This paper reports the effect of cooling rate on the microstructure and hardness of a kind of medium carbon steel microalloyed with two levels of V content (0.15% and 0.28%) after hot deformation by using single compression tests on a Gleeble-3800 thermal simulator. The results show that cooling rate has a significant effect on the microstructure and hardness of the tested steels. Both the fraction of pearlite and hardness increase with increasing cooling rate, whereas a further increase of the cooling rate above a critical value promotes the formation of acicular ferrite (AF), and thus leads to a decrease of hardness mainly owing to the decrease of pearlite fraction and replacing it by AF and the less effective precipitation strengthening. Increasing V content results in a significant increase of hardness, and this tendency enhances with increasing cooling rate until the formation of AF. Furthermore, increasing V content also significantly enhances the formation of AF structure at a lower cooling rate. The results also suggest that by controlling microstructure, especially the precipitation of fine V(C,N) particles through adjusting post-forging cooling, the strengthening and gradient function in one hot-forging part could be obtained.
Microalloyed (MA) medium carbon steels have been widely used as substitutes for quenched and tempered (Q& T) carbon and alloy steels to manufacture a variety of automotive forging components[1]. However, the use of this kind of steels is often limited to components such as crankshafts and connecting rods, which do not require high impact toughness. Therefore, many attempts have been made to improve the toughness of MA medium carbon steels in the applications of safety parts such as steering shafts, steering knuckles, drive couplings and drive flanges[1, 2, 3, 4, 5, 6]. The application of microalloying elements such as V, Ti and Nb offers an important cost-effective approach to obtain a good combination of high strength and high toughness mainly through precipitation hardening and grain size refining[1, 7].
The forging parameters, post-forging cooling rate as well as compositions have significant effect on the microstructural characteristics and thus final mechanical properties of MA steel forged components[3, 4, 5, 6]. Increasing cooling rate generally increases strength level mainly due to the increase of pearlite proportion or the formation of high hardness phases, such as bainite and martensite, as well as grain refinement. For MA steels, it should be emphasized that cooling conditions also significantly influence the precipitation hardening effect of microalloying elements[8]. Therefore, different post-forging cooling strategies have been used to modify the microstructure and mechanical properties of MA steels[1, 9, 10]. Owing to the inherent cleavage fracture mode of pearlite phase, there has been a trend to modify the ferrite-pearlite microstructure with high toughness microstructure such as acicular ferrite (AF) by obtaining a moderate post-forging cooling rate[4, 11, 12, 13, 14]. This kind of AF microstructure possesses a good combination of strength and toughness, because the fine interlocking plates of ferrite makes crack propagation difficult. The main difference between AF and bainite is their nucleation sites[14]. It is accepted that the nucleation of randomly oriented AF takes place inside the austenite grains at certain non-metallic inclusions and/or precipitates, while parallel bainitic sheaves nucleate at the austenite grain boundaries[13, 14].
Microalloying of V is commonly preferred to other microalloying additions, Ti and Nb, in medium carbon steels for the purpose of strong and easily controllable precipitation hardening, mainly owing to the relatively large solubility of its carbonitride V(C, N) in austenite at convenient heating temperature[15]. Moreover, it has been observed that either intragranular ferrite (IGF) idiomorphs or AF plates were nucleated at V(C, N) particles, which precipitated on MnS inclusions within austenite grains during cooling[2, 16, 17]. The normal addition of V is less than 0.15% in conventional MA medium carbon forging steels[1, 3, 5, 6, 9, 11, 18]. Recently, there has been an increasing need for the development of higher strength of automobile parts to improve fuel consumption through weight reduction[19, 20]. As an efficient method of strengthening medium carbon forging steels, there has been a tendency to further increase V content up to about 0.30%[19, 20, 21]. Therefore, the purpose of this investigation is to study the variations of microstructure and hardness under different cooling conditions of medium carbon steel microalloyed with 0.15% V and 0.28% V, in an attempt to clarify the effect of cooling rate and V content on transformation behavior and mechanical properties, as well as to further understand the relationship between microstructure and manufacturing processes of MA steels.
Medium carbon steels with two different contents of V (0.15%, 0.28%), which were designated as V1 and V2, were melted in a laboratory vacuum induction furnace and casted into 150 kg ingots. Table 1 shows the chemical compositions of the tested steels. These ingots were reheated to 1200-1220 ° C and held at that temperature for at least 1 h and then press forged to 50-mm diameter bars. Samples for hot compression tests were machined from half radius of the bars with their axis parallel to the bar axis. The size of the cylinder samples is 8 mm in diameter and 15 mm in height. In order to investigate the influence of V content on transformation temperatures, samples with a diameter of 4 mm and a length of 10 mm were also machined for dilatometry tests.
| Table 1 Chemical composition and phase transformation temperatures of the tested steels |
Uniaxial hot compression tests were carried out by using a Gleeble 3800 thermomechanical simulator. Graphite was used between the samples and the compress anvils to reduce friction. Argon was also used to protect the samples from surface oxidation. Samples were heated up to 1200 ° C at a constant rate 10 ° C/s and soaked at this temperature for 60 s, and then they were cooled at 10 ° C/s to the selected deformation temperature 1150 ° C. To minimize temperature gradient, the samples were kept at 1150 ° C for 10 s. Then the samples were deformed to a true strain of 0.92 (reduction in height of 60%) at a strain rate of 10 s-1. Afterwards, the deformed samples were first fast cooled to 800 ° C at a cooling rate of 10 ° C/s, and then cooled to 500 ° C at a selected constant cooling rate in the range of 0.2-10 ° C/s. Finally, all the deformed samples were still air cooled to room temperature. To identify the austenite grain size after deformation, parts of the samples were water-quenched just after deformation.
After the compression tests, the samples were sectioned parallel to the long axis for microstructural observation and hardness measurement. Metallographic samples were etched with 3% nital solution. An optical microscope instrumented by image analysis software SISC IAS V8.0 (accuracy: < 5%) and a scanning electron microscope (SEM) were used for microstructural characterization. A saturated picric acid solution was employed to reveal the austenite grain boundaries of the water quenched samples. Automatic image analysis using linear intercept method was carried out to determine the average austenite grain size. Vickers hardness of the specimens was measured with a 5 kg load, and Vickers microhardness of both the ferrite portion and the pearlite portion was also measured with 10 g and 50 g load, respectively. The hardness results are the average values of at least 10 measurements for each sample. Dilatometry tests were carried out using a Baehr DIL805L dilatometer at a heating rate of 200 ° C/h to obtain phase transformation temperatures during heating (Ac1 and Ac3). A transmission electron microscope (TEM, Hitachi H-800 and FEI Tecnai G2 F20) with energy dispersive X-ray spectrometer (EDS) was used to study the precipitate characteristics of typical samples. The operating voltage was 200 kV. Standard chromium trioxide-acetic acid solution was used for the preparation of thin foils in a twin-jet electropolishing apparatus.
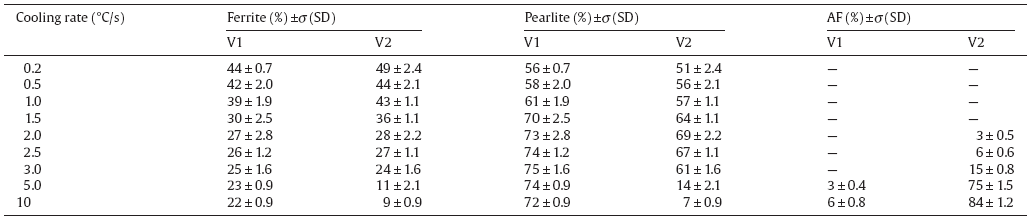
Phase transformation temperatures during heating (Ac1 and Ac3) of the tested steels are given in Table 1. There is a slight increase of both Ac1 and Ac3 temperatures with increasing V content. The austenite grain size after deformation for steels V1 and V2 are 28 m and 23 m, respectively, which indicates that austenite grain size decreases with increasing V content, and thus would influence on the following phase transformation during cooling. Cooling rate after hot deformation has a remarkable influence on the resulting microstructural characteristics of the tested steels. Fig. 1 and Fig. 2 show the evolution of optical microstructures for the tested steels at different cooling rates. Table 2 shows variations of the volume fraction of phases of ferrite, pearlite and/or AF with cooling rate. As can be seen, all the samples consist of proeutectoid ferrite and pearlite at the lower cooling rate. Proeutectoid ferrite was formed either at austenite grain boundaries or within austenite grains. An increase in cooling rate lowers transformation temperature and shortens the diffusion time at high temperature, and thus the fraction of proeutectoid ferrite decreases and pearlite becomes more dominant. The fraction of ferrite especially intragranular ferrite (IGF) also increases with increasing V content. IGF idiomorphs were often associated with MnS inclusions and V(C, N) particles[16]. High content of V, N and S of the tested steels favors to promote the IGF formation. Fig. 3(a, b) gives an example showing an IGF idiomorph precipitated on MnS inclusion within austenite grain. As a result, the formation of fine V(C, N) particles during forging not only lowers the carbon solution content in austenite but also promotes the formation of IGF, and therefore the volume fraction of ferrite increases with increasing V content[22].
| Table 2 Volume fraction of ferrite, pearlite and AF at different cooling rate after deformation |
 | Fig 1. Optical micrographs of V1 steel cooled at different cooling rates after deformation: (a) 0.5 ° C/s, (b) 1.0 ° C/s, (c) 2.0 ° C/s, (d) 5.0 ° C/s, (e, f) 10 ° C/s. |
 | Fig 2. Optical micrographs of V2 steel cooled at different cooling rates after deformation: (a) 0.5 ° C/s, (b) 1.0 ° C/s, (c) 2.0 ° C/s, (d) 5.0 ° C/s, (e, f) 10 ° C/s. |
Further increasing cooling rate leads to even less ferrite and promotes the formation of AF within austenite grains at the expense of pearlite (Figs. 1(d) and 2(c)). However, the tendency of AF formation is significantly different for the two tested steels. That is to say, for steel V2, increasing cooling rate to ~2.0 ° C/s, a certain amount of AF was first detected; further increasing cooling rate leads to a significant increase of AF. At a cooling rate of 5.0 ° C/s, the AF becomes dominant morphology, alongside with proeutectoid ferrite delineating the prior austenite grain boundaries and/or some adjacent pearlite (Fig. 2(d)), whereas for steel V1 only a small amount of AF was detected at 5.0 ° C/s (Fig. 1(d)), and this tendency increases slightly even at 10 ° C/s (Fig. 1(e, f)). These results clearly indicate that increasing V content could significantly promote the formation of AF. Both the N and S contents in the tested steels are considerably high, for the precipitates of VN or N-rich V(C, N) on MnS inclusions could already exist in the deformed austenite condition[2, 23]. It has been confirmed that those VN or V(C, N) particles are highly potent for intragranular nucleation of AF (Fig. 3(c, d))[2, 5, 17, 24]. Therefore, increasing V content could significantly enhance the formation of AF structure at a moderate cooling rate. Moreover, as can be seen in Fig. 2(e, f), a few amount of grain boundary ferrite and surrounded pearlite still exists even at a rather high cooling rate of 10 ° C/s. This result implies that a cooling rate of 10 ° C/s could not suppress ferrite-pearlite formation. By the way, the presence of proeutectoid ferrite at austenite grain boundaries could also suppress the nucleation of grain boundary bainite and thus promote the formation of AF[5, 25].
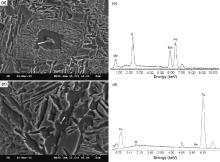
As mentioned previously, cooling rate could also significantly influence the precipitation hardening effect of microalloying elements. The higher the cooling rate, the lower the temperature at which the precipitate forms. The rate of cooling can then determine the forms of precipitate in austenite or ferrite and thus its size and amount[8]. Examination of the samples with different cooling rates using TEM revealed different sizes and amounts of precipitates. Fig. 4(a, b) shows the dark field TEM micrographs in proeutectoid ferrite region of steel V2 corresponding to cooling rates of 0.2 ° C/s and 2.0 ° C/s, respectively. The precipitate was identified as V(C, N) through selected area electron diffraction pattern (inset in Fig. 4(a)) and EDS analyses (Fig. 4(d)). V(C, N) precipitates were also observed in pearlitic ferrite (Fig. 4(c)).
As can be seen, the amount of V(C, N) precipitates is much higher and the size of the precipitates is much finer for the 2.0 ° C/s cooled sample than those for the 0.2 ° C/s cooled sample. That is to say, increasing cooling rate increases the driving force for the dissolved V(C, N) particles to precipitate again, thus promotes a more refined precipitate dispersion[26, 27]. Fig. 4(e, f) shows bright TEM images of steel V2 at a cooling rate of 5.0 ° C/s. It is clear that no obvious V(C, N) precipitates were found within both proeutectoid ferrite and AF plates, which indicates that high enough cooling rate could reduce or inhibit the precipitation of V(C, N) particles.
These results were confirmed by further microhardness measurements of both the proeutectoid ferrite and pearlite phases, as shown in Fig. 5. It was observed that the microhardness of both proeutectoid ferrite and pearlite increase with increasing cooling rate at first, indicating more fine V(C, N) particles were obtained within both proeutectoid ferrite and pearlitic ferrite with increasing cooling rate (not exceeding critical cooling rate). As more V(C, N) particles precipitate within proeutectoid ferrite, the hardness increase of proeutectoid ferrite with cooling rate is much higher than that of pearlite. Further increasing cooling rate to ~5 ° C/s for steel V1, and ~2 ° C/s for steel V2, causes a decrease of the microhardness of both proeutectoid ferrite and pearlite, implying that less precipitation hardening was obtained. When cooling rate is higher than 2 ° C/s, the decrease of the microhardness of ferrite for steel V2 is more significant than that of steel V1.
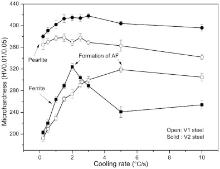
Fig. 6 shows the variations of hardness of the tested steels with cooling rate after deformation. As can be seen, the hardness of both steels increases nearly linearly with increasing cooling rate before the formation of AF. This is mainly due to the increase in the fraction of pearlite as well as the formation of more fine dispersed precipitates. It should be noticed that too fast cooling may not give enough time for complete precipitation of V(C, N) within either ferrite or AF. That is to say, the beneficial effect of V(C, N) precipitates depends on cooling rate[3]. Our previous investigation of the tested steels in the as-forged condition showed that only 48%-64% of total V is present as V(C, N) precipitates, the others is in the solution condition[28]. Other results also showed that the cooling rate during air cooling was not sufficiently slow to allow full precipitation of all carbides[8]. Therefore, there exists an optimum cooling rate to obtain the highest precipitation hardening effect for the tested steels in the precondition of ferrite-pearlite microstructure. According to this study, the optimum cooling rate is about 3-5 ° C/s for steel V1, which is in accordance with conventional MA medium carbon forging steels[29], and about 1.5-2.0 ° C/s for steel V2.
As shown in Fig. 6(a), the hardness of the tested steels tends to decrease with increasing cooling rate above a critical value (here ~5 ° C/s for steel V1 and ~2 ° C/s for steel V2), which corresponds to the start of the formation of AF. The hardness of steel V2 decreases slightly at first and then decreases remarkably at the cooling rate higher than 3 ° C/s, which corresponds to a sharp increase of AF content, whereas the hardness of steel V1 decreases slightly at the cooling rate higher than 5 ° C/s, which corresponds to a small increase of AF content (Table 2). This implies that the formation of AF tends to decrease the hardness of the tested steels. Previous studies have revealed that the formation of bainite or AF might lower the hardness and strength level of V-containing MA steels[5, 19, 30]. For example, Miyamoto et al.'s investigation of V-containing medium carbon steels transformed isothermally showed that the effect of V on hardness remarkably decreases at isothermal temperature of 550 ° C where bainite transformation takes place partly[19]. The investigation of the influence of reheating temperature and V content on transformation behavior and mechanical properties of medium carbon steels showed that V in solid solution promotes the formation of bainite following low reheating temperatures (950-1150 ° C), whereas for high reheating temperatures (1250-1300 ° C) AF becomes dominant structure with a decrease of tensile strength[5]. Investigation by Chiba et al. about the relationship between hardness and alloying elements of V-microalloyed MA steels showed that the effect of V on hardness in bainite type structure is not as obvious as that in ferrite-pearlite type structure. Their further precipitate quality analysis revealed that V does not form V(C, N) precipitates and only act as a solid solution hardening element in bainite type structure[30]. That is to say, the contribution of V is precipitation hardening in ferrite-pearlite type structure and solid solution hardening in bainite type structure.
As can be seen in Fig. 1 and Fig. 2 and Table 2, pearlite was mainly replaced by AF. Pearlite is the most important carbon-consuming constituent in the microstructure and the microhardness of pearlite is higher than that of AF, which has very low solubility for carbon[12]. As mentioned above, careful TEM examination of both the ferrite and AF regions found no V(C, N) precipitates (Fig. 4(e, f)). This is regarded that too fast cooling (higher than critical cooling rate) may not give enough time for complete precipitation of fine V(C, N) particles. Therefore, it is suggested that the decrease of pearlite content and replacing it by AF and the less effective precipitation hardening of V(C, N) precipitates are the main reasons for this hardness decrease above the critical cooling rate.
As indicated in Fig. 6(a), the increase of V content results in a significant increase of hardness, and this tendency enhances with increasing cooling rate until the formation of AF (Fig. 6(b)). For instance, the increase of hardness Δ HV5 (Δ HV5 refers to the hardness of steel V2 subtracting that of steel V1) is about 26 HV5 at 0.2 ° C/s, while it is about 57 HV5 at 2.0 ° C/s. This hardening by increasing V content is principally due to the precipitation hardening by more fine V(C, N) particles with increasing cooling rate (Fig. 4(a, b)). The increase of hardness noticeably decreases at the formation of AF. Therefore, it is concluded that increasing V content from 0.15% to 0.28% could significantly promote the formation of AF at a moderate cooling rate. These microstructural variations are expected to improve impact toughness without noticeable penalty on strength levels. Therefore, further thorough work needs to be carried out.
(1)Increasing cooling rate after hot deformation leads to an increase in the volume fraction of pearlite at the expense of ferrite and more fine V(C, N) precipitates. A further increase of cooling rate above a critical value leads to even less ferrite and promotes the formation of AF; at this moment no obvious precipitates were found within both pro-eutectoid ferrite and AF.
(2)Vickers hardness increases nearly linearly with increasing cooling rate up to the critical value, mainly due to the increase of pearlite fraction and precipitation hardening of fine V(C, N) precipitates. Hardness tends to decrease with further increasing cooling rate, which is ascribed to the decrease of pearlite content and replacing it by AF and the less effective precipitation hardening.
(3)Increasing V content from 0.15% to 0.28% results in a significant increase of hardness due to increased precipitation hardening effect, and this tendency enhances with increasing cooling rate until the formation of AF.
(4)Increasing V content could significantly enhance the formation of AF structure at a moderate critical cooling rate. The critical cooling rate corresponding to the formation of AF is ~5 ° C/s for the 0.15% V steel and ~2 ° C/s for the 0.28% V steel.
(5)The phase transformation and corresponding hardness variation behavior of both the tested steels is similar, i.e. mechanisms are the same, but the extent of processes is slightly different.
This work is financially supported by the National High-Technology Research & Development Program of China (No. 2013AA031605).
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|