Zirconia-based bioceramics have been widely applied in the field of prosthodontics owing to its desirable mechanical performance, biocompatibility and aesthetics. However, the low-temperature degradation (LTD) of tetragonal zirconia (ZrO2) under intraoral condition can lead to the deterioration of mechanical properties of ZrO2 dental crowns, which contribute to many clinical failures in long-term observations. The long-term tetragonal phase stability and mechanical properties of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) are influenced by grain size of ZrO2 crystals, distribution and properties of stabilizers, the humid environment, etc. However, it is still difficult to control the abovementioned factors at the same time. This review summarizes the major advances in researches dealing with LTD and clarifies the obstacles to stabilization of the tetragonal ZrO2. Furthermore, the suggestions on improving the LTD resistance of tetragonal ZrO2 are proposed, which is the catalyst to promote the long-term stability of ZrO2-based all-ceramic crowns.
Zirconia-based bioceramic has attracted significant attention as a desirable all-ceramic crown material due to its excellent mechanical properties (hardness, fracture toughness and strength), biocompatibility and aesthetics, etc., which are more auspicious relative to other bioceramics, e.g., alumina[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Especially, yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) can further enhance the bending strength and fracture toughness of crowns remarkably by neutralizing the external strain through phase transformation toughening (PPT) of zirconia (ZrO2) from tetragonal phase to monoclinic phase[24, 25, 26]. As a result, Y-TZP has been widely applied in the field of prosthodontics.
To date, many manufacturers, e.g., Vita Zahanfabirk, IvoclarVivadent and 3M ESPE, have devised various techniques such as In-Ceram and CAD/CAM to prepare Y-TZP bulks in mass production for dental crowns (bending strength ≥ 900 MPa, fracture toughness ≥ 5 MPa m1/2)[27]. However, the long-term tetragonal phase stability of Y-TZP deteriorates under intraoral condition due to the low-temperature degradation (LTD) of tetragonal zirconia (ZrO2), which contributes to many clinical failures of Y-TZP dental crowns[27, 28, 29, 30, 31]. Therefore, it is of great importance to improve the LTD (also referred as aging) resistance of tetragonal ZrO2 for long-term stability of ZrO2-based all-ceramic crowns.
Although the specific mechanism of LTD is still controversial, there are several reasonable speculated explanations on LTD phenomenon of Y-TZP, including[32]: (1) Y2O3 stabilizer in the neighboring tetragonal ZrO2 grains is depleted through the reaction of the stabilizer with water, resulting in the tetragonal-to-monoclinic (t-m) transformation of zirconia; (2) Zr-O bond is attacked and broken by water vapor, and stress is accumulated due to the movement of -OH, leading to the lattice defects acting as nucleation sites for t-m phase transformation; (3) O2- originated from water will fill oxygen vacancies of matrix, resulting in the t-m phase transformation. Overall, the following view has reached consensus: Y-TZP is prone to generate t-m phase transformation in the presence of water, and the transformation initially originates from the surface of the sample and then penetrate underneath, resulting in LTD of Y-TZP and cracks in the crown. To enhance the LTD resistance of TZP, numerous studies have been carried out experimentally and theoretically[33, 34, 35, 36, 37, 38]. In particular, the grain size of TZP, and types and contents of stabilizers are considered as two major factors to influence the tetragonal phase stability and aging performance of the sample.
From the thermodynamic perspective, the driving force of phase transformation can be reduced by decreasing the grain size of TZP, which is favorable for the stabilization of the tetragonal phase and LTD resistance; while from kinetic perspective, more grain boundaries acting as nucleation sites for t-m phase transformation will be generated[32]. Therefore, grain size will significantly affect the tetragonal phase stability and aging performance of the sample. A number of reports suggest that the critical grain size (GS) of stabilized tetragonal phase for pure ZrO2 powder is 5‒10 nm at room temperature, under which the tetragonal phase is thermodynamically stable and would have good LTD resistance[27, 33]. While for Y-TZP system, the critical GS is closely related with the amount of Y3+, yet it is still controversial in the detailed critical GS even for the same amount of Y3+[27, 29, 34]. Denry and Kelly indicated that such controversy may be due to the different distribution Y3+ in the matrix, which will affect the t-m phase transformation of ZrO2grains[27]. However, to the best of our knowledge, there are virtually few undisputed systematical studies involving the relation between Y3+ distribution and GS of ZrO2 to stabilize the tetragonal phase, which is definitely unfavorable to investigate the stabilization of tetragonal Y-TZP and its LTD resistance theoretically and experimentally. The critical GS of powder sample is quite different from that of solid sample, and the distribution of Y3+ in the matrix may also influence GS.
In fact, it is particularly difficult to rigorously control the GS of ZrO2 and distribution of Y3+ at the same time through conventional methods[38]. Till now, the liquid method, such as sol‒gel method and hydrothermal method are the most popular methods to prepare Y-TZP due to their simplicity and low cost, yet both methods have their restrictions. For sol‒gel method, although the homogenous distribution of Y3+ in ZrO2 matrix can be achieved, it is very difficult to control the GS of samples especially to several or dozens of nanometers. While for hydrothermal method, the GS of samples can be controlled to as low as several nanometers owing to the relatively low reaction temperature, but the distribution of Y3+ in ZrO2 matrix is uncontrollable due to the different precipitation consequence of ZrO2 and Y2O3 during the hydrothermal process. Therefore, it is of great importance to overcome such restrictions to further enhance the LTD resistance of Y-TZP for dental applications.
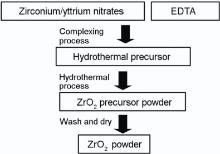
Our group has been working on dental materials[38, 39, 40, 41], and we devised a novel “ complexing-hydrothermal” method to prepare ZrO2-based materials by integrating complexing sol‒gel method and hydrothermal method. The flowchart of the so-called “ complexing-hydrothermal” is shown in Fig. 1. Zirconium nitrate and/or yttrium nitrate were used as reactants and ethylenediaminetetraacetic acid (EDTA) as chelating agent to prepare the EDTA-zirconium and EDTA-yttrium monomer complexes. With different time of gelation process, the mixed solution was adopted as the precursor of hydrothermal reaction. After the hydrothermal reaction, the pure ZrO2 powders, whose GS is lower than 10 nm, and Y-TZP powders, whose GS is lower than 15 nm, were obtained by washing and drying the precursor powder. In addition, the stability of tetragonal phase could be enhanced even if no Y3+ stabilizer was added, which was favorable to enhance the LTD resistance of samples. The reactions related to the “ complexing-hydrothermal” process are as follows:
EDTA+Zr4+⇔ [EDTA-Zr]+H+, EDTA+Y3+⇔ [EDTA-Y]+H+(1)
Zr4++OH-⇔ Zr(OH)4, Y3++OH-⇔ Y(OH)3(2)
Zr(OH)4⇔ ZrO2+H2O, Y(OH)3⇔ Y2O3+H2O (3)
where reactions (1) and (2) are the complexation reaction of metal ions and complexing agents, i.e., EDTA and OH-, respectively. Reaction (3) is the hydrothermal reaction of the hydroxide of Zr4+ and Y3+. Unlike conventional hydrothermal process, whose reaction is just reactions (2) and (3), a strong complexing agent, i.e., EDTA, was adopted to complex both Zr4+ and Y3+. In this way, the competition of complexation reactions of reactions (1) and (2) can delay the precipitation of ZrO2 and Y2O3 during the hydrothermal reaction, which can improve the control of GS and distribution of Y3+ in ZrO2 matrix. The preliminary energy spectrum analysis also confirmed the speculation, although the ratio of Zr4+ and Y3+ was still nonstoichiometric. Therefore, further investigations on the competition and balance of different reactions are in need in this promising method to clarify the relation mechanism between GS and distribution of stabilizer in stabilization of tetragonal Y-TZP, and then to improve the LTD resistance of Y-TZP.
It was reported that the LTD resistance of Ce-TZP was bigger than that of Y-TZP after the International Standard Organization (ISO) aging cycles test in water vapor conducted at 134 ° C and 2 × 105 Pa (2 bar) for 5 h[42]. Unlike the stabilization mechanism of yttria, ceria (CeO2) stabilizes the tetragonal zirconia by generating the O2- vacancies due to the different ionic radius of Ce4+ and Zr4+. Generally speaking, the amount of CeO2 should be in the range of 8‒12 mol% to obtain the stabilized tetragonal zirconia[32]. However, the stabilization mechanism of the concentration of Ce4+ on tetragonal zirconia is still unknown. Furthermore, Ce4+ can be reduced to Ce3+ under the reaction of many factors, e.g., sintering, local stress or glucose in oral cavity. As a result, the phase stabilization ability of CeO2 will deteriorate and the color of Ce-TZP will change, which are unfavorable for Ce-TZP crowns from both mechanical and aesthetic point of views. As an alternative, CeO2 can be applied as a secondary stabilizer of Y-TZP to prepare Ce4+ doped Y-TZP, i.e., Ce/Y-TZP, which can improve the LTD resistance of Y-TZP without affecting the mechanical properties and aesthetics obviously. This promising system, however, cannot solve LTD of tetragonal zirconia in the long term.
It is of great importance to restrict the contact and reaction between water molecules and tetragonal zirconia to improve the LTD resistance. From this perspective, it can shield tetragonal zirconia from water effectively if we can coat Y-TZP grains with shell. The choice of shell materials should base on the following: the shell should restrict the penetration of water to affect the stability of tetragonal zirconia; the introduction of shell should not deteriorate the mechanical properties of the Y-TZP system remarkably; the biocompatibility of the shell materials should be good enough for the clinical application.
Azimi et al.[43] found that surfaces of some rare earth oxides (e.g. CeO2 and Er2O3) are hydrophobic as shown in Fig. 2. Accordingly, it is of particular interest if we can combine both the hydrophobicity of CeO2 and its stabilization functions on Y-TZP to restrict or diminish the influence of water on tetragonal zirconia. In this respect, the core-shell Y-TZP@CeO2 composite may be a promising dental material with better LTD resistance compared to Y-TZP or Ce/Y-TZP. To the best of our knowledge, there are few studies investigating TZP coated with Y2O3, which was based on physical mixing method[44], and no report has been published on the study of the influence of hydrophobic shell on LTD resistance and mechanical properties of ZrO2-based crowns. Fig. 3 is the schematic flowchart of our ongoing work, in which core‒shell structure is constructed by electrostatic attraction of Zr(OH)4-Y(OH)3 colloid particles and Ce4+, and Y-TZP@CeO2 composite can be obtained by hydrothermal process.
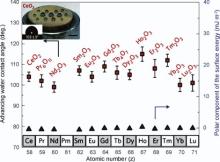 | Fig. 2. Hydrophobicity of surface of rare-earth elements oxides[43]. The inset is the photograph of water droplet on ceria surface. |
Meanwhile, nanotechnology has been widely applied to prepare ZrO2-based materials to improve the mechanical properties of the crowns[45], which may bring the potential biological toxicity of the materials. Especially, the toxicity of CeO2 is controversial[46], which is yet to be clarified for the clinical application of Y-TZP@CeO2 composite.
LTD of tetragonal zirconia is a hot issue for the clinical application of ZrO2-based all ceramic crowns. It is essential to clarify the following two aspects to improve the LTD resistance of Y-TZP: (1) role of distribution and properties of stabilizers and GS of ZrO2grains on the stability of tetragonal zirconia; (2) structural optimization to restrict the influence of water on tetragonal zirconia. It is believed that the LTD resistance and long-term performance of ZrO2-based crowns will be improved remarkably in the near future with the efforts of both researchers and manufacturers.
This work was financially supported by the National Natural Science Foundation of China(No. 81500897), China Scholarship Council (No. 201408210385), Foundation of the Education Department of Liaoning Province in China (No. L2013285) and Science and Technology Planning Project of Shenyang City (No. F11-262-9-16).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|