Corresponding authors:
Received: 2019-03-27
Revised: 2019-04-19
Accepted: 2019-04-22
Online: 2019-09-20
Copyright: 2019 Editorial board of Journal of Materials Science & Technology Copyright reserved, Editorial board of Journal of Materials Science & Technology
More
Abstract
The thermal and environmental barrier coatings (T/EBC) are technologically important for advanced propulsion engine system. In this study, RE4Hf3O12 (RE=Ho, Er, Tm) with defect fluorite structure was investigated for potential use as top TBC layer. Dense pellets were fabricated via a hot pressing method and the mechanical and thermal properties were characterized. RE4Hf3O12 (RE=Ho, Er, Tm) possessed a high Vickers hardness of 11 GPa. The material retained high elastic modulus at elevated temperatures up to 1773 K, which made it attractive for high temperature application. The coefficient of thermal expansion (CTE) of RE4Hf3O12 (RE = Ho, Er, Tm) laid in the range between 7 × 10-6 K-1 to 10 × 10-6 K-1 from 473 K to 1673 K. In addition, the rare earth hafnates exhibited lower thermal conductivity which rendered it a good candidate material for thermal barrier applications.
Keywords:
The operating temperature of turbine engine increases continuously to pursue higher efficiency and thrust-weight ratio, while the superalloy materials gradually reach the upper temperature limit [1]. Lightweight, high-temperature capable SiCf/SiC ceramic matrix composites (CMC) have been implemented for turbine engine hot section components [1,2]. Thus, environmental barrier coatings (EBCs) are essential to protect CMC components from recession by high-temperature water vapor and degradation by silicate deposits (generically known as CMAS) in harsh combustion environments [1,3]. Nowadays advanced multilayer coating architectures named of thermal and environmental barrier coatings (T/EBCs) have been proposed and manifested to extend the temperature capability of CMC components to 1650 °C [1,4,5]. As the exterior TBC, it is required to possess good temperature capability including high melting point, phase stability and sintering resistance; besides, sufficient thermal and mechanical properties to ensure heat insulation and strain tolerance, as well as good corrosion resistance against CMAS degradation and hot gas volatilization.
Rare earth hafnates are promising materials for the overcoat in T/EBC systems. There are two stoichiometric compounds in RE2O3-HfO2 system, the RE2Hf2O7 pyrochlore with light rare earth element (from La to Dy) and the RE4Hf3O12 δ-phase with heavy rare earth element (from Ho to Lu) [6]. They both belong to oxygen-deficient fluorite structural derivative compounds (MO2-x) [7,8]. δ-RE4Hf3O12 has accomplished wide attentions owing to their higher rare earth element ratio than that of RE2Hf2O7 and the rare earth element was considered as predominant constituent to crystallize the silicate deposits [4,[9], [10], [11]]. In the temperature range from 1500 to 1700 °C, some δ-RE4Hf3O12 compounds (RE = Er, Tm, Yb, Lu) undergo phase transformation to defect fluorite structure [12,13]. Structurally, the defect fluorite is regarded as a solid solution of REO1.5 in the fluorite-structured HfO2. In this defect structure, a large range of REO1.5 could be accommodated [14,15]. It is reasonable to speculate that deviation in the composition between the plasma sprayed defect fluorite coating and its feedstock do not induce phase separation which would benefit for the coating integrity. Moreover, in the rare earth hafnates with defect fluorite structure, the oxygen vacancies exhibit disordered arrangements. It is expected the defect fluorite phase would manifest low thermal conductivity resulting from phonon scattering of disordered lattice as well as defects.
In the present study, we focused on the defect fluorite with the formula of RE4Hf3O12 (RE=Ho, Er, Tm) inspired by the extensive researches on δ-RE4Hf3O12 [4,5,8,12,14,16,17]. The pellets were fabricated by hot press sintering. The mechanical and thermal properties were comprehensively investigated, including the hardness, elastic modulus, flexural strength, coefficient of thermal expansion (CTE), thermal shock resistance, and thermal conductivity. It is expected to facilitate the application of defect fluorite structured rare earth hafnates as the advanced T/EBCs.
The commercially available powders of HfO2 (99.95% purity) and RE2O3 (99.995% purity) were used as raw materials and mixed at a molar ratio of 3:2 for 24 h in a planetary mill using Si3N4 containers and Si3N4 milling balls with ethanol as the medium. RE4Hf3O12 (RE = Ho, Er, Tm) powders were synthesized by heating of the HfO2 and RE2O3 powder mixtures at 1600 °C for 1 h in air. Subsequently, the synthesized powders were wet grounded and milled for 24 h, and then dried at 80 °C. The RE4Hf3O12 bulk samples were obtained by hot pressing the as-synthesized powders in a flowing Ar atmosphere under 30 MPa for 1 h at 1700 °C, at a heating rate of 10 °C/min and a controlled cooling rate of 10 °C/min to 1200 °C to eliminate possible micro-cracks.
The density of sintered samples was determined by the Archimedes method. Phase composition was identified by X-ray diffraction (XRD; D/max-2400, Rigaku, Tokyo, Japan) with CuKα radiation. Microstructure observations were conducted using a SUPRA 35 scanning electron microscope (SEM; LEO, Oberkochen, Germany). The grain size analysis of the sintered pellets was performed after thermal etching at 1500 °C for 1 h.
The Vickers hardness was measured under loads of 3, 5, 10 and 30 N, respectively with a dwell time of 15 s. The flexural strength was determined via the three-point bending method using samples with dimensions of 3 mm × 4 mm × 36 mm in a universal testing machine (CMT4204, SANS, China). The dynamic elastic modulus was measured for rectangular beam-like samples with dimensions of 3 mm × 15 mm × 40 mm using the impulse excitation technique in a graphite furnace (HTVP 1750C, IMCE, Diepenbeek, Belgium) at a heating rate of 4 °C/min in Ar atmosphere. The first resonance mode was selected to determine the elastic modulus and internal friction. The vibration signal, captured by a laser vibrometer (Polytec, Waldbronn, Germany), was analyzed with the resonance frequency damping analyzer (Resonance frequency damping analyzer, IMCE, Diepenbeek, Belgium).
The thermal expansion behavior was studied with a vertical high-temperature optical dilatometer (ODHT, Modena, Italy) in an air atmosphere from room temperature to 1673 K. The data were recorded continuously while heating at a rate of 5 K/min. The CTE can be calculate by ΔL/(LΔT), where ΔL = L-L0 (L0 represents the length of sample at 300 K and L is the length measured at each temperature) and ΔT is the temperature interval where the data were collected (ΔT = T-300 K). The dimensions of the samples used to obtain the CTE measurements were 3 mm × 4 mm × 14 mm. Thermal diffusivity measurements were acquired with a laser flash analyzer (Netzsch LFA 427, Bavaria, Germany) from 300 to 1673 K. Before the thermal diffusivity test, the disk samples (Φ12.7 mm × 1.5 mm) were coated with a layer of Mo by magnetron sputtering to minimize thermal irradiation through the sample, and then sprayed with a thin colloidal graphite layer to ensure complete and uniform absorption of the laser pulse. The temperature dependences of the heat capacities are calculated based on the Neumann-Kopp rule [18]. Based on the measured thermal diffusivity and calculated heat capacity, the thermal conductivities at different temperatures were obtained according to the following equation:
κ=αCpρ (1)
where κ is the thermal conductivity, α is the thermal diffusivity, Cp is the specific heat, and ρ is the bulk density.
Fig. 1 shows the XRD patterns of the prepared RE4Hf3O12 (RE = Ho, Er, Tm, with defect fluorite donates as F*) ceramics. There are five characteristic peaks in fluorite crystal structure in the angular range between 10° and 70°. It agrees with the fact that defect fluorite crystallizes in Fm-3 m space group, corresponding to the CaF2 structure [13]. The peaks shifted slightly toward higher angles with the decrease of the ionic radius from Ho3+ to Tm3+, indicating the decreasing of lattice parameters from F*-Ho4Hf3O12 to F*-Tm4Hf3O12. The apparent densities of F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12 bulk sample were measured to be 9.15, 9.18 and 9.42 g/cm3, with the relative densities over 97%. Fig. 2 presents the typical microstructures of the thermal etched samples. Equiaxial grains were revealed and the average grain sizes were calculated to be 1.03, 1.27 and 1.34 μm for F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12, respectively.
Fig. 1. XRD patterns of as-synthesized (a) F*-Ho4Hf3O12, (b) F*-Er4Hf3O12 and (c) F*-Tm4Hf3O12.
Fig. 2. Microstructures of polished and etched surfaces of (a) F*-Ho4Hf3O12, (b) F*-Er4Hf3O12 and (c) F*-Tm4Hf3O12 pellets.
3.2.1. Vickers hardness and fracture toughness
Vickers hardness (HV) of F*-RE4Hf3O12 (RE = Ho, Er, Tm) ceramics as a function of the indentation load are plotted in Fig. 3(a). The hardness values decreased as the load increased and stabilized at approximately 11 GPa when the applied load was over 10 N. The typical Vickers indentation morphology of the tested sample is illustrated Fig. 3(b), which shows that cracks initiate from the corners of the indentation when a load is applied. The fracture toughness (KIC) is related to the length of cracks and according to the general rule, that if c/a > 2.5, where a is half length of the indentation diagonal and c represents the half length of the cracks obtained in the tips. Then the KIC can be calculated by the indentation fracture method using the following equation [19]:
KIC=0.018E/HV1/2(P/c3/2) (2)
Fig. 3. (a) Vickers hardness as a function of indentation load and (b) typical indentation morphology of sample under load.
where E is elastic modulus, HV is the Vickers hardness, P is the indent force. The fracture toughness values for F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12 were calculated to be 1.13, 1.07 and 1.05 MPa m1/2, respectively. The fracture toughness of F*-RE4Hf3O12 are slightly lower than that of yttria-stabilized zirconia (YSZ) obtained use the same method (1.47 MPa m1/2 for 8YSZ [20]) which has been widely used as TBC for Ni-based superalloy.
3.2.2. High-temperature elastic modulus
Fig. 4 shows the temperature-dependent elastic moduli and internal friction of the F*-RE4Hf3O12 samples. The room-temperature elastic moduli were measured to be 210, 205 and 214 GPa for F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12, respectively. The elastic modulus decreases slowly with increasing temperature up to about 1773 K. The retained elastic modulus of F*-RE4Hf3O12 at 1773 K is about 72% of that at room temperature. At the temperature around 800 K a transition in modulus was observed and corresponds to an internal friction peak, after which the decrease of elastic modulus slow down (Fig. 4). Similar behavior for zirconia stabilized with various amounts of Y2O3 has been reported and the internal friction peak is related to the relaxation of oxygen vacancies within a cluster of two (or more) rare earth ions [[21], [22], [23]]. There is no increase of internal friction when the temperature reaches about 1773 K, demonstrating the excellent retention of the high-temperature elastic modulus of rare earth hafnates [24].
Fig. 4. Temperature dependence of elastic modulus for F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12 and internal friction for F*-Tm4Hf3O12.
3.2.3. Flexural strength
The flexural strengths of F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12 were determined as 204 ± 20, 195 ± 5 and 217 ± 22 MPa, respectively. For comparison, the flexural strength of δ-Yb4Hf3O12 and δ-Lu4Hf3O12 [17] are also given in Fig. 5(a). The flexural strength of F*-/δ- RE4Hf3O12 phase is similar and around 200 MPa. Fig. 5(b)-(d) shows the typical fracture surfaces of F*-RE4Hf3O12, where predominantly intergranular fracture mode was identified. In some areas, sharp steps or layered cleavage characteristics could also be observed due to crack deflections as the cracks penetrated inside the grains and consumed the fracture energy.
Fig. 5. (a) Comparison of room temperature flexural strength of F*/δ-RE4Hf3O12 (RE=Ho, Er, Tm, Yb, Lu) and fractured surface morphology of (b) F*-Ho4Hf3O12, (c) F*-Er4Hf3O12 and (d) F*-Tm4Hf3O12.
3.3.1. Thermal expansion
A thermal expansion mismatch between the top layer and the underlying layers may result in coating cracking and spalling during thermal cycling [25]. Therefore, the CTE is an important factor in multilayer coating design. The results from optical dilatometer measurement are shown in Fig. 6(a). With the increasing of temperature from 473 K to 1673 K, the linear expansion can be observed for the all samples. The linear thermal expansion coefficient versus temperature is illustrated in Fig. 6(b), which shows that the CTE of RE4Hf3O12 increases with the temperature increasing. As listed in Table 1, the CTEs of Ho4Hf3O12, Er4Hf3O12 and Tm4Hf3O12 at 1473 K are 9.51 × 10-6 K-1, 9.49 × 10-6 K-1 and 9.57 × 10-6 K-1 respectively, which are larger than those of δ phase (CTEs of δ-Yb4Hf3O12 and δ-Lu4Hf3O12 are 7.64 × 10-6 K-1 and 7.64 × 10-6 K-1, respectively [17]). In the design of multilayer T/EBC systems, RE monosilicate has been proposed to be the layer beneath the RE hafnates [26]. Tian et al [24] have studied the thermal expansion of a series of RE monosilicates and the reported CTEs lie in the range from 6.94 × 10-6 K-1 to 8.84 × 10-6 K-1 at 1473 K. The CTEs of defect fluorite RE4Hf3O12 are slightly larger than those of the pure RE monosilicates. From the CTE point of view, F*-RE4Hf3O12 would undergo challenges in residual stresses during thermal cycling, it calls for the further coating morphology optimization with columnar or vertical cracks. Furthermore, doping with the proper element supplement in F*-RE4Hf3O12 may benefit for decreasing the CTE. For instance, it was reported that ThO2 and Y2O3 co-stabilized ZrO2 resulted in a distortion of the lattice and that lead to the change of the CTE [27]. From another point of view, the CTEs of F*-RE4Hf3O12 are close to those of Ni based superalloys and make the materials suitable in thermal barrier coatings for superalloys.
Fig. 6. (a) Linear thermal expansion and (b) thermal expansion coefficient of F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12 in temperature range 473-1673 K.
Table 1 Values of CTE of F*-RE4Hf3O12 in temperature range 473-1673 K.
| Temperature (K) | Ho4Hf3O12 (10-6 K-1) | Er4Hf3O12 (10-6 K-1) | Tm4Hf3O12 (10-6 K-1) |
|---|---|---|---|
| 473 | 7.59 | 7.40 | 7.20 |
| 673 | 8.50 | 8.27 | 8.13 |
| 873 | 8.92 | 8.71 | 8.73 |
| 1073 | 9.23 | 8.95 | 8.97 |
| 1273 | 9.29 | 9.15 | 9.21 |
| 1473 | 9.51 | 9.49 | 9.57 |
| 1673 | 9.54 | 9.59 | 9.67 |
3.3.2. Thermal shock resistance
The thermal shock of a coating material is the indication of its mechanical robustness. The thermal shock resistance relates to a number of properties including the flexural strength σ, Poisson’s ratio ν, elastic modulus E and the thermal expansion coefficient α, In particular, the thermal shock resistance parameter R is calculated as [28]:

The higher flexural strength, lower elastic modulus, and smaller CTE of ceramic are favorable for higher R value. The parameter R were calculated for F*-Ho4Hf3O12, F*-Er4Hf3O12, and F*-Tm4Hf3O12 to be 74, 72 and 73 K, respectively. F*-RE4Hf3O12 (RE=Ho, Er, Tm) shows lower R value than that of δ-Yb4Hf3O12 (81 K) and δ-Lu4Hf3O12 (98 K) [17] because of their comparable bending strength, modulus but higher CTEs.
3.3.3. Thermal conductivity
One of the most important requirements that a top layer must fulfil is pocessing low thermal conductivity. The fluorite derivate pyrochlore and defect fluorite zirconia have been widely used as thermal insulating materials [29,30]. In the present work, the temperature dependences of the heat capacities are calculated based on the Neumann-Kopp rule [18], which is known to be the simplest universal method for assessing the heat capacity of complex oxides, where the deviations are typically within ±3% compared with the experimental measurements [31]. The temperature dependence of the specific heat capacity of the hafnates is illustrated in Fig. 7(a). The measured thermal diffusivity of the investigated materials is plotted in Fig. 7(b). The thermal diffusivity decreased moderately with increasing the temperature for all samples. The dramatical increase of thermal diffusivity of F*-Ho4Hf3O12 above 1000 K is attributed to an effect of thermal radiation. The dense bulk samples were transparent in the near infrared, and thus the effects of radiative transport were more pronounced at high temperatures [32]. The thermal conductivities of F*-Ho4Hf3O12, F*-Er4Hf3O12, and F*-Tm4Hf3O12 are calculated according to Eq. (1) and presented in Fig. 7(c). The values of δ-Yb4Hf3O12 [17], δ-Lu4Hf3O12 [17], Zr3Y4O12 [32], dense YSZ [33], La2Zr2O7 [34], Gd2Zr2O7 [31] and Yb2Zr2O7 [34] are also included for comparison. It appears that the defect fluorite RE4Hf3O12 (RE=Ho, Er, Tm) exhibits a much lower thermal conductivity.
Fig. 7. (a) Heat capacity, (b) thermal diffusivity and (c) thermal conductivity for F*-Ho4Hf3O12, F*-Er4Hf3O12 and F*-Tm4Hf3O12. The thermal conductivity values of La2Zr2O7 [
Theoretically, at high temperatures, the thermal conductivity is independent of the temperature and it approximates the minimum thermal conductivity (κmin), which can be used as a selection guideline for low-thermal-conductivity materials. For pure materials, κmin can be estimated using the following equation [35]:
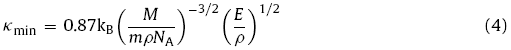
where kB is the Boltzmann constant, NA is Avogadro’s constant, m is the number of atoms in the primitive cell, ρ is the density and M is the molecular weight. Liu et al. [35] modified Eq. (4) by introducing elastic anisotropy and calculated κmin from the crystal structural information and elastic parameters:
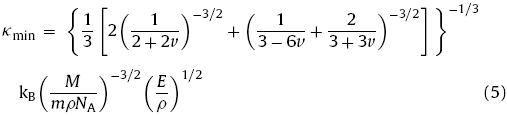
where v is Poisson ratio. The κmin values for F*-Ho4Hf3O12, F*-Er4Hf3O12, and F*-Tm4Hf3O12 were calculated as 0.83, 0.81 and 0.83 W m-1 K-1, respectively. The values are lower than those of La2T2O7 [36] and RE2SiO5 [21]. The low theoretical minimum thermal conductivities of hafnates can be ascribed to their higher molecular weight, complex crystal structure, and large number of atoms per molecule [35]. It has been demonstrated that F*-RE4Hf3O12 ceramics are a potential candidate for high-temperature thermal insulation applications.
Single phase and dense RE4Hf3O12 (RE = Ho, Er, Tm) ceramics with defect fluorite structure were successfully synthesized by solid-state reaction and hot pressing methods. The rare earth hafnates exhibited a hardness as high as 11 GPa but the toughness was relatively low, which would induce the tendency of crack propagation and damage during thermal cycling. The F*-RE4Hf3O12 retained high elastic modulus at elevated temperatures up to 1773 K, which endowed it with good high temperature mechanical property. The CTEs of RE4Hf3O12 (RE = Ho, Er, Tm) laid in the range between 9 × 10-6 K-1 to 10 × 10-6 K-1 in the temperature range from 473 K to 1673 K. The CTE mismatch between F*-RE4Hf3O12 and the under layers could be effectively depressed by featuring with loosely bonded columnar grains or by co-doping of F*-RE4Hf3O12 composition. In addition, the rare earth hafnates exhibited relatively low thermal conductivity and rendering it a good candidate material for thermal insulation applications.
This work was supported financially by the National Key R&D Program of China (No. 2017YFB0703201), the National Natural Science Foundation of China (Nos. 51402311, 51372252 and 51772302) and the International Cooperation Key Program (No. 174321KYSB20180008).
The authors have declared that no competing interests exist.

/
| 〈 |
|
〉 |