on 2-Dimensional surface morphologies.
3.2. Morphologies and chemical compositions on superhydrophobic meshes
In order to reveal the mechanic relation of pore size of superhydrophobic meshes with the oil-water separation efficiency towards various organic liquids. As shown in Fig. 4(a-f), many PET meshes with different pore sizes of diameter (from 0.25 mm to 0.038 mm), as substrate materials, were selected to take some surface modifications with superhydrophobicity for the ability of effective oil-water separation. It should be noted that the diameter refers to the inscribed circle inside the various PET meshes, as marked by yellow line in Fig. 4(a). Furthermore, the local higher resolution images depict the microscopic morphologies after and before superhydrophobic modification, as shown in Fig. 4(g, h). Due to the addition of nanoparticles, the agglomerated particles with micro-nano-level roughness appear on the surface of the treated PET meshes compared with the untreated PET meshes. The roughness contributes to capturing many air pockets, which make the PET meshes obtain higher superhydrophobic property. From macro perspective, the change in roughness does not lead to a change in the shape of the mesh structure, which ensures that the application field of the mesh is not limited.

Fig. 4.
SEM images of superhydrophobic F-SiO2/PDMS@PET meshes, (a) d = 0.25 mm; (b) d = 0.12 mm; (c) d = 0.075 mm; (d) d = 0.058 mm; (e) d = 0.048 mm; (f) d = 0.038 mm. Higher-magnification images of superhydrophobic F-SiO2/PDMS@PET meshes with d = 0.038 mm (g) and uncoated PET meshes with d = 0.12 mm (h).
In addition, the EDS energy spectrum in Fig. 5 shows that the fluorine content of the superhydrophobic F-SiO2/PDMS@PET meshes is significantly increased compared with the uncoated PET meshes. Also, the experimental process indicates that the sole source of fluorine is the PFDTES molecule, and therefore the modified fluorine-containing groups are successfully grafted onto the surface of SiO2 nanoparticles. Similarly, silicon element is detected on the surface of the superhydrophobic PET meshes, which is mainly derived from the SiO2 molecule. Finally, the synergistic action of the micro-nanoscale structures and the cavities brings the surface of the PET meshes great superhydrophobic property.

Fig. 5.
SEM images and elemental compositions of PET meshes surface, (a, b) uncoated PET meshes; (c, d) Superhydrophobic F-SiO2/PDMS@PET meshes.
To further determine whether the low surface energy groups successfully modified the PET meshes, the XPS spectra of the coated PET meshes and the high-resolution spectra of the various elements are depicted as shown in Fig. 6. The high resolution C1s spectrum of the superhydrophobic F-SiO2/PDMS@PET meshes has four peaks at 284.0 eV (corresponding to CC/CH bond), 282.4 eV (corresponding to CSi bond), 292.2 eV (corresponding to CF bond), and 289 eV (corresponding to CO bond), where the CF bonds are attributed to the PFDTES molecule, and the CO bonds are derived from the PET meshes. Besides, the absorption peaks at 689.3 eV and 684.9 eV, corresponding to the CF2 and CF3 chemical bonds respectively, are also found in the high resolution F1s spectrum of the superhydrophobic F-SiO2/PDMS@PET meshes. In Fig. 6(d), the high-resolution Si2p spectrum has shown three peaks of SiC (at 100.8 eV), SiOSi (at 103.4 eV), and SiOH (at 104.5 eV), which further indicates the PFDTES molecule having grafted onto the surface of SiO2 nanoparticles during fluorination modification. Furthermore, the SiOSi (at 288.5 eV) and SiOH (at 290 eV) chemical bonds demonstrate the occurrence of chemical reaction between PFDTES and SiO2 nanoparticles. Based on the above analyses, it is demonstrated that the PFDTES molecule and SiO2 nanoparticle have successfully undergone polycondensation reaction.

Fig. 6.
(a) XPS full spectrum of superhydrophobic F-SiO2/PDMS@PET meshes, PDMS-coated PET meshes and uncoated PET meshes. (b-d) High resolution spectra of each element.
3.3. Non-wettability
Non-wettability is an important factor to produce the function of oil-water separation. As shown in Fig. 7(a), the water CA reaches 153.3°(for the mesh with the pore size of 0.048 mm), demonstrating the as-prepared F-SiO2/PDMS@PET meshes possessing the great superhydrophobicity. Additionally, the as-prepared mesh exhibits not only superhydrophobicity but also ultralow adhesion to a water droplet. The water droplets dripping on the superhydrophobic F-SiO2/PDMS@PET meshes can easily roll away from the surface. This superhydrophobic state can be well explained by the Cassie-Baxter wetting model where the air pockets are trapped in the valley of the rough structures and greatly curtails the contact area between solid surface and water droplet. When the superhydrophobic F-SiO2/PDMS@PET meshes are immersed in deionized water, the flooded portion turns into a mirror-like surface, as shown in Fig. 7(b). Owing to the air pockets from the cavitation on the surface of the as-prepared meshes, the contact interface below the liquid surface is mostly a water-air interface instead of water-solid one. Besides, by comparing the hydrophobicity of the superhydrophobic F-SiO2/PDMS@PET meshes (Fig. 7(c)) with the uncoated PET meshes (Fig. 7(d)), it can be observed that the uncoated PET meshes cannot reach the superhydrophobic state, and the droplet forms a hemisphere shape. Another interesting phenomenon is that when the superhydrophobic F-SiO2/PDMS@PET meshes were placed in deionized water, the buoyancy of the PET meshes were increased greatly to cause it floating on the surface of the water. Correspondingly, the water droplets can remain spherical on the surface of the mesh, as shown in Fig. 7(e).
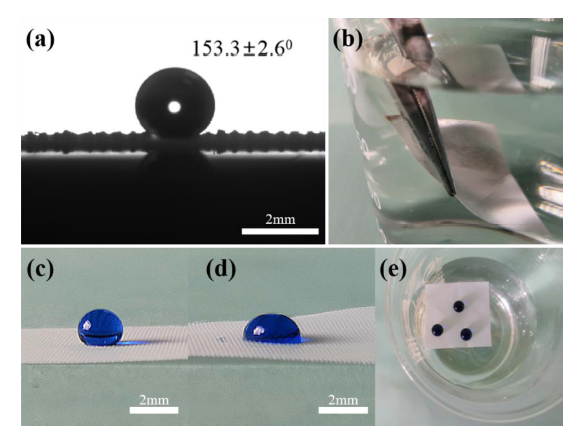
Fig. 7.
(a) Static water CA on superhydrophobic F-SiO2/PDMS@PET mesh. (b) Superhydrophobic F-SiO2/PDMS@PET mesh being immersed in water. (c, d) Water droplets on the superhydrophobic F-SiO2/PDMS@PET mesh and uncoated PET mesh. (e) Superhydrophobic F-SiO2/PDMS@PET meshes floating on water surface. The related videos (Video S1-S3) are provided in the supporting materials.
To better analyze the apparent non-wettability of the as-prepared superhydrophobic F-SiO2/PDMS@PET meshes, we further discuss the geometric relationship between the pore size of the superhydrophobic F-SiO2/PDMS@PET meshes and non-wettability, as shown in Fig. 8. Obviously, six different pore sizes of F-SiO2/PDMS@PET meshes can achieve excellent superhydrophobic property. Among them, as the pore size of the mesh is continuously reduced, the apparent water CA of the mesh surface is continuously increased. This is mainly attributed to that the water droplet will embed into the pore of the superhydrophobic PET mesh, and the water droplet can be stably supported with a smaller value of water CA. Oppositely, the superhydrophobic meshes with smaller pore size will be closer the flat surface, leading to a higher water CA on the surface. Regarding the different CA of several superhydrophobic meshes, the matching degree between mesh and oil plays an important role in oil-water separation. As the pore size of the mesh decreases, the more nanoparticles are coated on the surface of the mesh and the increase in rough structure leads to an improvement in non-wetting performance. Therefore, the superhydrophobicity and superlipophilicity of the mesh are enhanced. Oil-water separation ability is highly depended on apparent surface energy (a synergistic factor between membrane microstructure and surface energy). When the surface non-wettability is increased, the apparent surface energy will decrease towards the value of the oil phase so that the efficiency of oil-water separation will be improved.

Fig. 8.
Water CA on the superhydrophobic F-SiO2/PDMS@PET meshes with six different pore sizes (a) 0.25 mm; (b) 0.12 mm; (c) 0.075 mm; (d) 0.058 mm; (e) 0.048 mm; (f) 0.038 mm.
3.4. Oil-water separation efficiency
According to the above detailed analyses, the as-prepared superhydrophobic F-SiO2/PDMS@PET meshes could be used for the separation of different types of oil-water mixtures. It is worth mentioning that after placing the PET meshes at room temperature for 30 days, their superhydrophobicity and superoleophilicity does not change significantly, which shows the excellent stability. Based on the excellent superhydrophobicity and superoleophilicity of the PET meshes, it could be utilized as a selective filter membrane to realize oil-water separation. Firstly, we place the superhydrophobic PET mesh in the separation device, and then pour 60 mL of oil-water mixture (Vwater =10 mL, Voil =50 mL) into the funnel with a glass rod. During the separation process, the oil droplets will slowly pass through the superhydrophobic PET mesh under the gravity action and subsequently be collected. Meanwhile, the water is also collected at the top container due to the superhydrophobicity of the PET mesh. Subsequently, we calculated the separation efficiency (E) by observing the volume change of the oil before and after the collection (Eq. (1)):
where V0 is the volume of oil before separation and Vc is the volume of oil after separation. At the initial period, the calculated separation efficiency is a little low because of the adsorption of the oil droplets by the superhydrophobic PET mesh and the wetting action on the surface of the collection container. After that, the adsorption of the oil on the PET mesh reaches a saturated state and the separation efficiency is increased to stable level.
In order to discuss the relationship between oil-water separation efficiency and mesh pore size, we also prepared six types of superhydrophobic F-SiO2/PDMS@PET meshes with different pore sizes of 0.25, 0.12, 0.075, 0.058, 0.048, and 0.038 mm, and subsequently performed oil-water separation experiments. To ensure the objectivity and accuracy of the experimental results, we performed five sets of experiments on the same superhydrophobic PET mesh with different oil-water mixtures, then calculated the separation efficiency, as shown in Fig. 9. It can be clearly observed that the separation efficiency of the six groups of superhydrophobic PET meshes with different pore sizes for the oil-water mixture is above 90%, of which the highest value even reaches 98%. Among them, as the pore size of the PET mesh changes smaller, the separation efficiency generally increases, which displays a same trend for different oil-water mixtures, as shown in Fig. 10. However, we can clearly notice that the superhydrophobic PET mesh with pore size of 0.038 mm exhibits lower separation efficiency comparing with that with pore size of 0.048 mm. This is contrary to the change trend of water CA on the superhydrophobic PET mesh. Therefore, we can conclude that the pore size of superhydrophobic PET mesh will produce a certain extent of impact on the oil-water separation ability.

Fig. 9.
Separation efficiency of different oil-water mixtures onsuperhydrophobic F-SiO2/PDMS@PET meshes with different pore sizes.

Fig. 10.
Separation efficiency of (a) kerosene-water and (b) trichloromethane-water mixtures on superhydrophobic F-SiO2/PDMS@PET meshes with different pore sizes.
Another phenomenon is also observed that, different types of oil in the oil-water mixtures also lead to the various values of the separation efficiency. It is considered that the phenomenon is caused by matching degree of surface energy between oil and superhydrophobic PET mesh. Here, we took the type of oil as a variable and discussed the separation efficiency of oil with different surface energy, i.e., trichloromethane (1.489 g/cm3), n-decane (0.73 g/cm3), kerosene (0.8 g/cm3), octane (0.703 g/cm3), and n-hexane (0.66 g/cm3), on the same superhydrophobic PET mesh, as shown in Fig. 11. The separation efficiency of the superhydrophobic PET meshes for the five oil-water mixtures is above 95% and the highest value is 98% for kerosene with the surface energy equal to 0.8 g/cm3. It demonstrates that the as-prepared superhydrophobic PET meshes exhibit a wide application range, and realize ideal separation towards various oil-water mixtures, which benefits the design and manufacture of ideal oil-water separation materials.

Fig. 11.
Separation efficiency of oil with different surface energy on the superhydrophobic PET mesh with pore size of 0.048 mm. The related separation process is clearly shown in Video S4 of supporting materials.
3.5. Geometric relationship of pore size
In order to analyze the underlying mechanism of PET mesh pore size on oil-water separation, we make some mechanic analyses on the droplets on the superhydrophobic PET mesh, as shown in Fig. 12. The cartoon villain vividly shows the support force of the superhydrophobic PET mesh to the water droplets. According to the balance of forces, it is not difficult to draw as Eq. (2):
By simplifying the model, as shown in Fig. 12(b), and assuming that each side of the PET mesh has equal support for the water droplets, it is concluded that:
where F represents the force applied to the droplet on each side of the grid, θ is the CA and G is the gravity of the droplet. Eq. (3) is re-given by:
where r represents the radius of water droplet, N is the number of meshes contacting with water droplet, and L is the mesh spacing distance. It can be easily deduced from the above equation that the value of N increases exponentially and the value of F gradually decreases correspondingly, as the PET mesh pore size decreases. The synergistic effect of the both factors will result in smaller support force for the PET mesh with larger pore size. Oppositely, as the PET mesh pore size decreases, the separation efficiency gradually increases due to lager supporting force to water. Besides, the more nanoparticles are coated on the surface of the mesh with the pore size of the mesh decreasing and superhydrophobic performance has different degrees of improvement. As we know, oil-water separation ability is highly depended on apparent surface energy. When the surface non-wettability is increased, the apparent surface energy will decrease towards the value of the oil phase so that the efficiency of oil-water separation will be improved. However, when the mesh pore diameter is small to a certain degree, the flow rate of oil penetration is hindered and the separation efficiency is also slightly reduced, which is consistent with the experimental results. If the pore size is too small, below the critical value, the separation efficiency will change lower, such as that on the superhydrophobic PET mesh with the pore size of 0.038 mm. It is mainly attributed to smaller pore size causes lower apparent surface energy of the superhydrophobic PET mesh, even below the value of desired-separation oil. Consequently, the superhydrophobic PET mesh displays a certain extent of oil-repellence, leading to a little reduction in the entire oil-water separation. Regarding separation efficiency towards various oil with different surface energy, it is still depended on the matching degree of apparent surface energy of superhydrophobic PET mesh to the value of desired-separation oil. If the difference value is smaller, even closing to zero, the superhydrophobic PET mesh will produce higher separation efficiency.

Fig. 12.
Model of water droplets on surface of superhydrophobic PET mesh. (a) Schematic diagram, (b) Mechanic relationship.
4. Conclusion
In conclusion, we successfully constructed the superhydrophobic F-SiO2/PDMS@PET meshes for the application of effective oil-water separation towards various organic liquids. Due to the introduction of fluorinated silica, the surface of PET meshes exhibited a certain extent of microscopic roughness, leading to the remarkable water repellence. The water CA reached 155.9°±1.0° on the coated PET mesh with the pore size of 0.038 mm, and the water droplets can exhibit perfect spherical shape. On the basis, the pore size of PET mesh materials was mainly discussed with the great oil-water separation efficiency towards various organic liquids. As the pore size of the PET mesh changed smaller, the separation efficiency generally increased to even 98%, yet the separation efficiency showed a certain extent of reduction after the pore size being lower than a critical value. Also, different types of oil in the oil-water mixtures leaded to the various values of the separation efficiency, which mainly concentrated in a range from 95 % to 98 %. This was mainly depended on the matching degree of apparent surface energy of superhydrophobic PET mesh to the value of desired-separation oil, where the apparent surface energy could be regulated by the macroscopic geometric size of size of the mesh pore.
Reference




 WeChat
WeChat