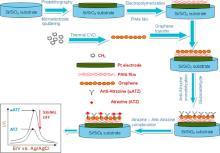
In this study, a novel layer-by-layer polyaniline/graphene (PANi/Gr) structure for electrochemical detection of atrazine was developed. Gr film was synthesized by thermal chemical vapor deposition (CVD) method and transferred onto the PANi-predeposited microelectrode. The properties of PANi/Gr film were thoroughly investigated by high-resolution transmission electron microscopy and Raman techniques. The most attractive feature of this system is a suitable microenvironment, which could provide an amplification of the conductive signal, thus may contribute to enhancing electron transfer and subsequently improve the sensitivity in electrochemical measurements. With low detection limit (∼ 43 × 10-12 g/L), acceptable stability and good reproducibility, the proposed electrochemical immunosensor could be advantageously extended for multiplexed detection of other agents of environmental pollution.
Atrazine (ATZ, 1-chloro-3-ethylamino-5-isopropylamino-2, 4, 6-triazine) is a triazine herbicide commonly used to control weeds in the production of corn and other agricultural crops. The mode of action of ATZ is binding to quinine-binding proteins, inhibiting electron transport and blocking photosynthesis of the weeds[1]. When ATZ was first released in 1958, it was thought that animals would be immune to any effects of ATZ since photosynthesis is limited to plants[2]. However, it was recently reported that the indiscriminate use of this herbicide may have harmful effects on the aquatic organisms, the environment and even the human health. Previous studies have suggested that the ATZ exposure with average exposure levels (< 10-4 g L-1 × 4 days) is sufficient to affect development, behaviors, and production in fishes and other aquatic organisms[3, 4]. In 2010, Hayes et al. have demonstrated that ATZ is a potent endocrine disruptor that turns male frogs into female ones at relatively low concentration (0.1 ppb)[5]. To humans, ATZ is slightly moderately toxic. Inhaling or swallowing this herbicide may cause abdominal pain, vomiting, eye irritation or irritation of mucous membranes. Several studies have found possible connections between ATZ exposure and higher rates of some birth defects and poor semen quality in men[6, 7]. Moreover, it must be also noticed that ATZ can be easily transported to non-target areas and infiltrated into ground water due to its high solubility in water (32 × 10-3 g L-1)[4]. As a result, ATZ is the most commonly detected herbicide contaminant of ground, surface, and drinking water. For those reasons, ATZ was banned in Europe by European Union in 2004[8]. Clearly, the development of rapid and reliable methods for determination of the toxins like ATZ has become increasingly important for human health and environment protection.
As a consequence of the ecotoxicological implications of atrazine residue in water sources, a growing number of methods have been developed. The traditional methods for the detection of atrazine such as high performance liquid chromatography-mass spectrometry (HPLC-MS), which show the best analytical performances, are time-consuming, expensive, and require highly skilled technicians. In the last two decades, electrochemical biosensor has been an increasing interest as one of the most promising technologies for the detection of bio-molecules in general[9] and of atrazine in particular. Some highly conductive materials could provide important improvements in sensitivity of electrochemical detection[10, 11, 12, 13, 14]. Several works have shown the enhanced sensitivity of atrazine sensors with using gold nanoparticles[12, 15, 16, 17, 18]. The use of conducting polymers was demonstrated to be important to increase biocompatibility and conductivity of biosensing systems[19]. Recently, graphene (Gr) was used for biosensing applications due to its high surface area (2600 m2 g-1)[20], extraordinary electronic transport properties and electrocatalytic activities[21]. Due to its atom-thick nature, the electrical properties of graphene are highly sensitive to the interaction between graphene surface and adsorbed foreign molecules[22]. However, one of the major challenges for the design of Gr-based sensors is how to incorporate this material into electrodes for the best electrochemical performance. In previous works, mixture of graphene oxide (GO) or reduced GO (RGO) and conducting polymers were spin coated on electrodes[23, 24, 25]. Currently, it was reported that Gr films grown by chemical vapor deposition (CVD) have better electrochemical properties and reproducibility in comparison with GO and RGO powders[22]. In our laboratory, we have recently developed a technique to deposit Gr multilayer film over polyaniline (PANi) pre-coated electrodes[26]. We suppose that the incorporation of graphene will provide a significant amplification of conductive signal, and thus may contribute to enhancing electron transfer and subsequently improve the sensitivity in electrochemical measurements. In addition, the layer-by-layer assembling offers easy control over the thickness of nanostructured films.
The aim of the present study was to develop a novel label-free, electrochemical immunosensor with graphene films coated with the PANi films for atrazine (ATZ) determination, taking advantage of the patterned graphene, layer-by-layer fabricated electrode, and excellent analytical quantification such as high sensitivity. Based on the specificity of immuno-reaction, the trace-concentration of ATZ would be determined by using monoclonal anti-atrazine antibody as probe. Furthermore, this promising electrode platform could be extended for the development of other electrochemical immunosensor and biomedical devices.
Atrazine (ATZ) and monoclonal anti-atrazine antibody (α -ATZ) (Mw = 150 kDa) were purchased from Thermo Scientific, USA. Aniline (ANi) and phosphate buffer saline (PBS, pH 7.4) were purchased from Sigma. All aqueous solutions were prepared in deionized water.
2.2.1. Microelectrodes fabrication
The microelectrode was fabricated on silicon oxide wafer by the planar microelectronics technology. The thickness of the covered silicon dioxide layer is 1 m. The silicon wafer was first spin-coated with a layer of photo-resist and the shape of the electrodes was defined by UV-photolithography. Then, chromium (Cr) and platinum (Pt) were sputtered on the top of the wafer with the thickness of 10 and 100 nm, respectively. The platinum working electrodes (WE) and counter electrodes (CE) were patterned by a lift-off process. A second photolithographic step was carried out to deposit the 500 nm silver (Ag) layer. Partial chlorination of the Ag layer, performed in 0.25 mol/L FeCl3 solution, was used for fabricating reference electrode (RE). The diameter of working electrode (WE) was 200 m.
2.2.2. Electrodeposition of PANi
The electrodeposition of PANi onto Pt working electrode was conducted by cyclic voltammetry performing between -200 mV and +800 mV at a scan rate of 50 mV/s. Anodic deposition was controlled in a 10 mL solution containing 0.1 mol/L H2SO4 and 0.05 mol/L aniline monomer. The resultant PANi films were then washed with distilled water followed by drying under nitrogen stream.
2.2.3. Sample characterizations
The Gr films were prepared by thermal chemical vapor deposition (CVD) method at high CVD temperature (1000 ° C) in a gas mixture of argon (Ar): hydrogen (H2): methane (CH4) = 1000:300:30 sccm (standard cubic centimeters per minute). The polycrystalline copper (Cu) scotch tapes with a purity and a thickness of 99.8% and 35 m, respectively, were used as substrates for graphene synthesis process. The CVD program for Gr film synthesis was previously described in Chuc et al.[27] and can be summarized as follows. At the first stage, the annealing temperature was raised to 1000 ° C and an argon flow of 1000 sccm was constantly flowed through the CVD chamber. The temperature was then kept constant and the hydrogen gas was added into the CVD chamber. This step helps to reduce the native oxidation of copper and to facilitate the growth of Cu grains on the Cu tape. After 30 min of annealing samples in (Ar + H2), a methane flow of 30 sccm was introduced to CVD system and the Gr started to grow on the Cu tape. The growth of Gr layer was preceded for 30 min. After the CVD process, the graphene films were cooled down to room temperature under a flow of Ar (1000 sccm).
2.2.4. Transfer graphene film from Cu tape to electrode
The synthesized Gr was removed from Cu tape and was transferred to Pt/PANi working electrode, using polymethyl methacrylate (PMMA) as sacrificial layer. Transferring process of Gr to Pt/PANi working electrode is described as follows: First, a thin layer of PMMA was coated on top of the obtained graphene films on Cu tapes. And then, the Gr/PMMA films were released from the Cu tapes by chemical etching of the underlying Cu in 0.5 mol/L Fe(NO3)3 for 10 min. Subsequently, suspended films were transferred to deionized water to remove the residual of Cu etching process. Next, graphene/PMMA films were transferred onto a Pt/PANi working electrode. For the purpose of better contact between the graphene film and the Pt/PANi working electrode, an appropriate amount of liquid PMMA solution was dropped secondly on the cured PMMA layer thus partially or fully dissolving the precoated PMMA. The re-dissolution of the PMMA tends to mechanically relax the underlying graphene, leading to a better contact with the Pt/PANi working electrode. Finally, the PMMA films were dissolved by acetone and the samples were cleaned by rinsing several times in deionized water (Fig. 1).
2.2.5. Immobilization of anti-atrazine (α -ATZ)
The immobilization of anti-atrazine (α -ATZ) onto the PANi/Gr films has been done using glutaraldehyde (GA) as a cross linker. The concentration of α -ATZ immobilized on electrode surface was chosen for a stoichiometric ATZ-α -ATZ reaction with 10-6 mol/L.
The graphene layer structure was studied by high-resolution transmission electron microscopy (HR-TEM, FEI Tecnai G20). Raman spectra were measured with a LabRAM Raman spectroscope (Horiba, Japan) under ambient condition with excitation laser of He-Ne at wavelength of 632.8 nm. Electrochemical behavior of the biosensor after each modification step during fabrication process was characterized using cyclic voltammetry performing in 1 mL PBS buffer between -200 mV and +800 mV at a scan rate of 50 mV/s. The response to atrazine concentration was observed by square wave voltammetry (SWV) measurement. The parameters for SWV were optimized as follows: frequency of 12.5 Hz; start potential of -0.6 V; end potential of +0.75 V; step of 10 mV; and amplitude of 25 mV.
We have developed a layer-by-layer atrazine immunosensor based on electrochemical microelectrode with PANi/Gr films. The whole procedure of sensor fabrication was schematically presented in Fig. 2. For each step, only the Pt working electrode was modified. The sensor was constructed with (i) conducting polymer film PANi at the bottom layer that acts as electron transfer promoter; (ii) Gr film at the middle layer that acts as signal amplifier; and (iii) anti-atrazine (α -ATZ) that acts as recognition element. The detailed configuration of each layer as well as their characteristics will be discussed below.
3.1.1. Characterization of PANi, Gr and PANi/Gr films
The conducting polymer PANi was first electrodeposited on microelectrodes to provide electroactive species for the electrochemical performances. Although many electrochemical methods were suitable for deposition of such conducting polymers on inert electrodes, cyclic voltammetry was chosen since this method produces more uniform and compact polymer films[26]. The Gr film was then transferred on top of polymer film to increase the conductivity of composite biochemical layer.
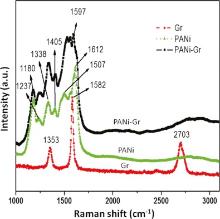
The crystalline of the Gr films before and after transferring onto Pt/PANi working electrodes previously modified with PANi (PANi/Gr) was evaluated by Raman spectroscopy. The Raman spectrum of PANi/Gr is presented in Fig. 3 and Raman spectra of neat PANi and neat Gr are also shown for comparison. The Raman spectrum of neat PANi (Fig. 3, green line) presents the bands associated with oxidized portions of ANi: 1180 cm-1 (C-H bending of quinoid rings), 1237 cm-1 (C-N stretching), 1338 cm-1 (C-N+ stretching, bipolaron), 1507 cm-1 (N-H bending, bipolaron), 1612 cm-1 (C-C stretching, benzenoid), and 1412 cm-1 (C-C stretching, quinoid rings). The spectrum of neat Gr (Fig. 3, red line) presents the characteristic bands of Gr sheets: The G peak at around 1582 cm-1 corresponds to the E2g phonon at the center of the Brillouin zone. The D peak, at 1353 cm-1, is due to the out-of-plane breathing mode of the sp2 atoms and is active in the presence of a defect [28, 29]. The D band is an efficient probe to assess the level of defects and impurities in graphene. A major fingerprint of graphene is the 2D peak at about 2703 cm-1. The shape, position and intensity relative to the D band of this peak depend markedly on the number of layers [30, 31]. The Raman spectrum of PANi/Gr (Fig. 3, black line) shows the bands attributed to the PANi and Gr, confirming the occurrence of both of these components in the film. The question here is if the Gr has firmly bonded by chemical bonding to PANi film or the Gr has only been mounted on this film temporarily. In the former case, the bands relevant to bipolarons and benzenoids should be strongly modified. In our case, it was found that the band situated at 1507 cm-1 (N-H bonding, bipolaron) has collapsed, and in the same time, the band located at 1612 cm-1 (C-C, benzenoid) red shifts to 1597 cm-1. These results clearly demonstrated the increase in concentration of benzenoid units; or on the other hand, the chemical bonding between PANi and Gr occurred. It was believed that those bondings are π -π bonding between quinoid rings of PANi and Gr. Such bondings can facilitate charge transfer between Gr and PANi, and therefore influence the charge-carrier transport properties of the material[32].
The thickness and structure of Gr film can also be predicted from Raman spectra and HR-TEM images, respectively. It is well-known that the ratio in intensities of 2D band to G band of Gr is dependent on the number of Gr layers. If the ratio I2D/IG ~ 2-3, Gr is monolayer; if 2 > I2D/IG > 1, it is bilayer; and if I2D/IG < 1, it is multilayer [33, 34]. In this study, the obtained ratio I2D/IG was 0.58, indicating that the Gr film grown on the Cu tape is multilayer.
HR-TEM is an excellent tool for structure analysis of Gr films, which are typically one or a few atoms thick. Here, this technique was used to provide direct evidence of the exact number of graphene layers. Fig. 4 shows HR-TEM image of the graphene layer after transferring from the copper tape to a copper grid. HR-TEM image has clearly revealed the multilayer structure of graphene film (4-5 layers) with parallel and well-aligned atomic layers.
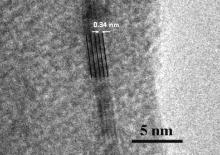 | Fig 4. HR-TEM image of the Gr layer after transferring from Cu substrate to a Cu grid for TEM examination. |
3.1.2. Immobilization of biological label
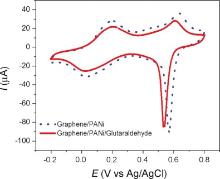
The immobilization of anti-atrazine (α -ATZ) onto the PANi/Gr films has been done using glutaraldehyde (GA) as a cross-linker. This compound is a dialdehyde capable to form a covalent bond between its aldehyde group and amine group of the other binding molecule. In this study, -CHO of GA reacts at the same time with NH2 group of PANi chains at one end and NH2 terminus of α -ATZ at the other, thus resulting in a stable and robust covalent bonding between them. The concentration of α -ATZ immobilized on electrode surface was chosen for a stoichiometric ATZ-α -ATZ reaction with 10-6 mol/L. The influence of GA on electrochemical behavior of working electrode is shown in Fig. 5. The shape of CV curves did not change but the current intensity was decreased slightly, suggesting the assembly of non-conductive organic compounds on microelectrodes. The fact is that the electroactivity of GA and α -ATZ is quite low; therefore the presence of these non-electronic materials reduced the output current.
Square wave voltammetry (SWV) is an excellent technique for monitoring biological binding events of immunosensor. In this method, the currents are measured in both positive and negative pulses successively; and the output current is the subtraction between oxidation and reduction peaks; thus the current density is much higher than classical cyclic voltammetry method. Additionally, the capacitive current as well as parasite current can be also reduced due to the reduction of dissolved oxygen. Here we used the reduction in SWV signal as indicator for the formation of complex between ATZ and anti-ATZ.
The as-prepared immunosensor operates in OFF-signal mode that is specially designed to detect ATZ. OFF-signal is defined as the reduction in SWV signal when the immunosensor contacts the source of targeted biomolecules (ATZ) [35]. The higher the concentration of ATZ is, the lower the SWV signal is and the higher the OFF-signal is. The working range of a targeted molecule is among the most important parameters defining biosensors. The two principles underlying the optimal concentration of analysis for operation of biosensors are to ensure the following criteria: (i) the analytic concentration should fall within the linear range; (ii) the electrochemical signals, acquired from immune reaction, should be strong enough and relatively well-distributed. Considering that the allowed concentration of ATZ in drinking water is less than 10-9 mol/L[36], we have decided to investigate the activity of prepared immunosensor with the analytic concentrations ranging from 10-11 to 10-7 mol/L (100 times greater and 100 times less). The concentration of α -ATZ chosen previously is in purpose to warrant an efficient and completed immune reaction between α -ATZ and ATZ in this range.
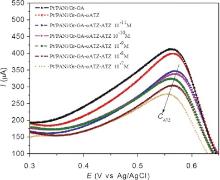
The developed immunosensor was used to detect ATZ in the chosen range. Fig. 6 shows the SWV curves of the immunosensor incubated at different concentrations of ATZ. It was found that the current response (at +0.57 V) decreased with increasing ATZ concentration. This is probably due to more ATZ binding to the immobilized antibodies at higher ATZ concentrations, which act as a definite kinetic barrier for the electron transfer. The deposition of non-electronic materials like ATZ on microelectrodes hinders the electroactive species to get onto the electrode and reduce the electron exchange between the electrode and solution. The variation of output current, I (intensity of SWV peak), with ATZ concentration, CATZ, is plotted in Fig. 7. This curve shows a linear immune response (current change at +0.57 V) against logarithm of ATZ concentration with a regression equation: I = 13.33logCATZ + 202 (A) (R2 = 0.9786). As seen here, the range of detection, over which we still have linear immune response of the immunosensor is relatively large; meaning that high precision can be easily achieved in a wide range of detection.
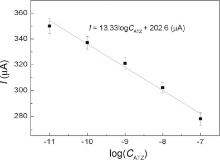 | Fig 7. Linear output of the fabricated immunosensor with ATZ concentration from 10-11 to 10-7 mol/L. |
The detection limit for the immunosensor with the PANi/Gr layer was 43 × 10-12 g L-1, far below the maximum residue level (10-4 g L-1) established by European Union. This value is much lower than detection limit of atrazine biosensors in previously reported works (see Table 1). The limit of detection of conductometric immunosensor with gold nanoparticles for atrazine was in the range from 10-10 to 10-3 g L-1[15, 16, 17, 18, 37]. The polyaniline-based immunosensor could detect significantly 10-8 g L-1 atrazine[19]. Only several studies have demonstrated the ability to fabricate electrochemical immunosensor for atrazine detection at the detection limit as low as our system[38, 39, 40].
| Table 1 Comparison of ATZ detection limits by different methods and electrodes |
Reproducibility of the biosensing performance was initially evaluated upon one homogeneous sample. The relative standard deviations of replicated measurements (one sample was measured five times) were less than 4% (Fig. 7). The relative standard deviations of three replicated sample preparations were less than 10%. The used biosensors can be stored in PBS buffer at 4 ° C with the loss of activity less than 20% after 30 days.
The label-free electrochemical sensor based on layer-by-layer PANi/Gr film was successfully developed for ATZ detection. The biosensor showed an excellent sensitivity (able to detect ATZ far below the EPA regulation). The layer-by-layer assembling appeared to be a good configuration to improve electrochemical performance of ATZ biosensor due to its easy control over the behaviors of nanostructured films. Apart from the pesticide detection, the proposed immunosensor might be extended for other sensing applications. Further works are under progress for selectivity improvement of the sensor for the real samples.
This work was financially supported mainly by the Vietnam National Foundation for Science and Technology Development (No. 103.99-2012.15). A part of this work was supported by VAST (VAST 03.06/14-15 and VAST.ÐLT.04/14-15). In addition, a part of the work was done with the help of devices from IMS Key Lab.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|