The present study focuses on the thermal response of carbon fiber-reinforced phenolic composites, where the matrix has been modified with different reinforcements. Two types of materials, multiwalled carbon nanotubes and zirconium diboride (ZrB2), were used in a new design of mixture to produce the heat-resistant ablative composite system. The CNT/ZrB2/carbon/phenolic nanocomposite (Z/NT-CR) system corresponding to CNT/carbon/phenolic nanocomposite (NT-CR) showed a reasonable decrease in mass loss and the ablation rate as compared to carbon/phenolic composite (CR). However, substantial drop in two factors was found for Z/NT-CR as compared to carbon/phenolic and NT-CR. Ablation mechanisms for all three composites were investigated by thermal gravimetric analysis in conjunction with microstructural studies using a field emission scanning electron microscope. The microstructural studies revealed that CNTs acted as an ablation resistant phase for protection against 2000 °C, and the conversion from ZrB2 to ZrO2 played an important role as an insulator in the performance of char layer in the ablation resistance.
In view of high temperature applications, an understanding of thermal stability is a key factor for the optimization of the material performances[1]. Nowadays, phenolic resins are indeed irreplaceable materials for many specific high-technology applications, e.g., thermal protection systems[2, 3]. High char yield, good thermal stability, low smoke production on burning, and low flammability are the characteristics that make phenolic resins an attractive candidate for various applications in the electrical, automotive, construction, and appliance industries[4]. Since phenolic resins are aromatic compounds with one or more hydroxyl groups attached to an aromatic ring, they typically exhibit good heat resistance, are rather flame resistant, and have good dimensional stability for ablative heat shields[5]. However, there is always a desire to further improve the erosion resistance of phenolic composites. Until now, some fillers are used to reduce flammability properties of phenolic compounds[6]. The most common ablative approach based on nanocomposites involves the use of silicates having a high aspect ratio[7, 8, 9]. However, other nanofillers such as polyhedral oligomeric silsesquioxane (POSS)[10, 11] and CNT are already used[12]. There are some works where good results were obtained using these nanofillers in combination in order to obtain a synergic effect[13, 14]. But relatively few studies have dealt with the processes of ablation for CNT in phenolic composites[12, 15].
Although CNTs exhibit remarkable thermal properties, owing to their unique structures, as the ablation goes on, the cracks spread, and oxygen penetrates into char layer through these voids and cracks, thus resulting in further oxidation of the surface[16]. To reduce the erosion phenomena of CNT/phenolic ablators, several kinds of improved phenolic composites have been evaluated[17]. Accordingly, the best way is the addition of another filler to improve thermal properties in phenolic composites. ZrB2 particles have been reported as effective additives in the thermal resistance of multiwalled carbon nanotubes with good performances. On the other hand, ZrB2 nanoparticles undergo appreciable oxidation in gaseous oxidants at temperatures as low as 700-800 ° C, which could be increased by attaching them to multiwalled carbon nanotubes (MWCNT)[18].
ZrB2-based materials, having marked properties of high melting point (3313 K), high thermal conductivity (65-135 W/(m K)), excellent thermal shock and ablation resistance, are identified as promising candidate for high temperature applications[19]. The addition of ZrB2 significantly improves the oxidation resistance of phenolic composites due to the formation of adherent compact scale without scale spallation[20]. When the gases escape in ZrB2/phenolic composites, they pass through ZrO2 particles (obtained from decomposition of ZrB2), leaving behind channels of porosity. ZrO2 skeleton is then dominantly responsible for the oxidation resistance of phenolic composites at this stage[21]. Because ZrO2 is not passivated and alone does not completely seal the surface of the sample, oxygen can diffuse through the cracks and porous oxide channels[22], and thus CNT/ZrB2 under ZrO2/CNT/char surface is subject to protect against the further oxidation.
The present work investigates the design of the ablative composites with particular significance for thermal protection of systems against high temperature exposure. Two types of nanostructures, multiwalled carbon nanotubes (MWCNTs) and zirconium diboride are used in a novel ablative composite to produce a system design with the great improvement in ablation quality. An oxygen-acetylene-flame torch and a field emission scanning electron microscope were used to evaluate the ablative properties and microstructures of these nanocomposites. The thermal properties of these phenolic composites were studied by thermal gravimetric analysis (TGA).
MWCNTs (filament diameter: 8-15 nm, filament length: 50 µ m) used for the preparation of nanocomposites were obtained from Neutrino. Phenolic resin (Density 1050 kg/m3) based on a phenolic resol type resin was received from Resitan. Zirconium diboride nanoparticles (ZrB2) were prepared according to previous work during carbothermal reduction process in a tube furnace (Azar Furnace - Iran, equipped with gas flow line)[23]. Carbon fiber (filament diameter: 7 µ m, filament length: 5-10 mm) used for the preparation of the samples was received from Uvicom.
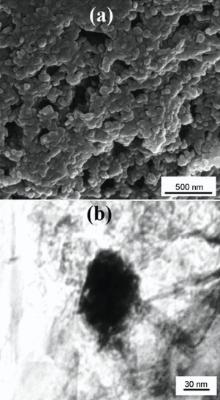
To achieve a better state of dispersion, two nanostructures were first dispersed separately in ethanol by sonication for 2 h. Phenolic resin was added to this nanostructure solution, and sonicated again. Then, carbon fibers were impregnated with phenolic resin-nanoparticles mixture to form a prepreg, using a carbon fiber to resin ratio of 1:1, and mechanical mixing was done for 3 h. After the dispersion and mixing process of phenolic matrix, the solvent was evaporated at 70 ° C for 3 h. Finally, the curing process was done at 150 ° C for 2 h under the hot-press condition. The concentrations of CNT and ZrB2 for the composites used in this study were 1 and 7 wt%, respectively. According to Table 1, three types of carbon fiber/phenolic, MWCNT/carbon fiber/phenolic and ZrB2/MWCNT/carbon fiber/phenolic composites were utilized for the preparation of the specimens. The ZrB2 nanoparticle morphology and internal crystalline structures were observed by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) in Fig. 1.
| Table 1. Physical and ablation properties of the composites |
The characterization of ZrB2 nanoparticles and the cured composites includes the following analysis:
(1) The phase analysis of ZrB2 nanoparticles after calcination was conducted by X-ray diffraction, GNR. Co, Explorer.
(2)Shore D hardness tests of the composite specimens were determined with a Zwick 3100 Shore D, which provides the measure of the surface hardness of the specimens. The measurement was carried out according to ASTM D2240.
(3)TGA was conducted on Linseys (STA 1600) equipment. Heating of the samples was done at a heating rate of 10 ° C/min from 25 to 800 ° C.
(4)To evaluate the ablation performance of the composites, the oxyacetylene flame test in MalekAshtar University of Technology, Tehran, Iran was carried out for simulating the extreme condition. The diameter and thickness of the samples were 25 mm and 20 mm, respectively. The specimens were fixed in a sample holder with the distance between the nozzle tip and the specimen of 5 cm. The flame temperature was estimated to be approximately 2000 ° C during the test.
(5)Morphological characterization of the samples was carried out using a field emission scanning electron microscope, Mira 2 Tescan with an energy dispersion spectroscopy (EDS) analyzer and a transmission electron microscope (Philips CM30).
Table 1 shows shore D hardness values and physical properties of the composites. NT-CR composite was found to have the highest value of hardness as compared to CR and Z/NT-CR. Hardness correlates well with compressive strength and primary effects of the reinforcement[24]. It indicates that the CNT significantly increases hardness of phenolic resin, whereas zirconium diboride nanoparticles tend to reduce hardness compared to CNT/C/phenolic. The hardness increase is attributed to the high hardness of the thermosetting binder resin with CNT after curing[25]. On the other hand, the decrease after the addition of ZrB2 may be due to insufficient binder resin and the voids developed in the carbon fiber and resin interface during post-curing. Also, the values of density calculated by Archimedes method are nearly the same for three composites. The open pore volume (the percentage porosity) was found to increase a little with adding ZrB2 and CNT. The increased pore volume could be explained by the fact that initially during hot press, the matrix still contains some solvent, which is gradually removed leading to contraction of the composite resulting in a net reduction of the pore volume. The CNT-containing polymer cannot contract as much as the solvent is removed, holding the structure open and resulting in a larger pore volume.
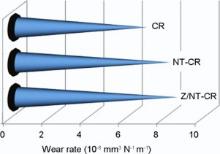
For evaluating the effects of nanoparticles on the mechanical properties of the phenolic composites, wear tests were performed on these specimens at a load of 90 N and distances of 300 m with stainless steel pin. The average of the wear rates was measured, as shown in Fig. 2. It is seen that the wear rate of the phenolic composites slowly decreases with the CNT loading first, and then rapidly decrease when the ZrB2 nanoparticles are added to the system B. The CNT-matrix interaction led to higher wear resistance of CNT/phenolic nanocomposite than the neat composite[26]. Also, wear rate reduces in the case of Z/NT-CR for a rougher surface.
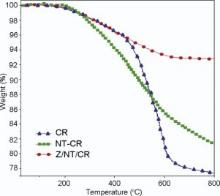
The effects of the nanoparticles on the thermal stability of phenolic resin were studied by TGA under a nitrogen atmosphere as shown in Fig. 3. The phenolic matrix exhibited a good thermal resistance, and was relatively stable below 150 ° C. Volatilization of phenolic resin started at about 150 ° C, and the fragmentation of phenolic network was nearly complete at 450 ° C. The zone of 100-350 ° C is the thermal decomposition zone of free or imperfectly cross-linked molecules[27]. These molecules include water, unreacted phenol and formaldehyde. The weight loss by the evolution of unreacted monomers contributes up to 5-8%. The second and third zones at 400-625 ° C and 625-900 ° C, respectively, can be attributed to the decomposition of the cross-linked nanocomposites with the evolution of carbon monoxide, carbon dioxide, methane, phenols, and cresols. The release of these molecules can be correlated to the post-cure reactions and the formation of dihydroxy benzophenone linkages. With further heating, quinones and carboxylic acids were formed before finally decomposing into cresols, carbonic oxide and carbon dioxide[28]. Thus, a residual mass of over 77% was obtained after heating at 800 ° C for neat carbon/phenolic. On the other hand, the increased thermal stability can be attributed to the resin matrix-CNTs interactions[29]. The residual mass of CNT/carbon/phenolic at 800 ° C was 81.45%, while it increased with doping of ZrB2 by 50% (92.78 wt%). However, ZrB2/CNTs in composite displayed a better thermal barrier effect than pristine CNTs. ZrB2 provides a barrier action against heat diffusion, which improves the thermal stability of the phenolic composites[20]. The stabilization was proven to be due to the formation of a thin protective film of CNT/ZrB2/ZrO2/carbon in char layer generated on the surface of the nanocomposites.
Ablation test was performed using an oxyacetylene flame. The surfaces of samples were exposed to the flame at 2000 ° C for 160 s. The entering of the pyrolysis gases into the boundary layer alters its properties through chemical reactions with the boundary layer gases, thus influencing the net heat transferred into the surface in consumption and the recession of the surface[30]. At higher temperatures, CNTs in Z/NT-CR are not alone responsible for the remarkable improvement in oxidation resistance because partial chars are blown away by mechanical denudation of the flame. Chemical erosion and mechanical denudation improvements[31] inoxidation resistance of CNT/carbon/phenolic composites have been achieved by incorporation of zirconium diboride nanoparticles due to the formation of zirconia and boria glass layer with low oxygen permeability, which provide an efficacious protective oxidation barrier[32]. The ablation resistance of ZrB2 in CNT/carbon/phenolic composites is believed to arise from the formation of a coherent passivating oxide scale on the surface, which acts as an effective barrier against the inward diffusion of oxygen. The main reaction products from the oxidation of ZrB2 are zirconia and amorphous boron trioxide (B2O3)[33]. B2O3 has an unusually low melting point (450 ° C) and a high vapor pressure[34]. Therefore, at high temperature B2O3 quickly vaporizes, resulting in the increase in oxidation of ZrB2[35]. For Z/NT-CR, the process of the growth of ZrO2 on the sidewalls of the MWCNTs might occur[36]; the occurrence of ZrO2-CNT could spread over the oxidation surface and prevent the inward penetration of O2, due to its low thermal conductivity, then provided a better protection on the oxidation layer than CNT alone. Furthermore, CNT in char could fill in the voids in the ZrO2 framework and endow the oxidation layer with a moderate strength, preventing it from being broken off. Furthermore, the introduction of ZrB2 remarkably improves the oxidation resistance of the CR composite at high temperature. However, the composites are covered with different char layers: only carbon char, CNT oxide layer and ZrO2-CNT oxide layer after ablation test. The protective oxides in Z/NT-CR can reduce oxygen diffusion into inner layer and the evaporation of the B2O3 consumes much heat. As the oxides are consumed out, the ZrB2 and CNT underlying layer are oxidized further. After ablation for 160 s, the ablation surface was covered with dense ZrO2 in carbon layer[37]. Finally, the increase of total smoke released might be due to limited volatilization of the charring polymer, while the surface protective char is being created. A thin protective film is rapidly formed already with the lowest MWCNT concentration used here (1 wt%). It is significant to note that the surface-oxidized Z/NT-CR composite consists of two distinctly different scales, namely, the white and black phases.
Time-temperature profiles used for exposed surface and back face of carbon/phenolic composites are shown in Fig. 4. In order to clarify the thermal resistance mechanism, it is usually useful to investigate the morphology and chemical structure of char residue after flame test. Fig. 5 shows photographs of the specimens before and after ablation test. The back surface temperature of CR was shifted toward higher values (600 ° C) in Fig. 4(b), and there was no evidence of formation of a stable charred surface in Fig. 5(b). Macroscopic images of each composite generally showed a smooth, black and uniform surface before flame exposure in Fig. 5(a). CR composite was eroded due to oxidation at high temperature in Fig. 5(b). But, during burning of NT-CR and Z/NT-CR, a nanotube char layer is formed, and this layer insulates the composites from the external radiant flux changing the transmission of heat from thermal conduction to radiative transfer[38]. The nanotube layer becomes a physical shield decreasing external flux. The surface polymer ablation in composite C, upon exposure to radiant heat from the cone, creates a thin surface layer rich of CNT and ZrO2, which protects the polymer from the heat source and hinders mass transfer. Thus, the back surface temperature of NT-CR and Z/NT-CR showed a maximum around 200 ° C and 100 ° C after 160 s in Fig. 4(b), respectively. By effectively transferring heat away from the composite surface through the CNT network, the thermal transferred into the sample is decreased, and subsequently reduced the back surface of the nanocomposites. MWCNTs can reflect a large amount of heat to reduce the surface temperature of the sample. The MWCNTs became a physical shield decreasing external heat flux changing by the transmission of heat from thermal conduction to radiation transfer[5, 39]. Also, the obtained zirconia-CNT completely covers the surface in the transition zone, then leads to lower thermal conductivity.
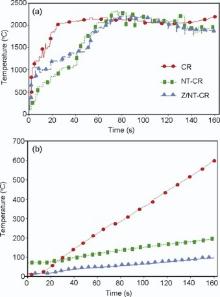 | Fig. 4. Time-temperature force profile used for (a) exposed surface and (b) back face of carbon/phenolic composites. |
 | Fig. 5. Morphology of carbon/phenolic composites: (a) before ablation for all composites, (b) CR, (c) NT-CR, and (d) Z/NT-CR after ablation for 160 s at 2000 ° C. |
There are large numbers of porous microstructures in the char layer after ablation as is evident in Fig. 5(c). It was not difficult to observe that the morphologies of char for NT-CR and Z/NT-CR were different, which demonstrated that the reaction between char residue contributed to the homogeneity of char residue. While for the char residue of composite C, more char was left, and this was much more compact relative to NT-CR without ZrB2. The bright phase on the surface of Z/NT-CR was distributed at the grain boundaries of the gray phase and sealed the cracks at the boundaries in Fig. 5(d). As illustrated in Fig. 6, the surfaces of NT-CR and Z/NT-CR nanocomposites were analyzed by XRD, and it turned out to be ZrO2 and amorphous carbon after ablation.
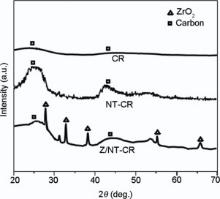 | Fig. 6. Diffraction pattern of pristine and reinforced CR composite with ZrB2 and ZrB2/MWCNT after exposure to the flame at 2500 ° C for 160 s. |
A comparison of ablation rates of the carbon-phenolic resin composites with and without the addition of CNTs and ZrB2 are shown in Table 1. The ablation rates for the neat carbon/phenolic resin, NT-CR and Z/NT-CR were 0.55, 0.21, and 0.13 mm/s, respectively. Compared to carbon/phenolic composite, both NT-CR and Z/NT-CR exhibited significantly improved ablation behavior. 7 wt% ZrB2 could result in a 38% reduction in the ablation rate of CNT/carbon/phenolic, while ZrB2 and CNTs reduced the ablation rate of carbon/phenolic by 76%. This indicated that Z/NT-CR has an oxidation rate about 1/4 of CR. Also, Table 1shows that the percentage of weight loss for CR, NT-CR and Z/NT-CR. Z/NT-CR composite lost 9% of its mass in 160 s, while CR and NT-CR lost 33% and 11% of their masses, respectively. In the present C/phenolic with CNT and ZrB2, the oxide particles remained on the surface of composite after ablation test would protect it from the further oxidation, making the surface oxidation reaction even slower[40]. Therefore, the oxidation rate and mass loss of the carbon/phenolic with these nanoparticles was controlled by the oxygen diffusion through the cracks and insulated layer.
Fig. 7 shows images from the FESEM investigations used to study the mechanism of the ablation process. The structure of the composites before ablation seen with FESEM is similar for all the samples, and shows the formation of a dense structure of carbon fiber and resin with nanoparticles (Fig. 7(a)). From the backscatter scanning electronic microscopy image (Fig. 7(a) for Z/NT-CR), uniform distribution of ceramic nanoparticles can be observed in the carbon/phenolic matrix. Under the high-pressure and shearing forces of the ablation flame, mechanical denudation happened, and the fibers appear to be denuded of the resin matrix in composite CR. But, Fig. 7(b) clearly indicates that NT-CR exhibited less homogenous erosion on the surface than the composite CR. From this, it might be concluded that the MWCNT provided better protection against flame, whereas CR suffered easier heat penetration that extended deeper into the composite. Further observation by FESEM showed the very different microstructures of the oxidized surface for NT-CR, and Z/NT-CR. Extensive char formation composed of particles was observed on the surface of Z/NT-CR. Combined with the EDS line analysis, these particles were ZrO2 grains with carbon. Boron trioxide phase was not observed on the oxidized surface of this composite. It was attributed to the high temperature flow acted on the sample surface, which could greatly evaporate the formation of B2O3 phase. Generally, an eventual protection from heat and mass transfer, decreasing rate of combustion due to the formation of a film on the surface of the specimens is in good agreement with FESEM observations.
In this study, we investigated the effects of pristine CNTs and CNTs/ZrB2 on the thermal oxidative stability, wear and hardness of carbon-phenolic materials. Although complete oxidation protection was not achieved with only CNTs, they can be improved with the incorporation of ZrB2 nanoparticles due to synergy reinforcing effect. Decrease in average weight loss was observed on addition of these two types of nanostructures to carbon fiber reinforced phenolic resin composite, and consequently the excellent ablation resistance was mainly attributed to the formation of a dense and continuous zirconia and char layer during the oxidation. In this situation, an average decrease of 76% and 72% in the values in ablation rate and mass loss was observed at 2000 ° C for 160 s, respectively. Developments in the improvement of the thermal properties of phenolic composites would rapidly lead to the generation of new high performance composites, which may find a wide range of uses for advanced high-technology purposes.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|