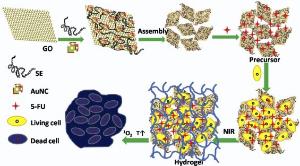
To combine localized drug release with multimodal therapy for malignant tumor, a composite hydrogel as an integrative drug delivery system was facilely prepared. The system contains spinach extract (SE), reduced graphene oxide (rGO) and gold nanocages (AuNCs). SE conduces to the formation of hydrogel, and also serves as a green material for improving the biocompatibility of hydrogel, and a natural photosensitizer for killing tumor cells under laser radiation (660 nm). AuNCs show obvious photothermy and can enhance the generation of cytotoxic singlet oxygen (1O2). The composite hydrogel shell on tumor cells exhibits several competitive advantages including enhanced antitumor effect by retaining the high concentration of drugs around cancer cell, excellent PDT/PTT compatibility as well as high loading and controllable release of fluorouracil (5-FU) for synergetic multimodal treatment. The survival rate of HeLa cells incubated with 5-FU loaded hydrogel under NIR radiation for 10 min sharply decreases to 1.2%, indicating remarkably improved antitumor effects. These results demonstrate that the hydrogel is an excellent delivery carrier for localizable, NIR-responsive and combined PTT/PDT/Chemo synergetic antitumor.
Cancer still remains a major threat to human health. Among all the anticancer treatments, surgery and chemotherapy continue to be the most fundamental and important means of treatments. However, surgical resection usually brings about very big trauma to the human body and only applies to cancer early. The metastases are considered to be unresectable. Chemotherapy is a common choice for treatment of metastases, but is subject to weak selectivity, strong side effects and drug resistance[1, 2]. Once resistance develops, higher doses of available drugs may be ineffective, causing stronger toxic side effects. An attractive option for overcoming the resistance is to generate a high concentration of singlet oxygen (1O2) in situ. Photodynamic therapy (PDT) and photo-thermal therapy (PTT) are both emerging and excellent minimally invasive therapeutic treatments for a variety of cancers. Compared with surgery and chemotherapy, PDT and PTT have unique advantages [3, 4], including remote controllability and minimal trauma. Unfortunately, most available photosensitizers or photothermal reagents are hydrophobic, which have low selectivity for the target sites and tumor cells, and absorb light energy only over wavelengths shorter than 600 nm. These problems hinder the broader clinical applications.
To overcome these problems in the clinical treatment, various delivery systems have been developed. Besides, another strategy of integrating discrete therapeutics and development of multimodal therapy leading to low systemic toxicity, synergistic effects and improved selectivity has received considerable research interest in cancer treatment[5, 6]. With great advancement in nano-biotechnology, it is important and of great potential to integrate chemotherapy with PDT or PTT for enhanced antitumor efficacy and reduced side effects[7, 8, 9]. Due to enhanced permeability and retention effect, nanomaterials are more suitable for intracellular applications such as imaging and drug delivery[10]. As photothermal transducers, Au nanostructures represent a promising platform for therapeutic applications[11, 12, 13, 14], and have been extensively investigated in cancer PTT. Especially, gold nanocages (AuNCs) with hollow interiors and porous walls, possess tunable surface plasmon resonance wavelengths between 700 and 1000 nm (NIR region)[15], which could be used as clinically ideal phototherapeutic window for malignancy, due to little photodamage to living organisms and highpenetration depths in tyears[16]. And AuNCs also exhibit more efficient cellular uptake and low cytotoxicity than Au nanorods and Au nanoparticles (AuNPs)[17]. Photo-thermal energy conversion by graphene[18] and its derivatives have also attracted considerable interest from biomedical researchers. The large surface area, strong optical absorption across the spectrum of graphene oxide (GO), and facile surface functionalization with biomolecules that could improve biocompatibility or targeting specificity[19, 20], are highly propitious to PTT applications. For instance, GO sheets[19] (typically larger than 0.5 µ m) loaded with photosensitizer have also been reported to achieve active targeting to cancer cells via folic acid. GO also has been accepted as good drug delivery system via hydrogen bond, hydrophobic and π -π interactions [21] with various chemotherapeutic, such as fluorouracil (5-FU), doxorubicin and paclitaxel.
However, co-assembly of functional components for synergetic cancer therapy encounters some difficulties, such as complex chemical synthesis process and extra steps to load drugs, which become increasingly difficult as the complexity of the system increases with corresponding decrease in yield and loading efficiency[18]. To solve these problems, composite hydrogels based on GO[22, 23] have been selected owing to their outstanding properties of super-hydrophilic, good biocompatibility and avid cell uptake[24]. Hydrogels also can prevent the flow of drugs from the intended area to reduce the side effect, control drug release by changing the gel structure in response to environmental stimuli, and protect drugs from hostile environments, e.g. the presence of enzymes and low pH. Despite these attractive advantages, hydrogels have some limitations as well. First, the formation of hydrogels usually uses potentially toxic cross-linking agents, which is difficult to be completely removed, and is unfavorable for the maintenance of the bioactivity of encapsulated chemotherapeutics; second, the quantity and homogeneity of drug loaded into hydrogels may be limited. Epidemiological studies have shown that spinach, a traditional leafy vegetable with high nutritional quality, contains relatively large amounts of natural photoactive pigments (Chlorophy II, β -carotene), ascorbic acid and folic acid (FA), which could be noncovalently bonded on rGO and AuNCs hybrids, and be internalized by cancer cells through folate receptor-mediated delivery[25, 26, 27, 28]. Castillo et al.[29]found that FA noncovalently bonded on single-walled carbon nanotubes could be internalized by folate receptors (FR) positive THP-1 cells through interaction with the FRs. Ascorbic acid is reductive. Chlorophy II and its derivatives in spinach extracts (SE) can serve as natural PDT drugs, and induce cell death in HuH-7 cells under laser irradiation[30, 31, 32], which highlights their potential roles for PDT. Therefore, SE is chosen as the green reductant and cross-linkers for the formation of hydrogel to avoid toxic additives, the other way round, GO also can overcome the inherent deficiencies of natural photosensitizer, such as, hydrophobicity and insolubility under physiological conditions.
In this study, we propose a facile approach for preparing a multifunction rGO/AuNCs/SE hydrogel with high 5-FU loading for localizable and multimodal killing of tumor cells. SE in the system contributes to the formation of hydrogel, and also serves as a green material for improving the biocompatibility of hydrogel, and a natural photosensitizer for killing tumor cells under laser radiation (660 nm). This study focuses on the influence of AuNCs and rGO on the near-infrared absorption, the production of 1O2 and photon-to-thermal energy transfer efficiency of the hydrogel. To our knowledge, this is the first report on a triune system (PDT, PTT and chemo are integrated into a multimodality therapy) with high photosensitizer and chemotherapeutics loading efficiency as well as excellent PDT/PTT compatibility. The hydrogel system exhibits an NIR response, cell-penetrating, 5-FU large payload and controlled release for synergistic antitumor. As illustrated in Fig. 1, when cultured with tumor cells, the rGO/AuNCs/SE precursor containing 5-FU was internalized by the tumor cells, and rapidly converted into hydrogel in situ by means of laser irradiation. The bioactive substances, such as SE, AuNCs and 5-FU, were confined in cross-linking network of hydrogel and localized on tumor cells to enhance the curative effect. During the hydrogel formation process, the tumor cells were killed by the 1O2 released from SE and oxygen, the local increase in temperature from both AuNCs and rGO, and chemotherapeutics. The intracellular uptake and conversion process were confirmed by the experiment of cellular uptake and drug releasing in vitro. The multimodal and localized therapy as well as the mode of photoregulation of cellular sensitive to 1O2 shows a remarkably improved and synergistic antitumor effect.
Baby spinach leaves were from a local market (Hefei, Anhui, China). Graphite powder was purchased from Huatai Lubricant Sealing S& T Co. Ltd (Qingdao, Shandong, China). 1, 3-Diphenylisobenzofuran (DPBF) was gotten from Acros Organics. 5-FU, dimethyl sulfoxide (DMSO), and polyvinylpyrrolidone (PVP, Mr ≈ 55 000) were obtained from Aladdin Reagent Database Inc. (Shanghai, China). Hoechst 33342, FITC-dextran (Mw 70 kDa), propidium iodide (PI), 3-(4, 5-dimethyl-2-thiazolyl)-2-5-diphenyl-2H-tetrazolium bromide (MTT), Dulbecco's Modified Eagle Medium (DMEM), double-resistant Fetal calf serum (GIBCO) and pancreatin (0.25% EDTA, GIBCO) were from Sangon Company (Shanghai, China). Potassium permanganate (KMnO4), hydrochloric acid (HCl), concentrated sulfuric acid (H2SO4), chloroauric acid (HAuCl4), ethylene glycol, AgNO3, NaNO3, Na2S∙ H2O, acetone and hydrogen peroxide (H2O2) were obtained from Chemical Shanghai Reagent Co. All chemical reagents were of analytical grade and used without further purification.
FTIR spectra were measured on a NEXUS-870 spectrophotometer (Thermo Fisher, USA, frequency range from 4000 to 500 cm-1). Raman spectroscopy was recorded on an inVia-Reflex Raman Microscope equipped with a 532 nm laser. X-ray diffraction (XRD) was scanned on an X-ray diffractometer (DX-2700) in the range between 5° and 70° . X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB-MKII spectrometer (VG Co., U.K.) with AlKr X-ray radiation as the X-ray source for excitation. The morphologies of the samples were examined by a Hitachi S4800 scanning electron microscope with energy dispersive X-ray (EDX) analysis. Transmission electron microscopy (TEM) images were obtained using a JEM 2100 instrument and the selected area electron diffraction patterns were recorded. UV-vis absorption spectra of the samples were recorded using a UV-3900 spectrophotometer (Hitachi) over the range of 200-1000 nm. IR thermal imaging was performed on an IR thermal camera (Fluke, USA). Cyclic voltammetry was performed in a three-electrode electrochemical cell using Pt wire and Ag/AgCl electrode as counter-electrode and reference electrode, respectively. Photoluminescence spectra were measured with an FL spectrophotometer (F-4500, Hitachi, Japan). The optical density (OD) values of the MTT assay were obtained using an RT-2100C spectrophotometric microplate reader (Rayto, Shenzhen, and PR China). Fluorescence images were recorded using a DMI3000B inverted fluorescence microscope (Leica, Germany).
GO was synthesized from graphite by modified Hummers method[33]. Fresh spinach leaves were cleaned and dried at room temperature, and then put into a juicer for squeezing juice. The dispersion was centrifuged at 4500 rpm for 10 min. Finally, the supernatant was collected, and SE was obtained by drying under vacuum at low temperature.
AuNCs were synthesized according to the galvanic replacement reaction[34]. Briefly, Ag nanocubes (5.0 mL, 3 nM) was added to homogenous PVP aqueous solution (50 mL, 1 mg mL-1) and was heated to boil for 10 min. Subsequently, the HAuCl4 (0.5 mM) was slowly added to the flask, and refluxed for another 30 min until the color of the reaction was stable. Then the precipitates were centrifuged and washed with saturated NaCl solution to remove AgCl and then with deionized water for several times to remove PVP and NaCl. Then 2 mg of the obtained AuNCs was added into a mixture of GO (5 mL, 3.0 mg mL-1) and SE solution (2 mL, 10 mg mL-1) to get a homogeneous solution, which is named as rGO/AuNCs/SE precursor. The precursor was placed into a glass vial, and then subjected to laser irradiation (660 nm, 200 mW cm-2) for 10 min to form the composite hydrogel.
DPBF was used as a probe to monitor the generation of singlet oxygen, which reacts with1O2 irreversibly causing the decrease of the absorption at 410 nm[35]. Briefly, DPBF (20 mL, 1.75 mmol) was mixed with 1 mL of precursor, and then irradiated with 660 nm laser. The absorption spectra of DPBF were recorded at predefined time (ranging from 0 to 25 s). All the experiments were repeated for three times.
5-FU loaded rGO/AuNCs/SE precursor was prepared by adding the mixture of 5-FU aqueous solution (1 mL, 4 mg mL-1) and 7 mL of rGO/AuNCs/SE precursor, and then the mixture was irradiated by laser light (660 nm, 200 mW cm-2) for 10 min to form the composite hydrogel. The drug loaded hydrogel was immersed into PBS buffer (20 mL, pH 7.4 or pH 5.0) at 37 ° C, which was used to simulate the slightly acidic environment of tumor cells and normal cell environment respectively. An aliquot (2 mL) of dialysis solution was taken out at predefined time interval (range from 6 to 96 h) and an equal volume of fresh PBS was added, maintaining the total volume constant. The absorption spectra of 5-FU were measured at 280 nm, and the cumulative release amount of 5-FU from hydrogel can be calculated according to standard calibration curve. All experiments were repeated for three times.
The cell viability was determined by MTT assay using HeLa cells or Chinese hamster ovary cells (CHO cells). The precursor containing SE, rGO/SE and rGO/AuNCs/SE was diluted by DMEM with the volume ratio of precursor to culture medium ranging from 1 to 0.01, respectively. For comparative study, the cell killing efficacies of 5-FU loaded rGO/AuNCs/SE precursor and free 5-FU with and without NIR irradiation were also carried out for manifesting multiple antitumor effects. In a typical procedure, HeLa cells were seeded (5 × 103/well) into 96-well plates and incubated for 24 h (37 ° C, 5% CO2). After removing the culture medium, 100 µ L of fresh culture medium that contained different concentrations of the precursor was added. The phototoxicity studies of the precursors were performed by 660 nm irradiation for 10 min. Following incubation for 24 h, the culture medium was replaced with MTT solution (20 µ L, 5 mg mL-1) and further cultured for 4 h. Then, 150 µ L of DMSO was added to dissolve formazan crystals, which were formed during the reductive cleavage of MTT by mitochondrial succinate dehydrogenases of living cells. The peak intensity of formazan crystals at 490 nm was recorded using a spectrophotometric microplate reader. Culture medium and cells only treated with culture medium served as blank group and a negative control group, respectively. The measured OD values of the blank, control and experimental groups were coded as ODb, ODc, and ODe. Cellular survival rates were calculated according to the following equation[36].
Survival rate(%)=
Fluorescence imaging was used to demonstrate the in vitro antitumor activities of samples. HeLa cells were seeded at a density of 5 × 104 cells per well in 6-well plates and incubated for 24 h. Afterward, 3 mL of fresh DMEM medium containing sample (1 mL) was added into each well. After incubating for 6 h, the plate was exposed to 660 nm laser light for 10 min. Then, the HeLa cells were dual stained in sequence using Hoechst 33342 (0.5 mL, 1 µ g mL-1) and PI (0.5 mL, 1 µ g mL-1) for 20 min in the dark. Fluorescence-stained cells were washed with PBS and observed under an inverted fluorescence microscope. All experiments were carried out in triplicate.
With regard to the endocytosis study of FITC-dextran[37], HeLa cells were seeded in 6-well plates and cultured for 24 h (37 ° C, 5% CO2). After complete adhesion, the cells were washed twice, and incubated with fresh serum-free medium containing FITC-dextran (1 mg mL-1) and the rGO/AuNCs/SE precursor at 37 ° C for 1 h. Then the cells were washed with PBS buffer and observed under a fluorescence microscope with a 488 nm laser.
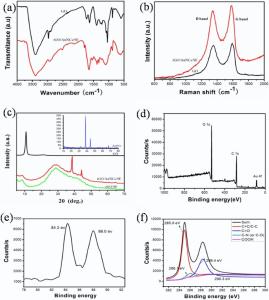
The rGO/AuNCs/SE hydrogel was prepared at mild environment using SE as a cross-linking agent and reductant under noninvasive laser (660 nm, 200 mW cm-2) irradiation. The assembly of materials and the formation of hydrogel can be ascribed to multiple interactions in the precursors: (1) the partial and green reduction of graphene oxide to rGO caused by excess SE, (2) the enhanced π -π stacking and hydrophobic interaction among rGOnanosheets, (3) positively charged AuNCs[31, 38] and negatively charged rGO sheets. To investigate the existence of multiple interactions in the precursors, FTIR spectrum, Raman spectra, XRD patterns and XPS spectra (Fig. 2) were measured. As shown in Fig. 2(a), the FTIR spectrum of GO presents the
The Raman spectrum of GO in Fig. 2(b) presents two strong peaks at 1355 and 1594 cm-1that are assigned to the typical D and G bands, respectively. It has been acknowledged that the appearance of D band and G band is due to disorder in the aromatic structure or the edge effect of graphene and the in-plane vibration of the sp2 carbon atoms[40]. The D band and G band for rGO/AuNCs/SE hydrogels shift downward to 1349 and 1585 cm-1, respectively. The small red-shift could be ascribed to the disturbance of the GO structure caused by the physical or chemical interactions between carboxyl, epoxy and hydroxyl moieties of GO and polar groups of SE, such as, amino, acylamino, carboxyl, etc. The results indicate that green reduction process has reduced the degree of chaos among the graphene layer.
XRD patterns of the pure GO, rGO/SE and the rGO/AuNCs/SE composite hydrogel are presented in Fig. 2(c). As shown in Fig. 2(c), the sharp (002) peak of GO is observed at 10.6° (d = 0.82 nm) [41]. The broad diffraction peak around 27.8° (d = 0.32 nm) appeared in both rGO/AuNCs/SE and rGO/SE hydrogel indicate that GO has been partly reduced into rGO with a random packing and less functionalities [42]. The diffraction peaks at 2θ value of 38.2° (111) and 44.5° (200) can be indexed to AuNCs (JCPDS No.4-0784). XPS spectrum for rGO/AuNCs/SE hydrogel is shown in Fig. 2(d). The survey scan spectrum reveals the existence of Au, C and O elements in the hydrogel. As can be seen fromFig. 2(e), the Au 4f7/2 and Au 4f5/2 peaks appear at a binding energy of 84.3 and 88.0 eV, respectively. The position of doublet peak and the peak-to-peak distance of 3.6 eV are due to metallic gold (Au0)[43]. The C 1s XPS spectra of rGO/AuNCs/SE in Fig. 2(f) can be fitted into four peaks centered at 284.8, 285.7, 288.8 and 290.4 eV, corresponding to C=C/C-C in aromatic rings, C-N or C-O (epoxy and alkoxy), carbonyl and carboxyl groups, respectively. The O 1s peak at 532.1 eV may be attributed to rGO sheets and SE. All these results suggest that the composite hydrogel contains rGO, SE and AuNCs.
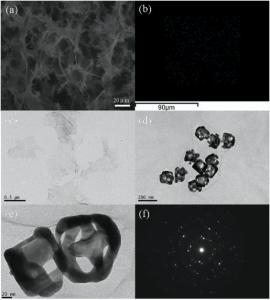
The SEM image of lyophilized rGO/AuNCs/SE hydrogel in Fig. 3(a) demonstrates a highly porous, interconnected three-dimensional structure, and these pores distribute regularly. The dots in red, green and blue stand for the elemental components O, C and Au, respectively, as analyzed by elemental mapping in Fig. 3(b) and Fig. S2. As shown inFig. 3(c), the GO sheets look like a wrinkled paper, and are about 0.5-1 µ m in lateral direction. TEM images in Fig. 3(d) and (e) show a hybrid morphology with cross-linked rGOnanosheets and AuNCs, and the AuNCs with good uniformity are decorated in the almost transparent rGO sheets. The size of AuNCs is about 80 nm and the pore size in the {100} or {111} faces of nanocube was about 5-8 nm. The selected area electron diffraction patterns of the AuNCs are presented in Fig. 3(f), which seems to be a multicrystalline nanoparticle. All the results confirm that the composite hydrogel is composed of AuNCs, rGO and SE; and AuNCs are evenly distributed throughout the three-dimensional macroporous structure of the hydrogel.
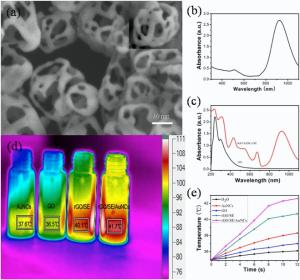
Fig. 4(a) presents the typical morphology of AuNCs with small holes at the corners and side facets. The UV-vis spectra of AuNCs, GO and rGO/AuNCs/SE precursor as well as SE are present in Fig. 4(b), (c) and Fig. S1(b), respectively. The as-prepared AuNCs has a surface plasmonresonance peak located at 925 nm. The characteristic absorption bands at 235 nm and 303 nm correspond to π → π * (C = C) and n→ p* (C=O) transition of GO, respectively as shown in Fig. 4(c). By comparison, the UV-vis absorption spectrum of rGO/AuNCs/SE precursor not only shows the characteristic bands of both SE and rGO, but also exhibits a broad peak at about 920 nm, which could be used to absorb NIR light for phototherapy of malignant tumor.
A time-dependent photothermal effect under irradiation by NIR laser (808 nm, 200 mW cm-2) was measured to study the potential use of rGO/AuNCs/SE as PTT agentia and the results are shown in Fig. 4(d) and (e). Temperature increments of GO, AuNCs, rGO/SE and rGO/AuNCs/SE precursors are 2.0, 3.3, 5.7 and 7.6 ° C, respectively, whereas the water temperature increases by only 1.1 ° C under the same conditions. These results demonstrate that AuNCs and rGO both have good photo-thermal effect, and the combination of rGO/AuNCs/SE could be used for efficient hyperthermia therapy.
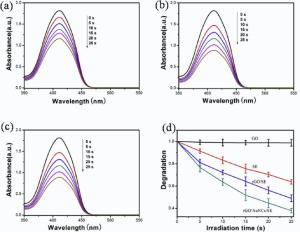
SE exhibits two characteristic absorbance bands corresponding to the absorption range of chlorophyll in Vis-NIR regions in Fig. S1(b), a bluish violet-absorbing band in 390-460 nm and a red-absorbing band in 630-690 nm. The emission maximum peak of photoluminescence spectrum in Fig. S1(c) is around 680 nm, and could be excited by using 660 nm laser[44], which suggests that SE can act as natural photosensitizer in photodynamic killing of cancer cells. Thus we compare the 1O2 generation abilities of the precursors. DPBF can react irreversibly with 1O2 and the reaction can be monitored by measuring the decrease in DPBF absorption intensity at 410 nm[33]. As can be seen clearly from Fig. 5(a)-(d), the DPBF with rGO/AuNCs/SE exhibits faster degradation than with SE or rGO/SE under irradiation. An explanation for the result may be that, as a good electron acceptor, rGO can decrease the recombination rate of the electron-hole pairs, and increase the quantum efficiency of the photosensitization process, thus enhance the generation of1O2. In contrast, the absorption of DPBF almost remains unchanged under irradiation in the presence of pure GO or nothing (Fig. S3). The result suggests that SE plays a major role in the whole photobleaching experiment of DPBF, and AuNCs and rGO both have a synergistic effect on accelerating the generation of 1O2. Therefore, the rate of singlet oxygen production may be controlled by adjusting the content of SE for achieving a better antitumor effect.
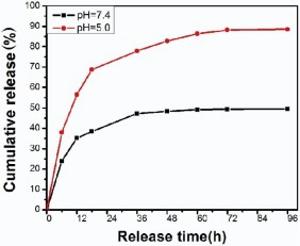
The release of 5-FU is carried out at 37 ° C in a pH of 7.4 or pH of 5.0 PBS buffer, which is used to simulate the neutral and acidic environment of normal and tumor cells, respectively. As shown in Fig. 6, the release curve of 5-FU from hydrogel at pH 7.4 shows a burst release within 24 h and then enters a slow release. The final releasing amount is only 49.5% after about 96 h. In contrast, the release rate of 5-FU is much faster at pH of 5.0, and about 88.5% of 5-FU is released over the same time period. The pH-dependent release may be because that the hydrogen bonding interactions between 5-FU and rGOunder neutral conditions are much stronger than that in acidic environment, which results in the lower release amount of 5-FU[45, 46]. The simulation of drug releasing result shows that the acidic microenvironment of tumor cells could promote drug release, that is, the pH-activated release of drugs can be used to reduce side effects toward normal tyears.
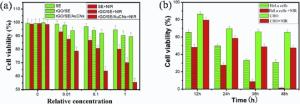
The relative viabilities of HeLa cells cultured with the precursors were examined by the standard MTT assay and the results are shown in Fig. 7(a). As expected, the cell survival rates with all the precursors show a downward trend as the concentrationincreases. However, even when the precursors containing SE, rGO/SE and rGO/AuNCs/SE were diluted with equivoluminal DMEM, the relative survival rates in the dark are still 94.5%, 90.3% and 89.5%, respectively. The results suggest that all the samples possess good biocompatibility. However, after 10 min of sequential NIR irradiation, the relative viabilities of HeLa cells treated by SE, rGO/SE and rGO/AuNCs/SE (the volume ratio of precursor to culture medium up to 1) decrease to 80.2%, 70.1% and 55.4%, respectively. Furthermore, rGO/AuNCs/SE demonstrates remarkably higher anticancer efficiency than rGO/SE and SE at each concentration. The results reveal that the presence of AuNCs or rGO in the precursors can accelerate cell death. For comparative study, the MTT assays of 5-FU loaded rGO/AuNCs/SE precursor were performed at different incubation period using HeLa cells and CHO cell, and the results are shown in Fig. 7(b). When being subjected to laser irradiation for 10 min and then incubating with 5-FU loaded rGO/AuNCs/SE for 24 h and 48 h, HeLa cells present a sharp decrease of survival rates to 27.5% and 1.2%, respectively. All the results indicate that the rGO/AuNCs/SE hydrogel could serve as a good platform for high-efficiency, localized and synergistic antitumor in multimodal therapy. As for CHO cells, the unconspicuous dose-dependent cytotoxicity and relatively low killing efficiency could be attributed to the minimal internalization of the precursors, owing to lack of epidermal growth factor receptor on the CHO cell surface[47].
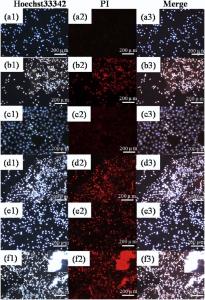
The antitumor effect of the rGO/SE, rGO/AuNCs/SE precursor without and with 5-FU were investigated by fluorescence microscope with PI/Hoechst 33342 double staining. Hoechst 33342 could freely pass through cell membranes and stain nuclear DNA blue, while PI can only enter late-phase apoptotic cells or necrotic cells to stain nuclear DNA red[48]. Fluorescence images of HeLa cells under different conditions are shown in Fig. 8 andFig. S4. Only faint red fluorescence signals can be observed for cells incubated with rGO/SE and rGO/AuNCs/SE precursor without NIR irradiation in Fig. 8(a) and (c), respectively. No significant cell damage indicates that the precursor possess good biocompatibility and low toxicity. With NIR irradiation for 10 min, strong red fluorescence image and purple merged image in Fig. 8(b) and (d) can be observed. As control groups inFig. S4(a) and (b), bare HeLa cells grow exuberantly before or even after laser irradiation, without their nuclei appearing red. It demonstrates that HeLa cells are effectively killed by the 1O2 and local hyperthermia. After being cultured for 3 h with free 5-FU or with 5-FU loaded rGO/AuNCs/SE precursor, a fairly strong red image in Fig. S4(c) or in Fig. 8(e) can be observed. While the same HeLa cells cultured with 5-FU loaded rGO/AuNCs/SE precursor are subjected to NIR irradiation for 10 min, nearly all the HeLa cells were efficiently damaged with the nuclei turning very strong red. And most cells lose their membrane integrity, as evident by the enlarged view shown in the inset of Fig. 8(f), which strengthens its penetrability of the cell membrane and promotes the transport of drugs to cell interiors. Moreover, HeLa cells incubated by rGO/AuNCs/SE with or without 5-FU loading were effectively killed after laser exposure while rGO/SE treated cells were less destroyed, which collectively demonstrate that rGO/AuNCs/SE composite hydrogel could serve as a potential PTT/PDT/Chemo platform for multimodal antitumor therapy. More importantly, hydrogel shells also could be facilely formed on the tumor cells in situ, which is thought to trap drugs on the lesion site.
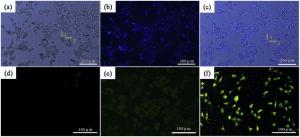
The optical and fluorescence microscopic images of the hydrogel shell on HeLa cells cultured with rGO/AuNCs/SE are presented in Fig. 9. The surface of HeLa cells is covered with a semitransparent hydrogel shell. Fig. 9(b) shows fluorescence microscopy image of HeLa cells encapsulated with hydrogel shells, and Fig. 9(c) is the merged image of Fig. 9(a) and (b). The thin and obvious hydrogel shells (indicated by straight 1) on the cells (straight 2) can be seen from Fig. 9(a) and (c). The results suggest that the hydrogel is successfully formed on the HeLa cells in situ, which conduce to maintain a high drug concentration on the tumor cells, and prevent them from migrating to normal tyear.
The intracellular uptake of precursors labeled by FITC-dextran was observed by fluorescence microscopy at excitation wavelength of 488 nm. The results indicate that almost no fluorescence within the cells in Fig. 9(d), faint green fluorescence around the HeLa cells in Fig. 9(e) and intense green fluorescence in the entire cell in Fig. 9(f) are observed. And the fluorescence signal increased during the formation process of hydrogel under laser irradiation, which implies that precursor with FITC-dextran labeling is successfully internalized into the cell interior. The efficient cellular uptake of rGO/AuNCs/SE precursor labeled by FITC-dextran is probably through the specific interaction between the FA in the precursor and the folate receptor on the surface of tumor cells[49], which is converted into the composite hydrogel, and prevents FITC-dextran emigrating from the labeled cell. And the interaction is dominated by hydrogen bonds and van der Waals forces between carboxyl groups and N-acetyl groups in rGO/AuNCs/SE precursor and receptors protein on the cell membrane[47]. For the same reason, the hydrogel shell on tumor cell also could retain a high concentration of drugs on lesions, and prevent them from migrating to normal tyear, thereby enhancing the curative effect.
We constructed a 5-FU loaded and controlled release composite hydrogel that contained SE, AuNCs and rGO for multimodal therapy of malignant tumor. SE plays multiple roles in the formation of hydrogel and killing tumor cell under NIR irradiation, and also is used as a natural platform for improving the biocompatibility of the system. When loaded with 5-FU, the composite hydrogel shells show good drug retention ability in neutral physiological environment, and also could achieve controllable release of 5-FU in the acidic environment of tumor cells. This hydrogel has several characteristics, such as simple preparation process, environmentally friendly and easy to scale up, and also could serve as a highly integrated platform for multimodal and enhanced cancer therapy.
Supplementary data to this article can be found online at
This work was financially supported by the National Natural Science Foundation of China(Nos. 21171001, 51372004, 21571002 and 21371003), the Anhui Province Key Laboratory of Environment-friendly Polymer Materials and the Anhui Provincial College Student Innovation Fund Project (No. 201510375048), Key Project of the Natural Science Foundation of the Provincial Education Department (No. KJ2016A679).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|