Biorecycling microbes, which have critical functionalities in natural cycles, are essential to sustain ecosystem of the earth. Any alterations in these cycles caused by the mutations of microbes could be a potential threat to life on earth. Antibiotics leached from pharmaceutical waste, animal food and agribusiness products are accumulating in the environment. Metal nanoparticles are also accumulating in environment because of their extensive use as biocidal agent in domestic products. Interaction of antibiotics and metal nanoparticles with eco-friendly microorganisms has a potential to alter the ecosystem of the earth. In this article, we have studied the antibacterial activities of silver and copper nanoparticles and their formulations with antibiotics, tetracycline, and kanamycin against biorecycling microbes, Bacillus subtilis and Pseudomonas fluorescens. Strong synergistic effect of metal nanoparticles on the antimicrobial activities of commercial antibiotics has been observed. Antimicrobial activity of tetracycline improves by 286%-346% and 0%-28% when being tested in the presence of 250 ppm of silver and copper nanoparticles, respectively. For kanamycin, the improvement is 154%-289% for silver and 3%-20% for copper nanoparticles. Irrespective of the antibiotics and tested organisms, synergy is more prominent for silver nanoparticles even at their minimum active concentration (100 ppm). This study demonstrates that the combination of metal nanoparticles with antibiotics could be more fatal to ecosystem than either the metal nanoparticles or the antibiotics alone.
Metal nanostructures have superior biocidal activities against all forms of microorganisms, so they are getting increasingly popular as anti-infective agents in products like bandages, antiseptic skin care and personal hygiene products[1]. Leaching of nanoparticles from these products will lead to their accumulation in land fields and water bodies. Use of conventional antibiotics in pest control and dairy products is also on surge, which will eventually accumulate in ecosystem[2, 3, 4]. Considering the critical role of microbial communities in organic matter and nutrient recycling in ecosystems, environmental exposure of nano and conventional antibiotics can alter ecosystem productivity[5]. Whether environmental accumulation of these antibiotics poses a threat to microbes essential for recycling in natural or engineered systems is an outstanding question of great relevance to ecosystem health and sustainable nanotechnology[6, 7].
Antimicrobial activities of metal nanoparticles are size dependent. Biocidal activities of metal nanostructures increase with the decrease in their hydrodynamic size[8]. Most of the studies found in literature on the biocidal activities of metal nanostructures are restricted to strains of pathogenic microorganisms and rarely extend to non-pathogenic eco-friendly microorganisms[9, 10]. Hence, the impacts of environmental accumulation of nanoparticles, especially on microbial communities residing in land fields and water bodies, which are critical to environmental recycling, are unknown. Further, there are isolated reports on synergistic effect of metal nanoparticles on the biocidal activities of conventional antibiotics[11, 12]. How far reaching the consequences of such synergies is not clear. It is absolutely essential to understand any synergistic effects of metal nanostructures on the biocidal activities of conventional antibiotics against microbes that are essential for environmental recycling. In the absence of this information, it will be hard to formulate regulatory protocols for the environmental pollution that is going to be originating from the simultaneous exposure of ecosystem to conventional and nanoantibiotics. Hence, detailed investigation on synergistic effect of metal nanoparticles on the antimicrobial activities of antibiotics against biorecycling microbes is vital.
Tetracycline and kanamycin are broad spectrum first-line antibiotics used to control spread of infectious microorganisms. Because of increasing use of these antibiotics in husbandry and dairy farming, substantial amount of these antibiotics are already accumulated in environment. In this work, we aimed to investigate synergistic effects of silver and copper nanoparticles on antibacterial activities of these antibiotics. Antibacterial activities of silver nanoparticles (SNPs), copper nanoparticles (CNPs) and antibiotics tetracycline and kanamycin adsorbed silver and copper nanoparticles have been evaluated by micro-dilution and disk diffusion tests on biorecycling microbes, Bacillus subtilis (B. subtilis) and Pseudomonas fluorescens (P. fluorescens). These microbes play a critical role in elemental recycling, bioremediation of pollutants and plant growth [13, 14]. Hence, it is absolutely essential to understand harmful effects of antibiotic pollutants on these microbes.
For this study, high quality aqueous colloidal dispersion of monodisperse silver and copper nanoparticles was prepared by chemical reduction technique. Antibiotics tetracycline and kanamycin were adsorbed on the surface of these nanoparticles and their antimicrobial activities have been tested on B. subtilis and P. fluorescens. Results of antibacterial tests are analyzed to understand the synergy between conventional antibiotics (tetracycline and kanamycin) and nanoantibiotics (silver and copper nanoparticles).
Silver nitrate (AgNO3) (99.8%) and diphenyl ether were procured from S.D. Fine-Chem. Ltd. Oleylamine (OA) (70%), pluronic F-127, copper (II) chloride dihydrate (≥ 99%), polyvinylpyrrolidone (PVP) (average mol. wt. 10, 000), sodium borohydride (NaBH4) (≥ 98%), tetracycline (hydrochloride salt) and kanamycin (sulphate salt) were procured from Sigma-Aldrich. Absolute ethanol, n-hexane (95%) and L-ascorbic acid (AA) were purchased from Merck. Mueller Hinton Agar (MM019) and nutrient broth (NM019) were purchased from Sisco Research Laboratories. Antibacterial activities have been investigated on B. subtilis (MTCC No. 441) and P. fluorescens (MTCC No. 1749), which were obtained from IMTECH, Chandigarh. Solutions were prepared in ultrapure Milli-Q water (ρ = 18.2 MΩ ). All the chemicals were used as received without any further purification.
Synthesis of uniform, monodisperse SNPs was carried out by chemical reduction technique by using oleylamine as capping and reducing agent[15]. It is a two-step process. In the first step, oleylamine capped hydrophobic SNPs were prepared by reducing AgNO3 with OA[15]. In the second step, hydrophobic SNPs were phase transferred into water by ligand exchange reaction using block copolymer, pluronic F-127[8]. In brief, 20 mL diphenyl ether was mixed with 15 mmol/L OA. Temperature was raised to 200 ° C and 3 mmol/L AgNO3 was added to it. Upon addition, OA immediately reduced AgNO3 to Ag0. The nucleation time was optimized to be 30 min, following which the reaction temperature was lowered to 150 ° C to have better control on the growth of individual crystallites. The nuclei were allowed to ripen at 150 ° C for 4 h. The colloid was subsequently cooled to room temperature and purified by precipitation-redispersion[15]. Water dispersible SNPs were obtained by facile phase transfer protocols[8]. Aqueous solution of 20 mL, 0.2 mol/L pluronic F-127 was mixed with equal volume of stock solution of SNPs in n-hexane. It was covered with a perforated aluminum foil. The mixture was magnetically stirred till the n-hexane evaporated completely. Phase transferred aqueous dispersion of SNPs was preserved at 4 ° C. The ligand exchange reaction was presented as a schematic in Fig. 1 along with photographs of colloidal SNPs before and after the phase transfer.
CNPs were synthesized by chemical reduction of copper chloride under mild reaction conditions. To achieve fast and homogenous nucleation of CNPs, aqueous solution of copper (II) chloride was added into the preheated mixture of reducing (NaBH4 + L-ascorbic acid) and capping (PVP) agents. In brief, 10 mmol/L each AA, PVP and NaBH4 were mixed and heated to 80 ° C. Aqueous solution of copper chloride (1.16 mmol/L) was added dropwise to this preheated solution of reducing and capping agents. Heating was continued till the color of the mixture turned bright red. The mixture was then cooled to 25 ° C. CNPs were collected by centrifugation @15471 × g. Well dispersed CNPs were obtained as supernatant and larger cluster of CNPs, if any, were discarded as sediment.
Chemical reactions that lead to the formation of CNPs are presented in Scheme 1. It is a multistep process. Precise control is essential at every step to obtain monodisperse, uniform, single phase CNPs. The first step in the reduction process of Cu2+ is the formation of PVP-Cu2+ complex[16, 17, 18]. This complex formation between PVP and Cu2+ is proposed on the basis of the structural features of PVP. It has polyvinyl skeleton with nitrogen and oxygen polar groups[16, 17, 18]. These polar groups can form coordinative bonds between PVP and Cu2+ ions by donating their lone pair electrons to Cu2+. In the second step, NaBH4 ionizes and forms a (BH4)- ligand, which then reacted with hydroxyl anions. During this reaction (step-II), eight electrons are liberated from the hydroxyl ions. These electrons react with Cu2+ in the PVP-Cu2+ complex and reduce it to Cu0. The execution of reduction reaction (Step-III) is evidenced from the change in the solution color from light yellow to dark red. In the oxidizing environment, Cu0 is not stable and oxidizes back to Cu2+. In the fourth step, AA oxidizes into dehydroascorbic acid with a release of two electrons[19]. These electrons react with the oxidized product of step-III and reduce Cu2+ back into Cu0. The oxidation by-product of AA, i.e. “ dehydroascorbic acid” simulates a dynamic equilibrium (Step-V) around the Cu0, which stabilizes CNPs against oxidation[19].
Antibiotic adsorbed SNPs and CNPs were prepared by vortex mixing of 10 mL aqueous colloidal dispersion of SNPs/CNPs with desired quantity of tetracycline or kanamycin. The final concentration of metal nanoparticles in antibiotics adsorbed nanoparticles was adjusted to 100 ppm and 250 ppm. The mixture was centrifuged at relative centrifugal force (RCF) of 15471 g (12, 000 rpm). Supernatant was removed and the final volume of the colloid was readjusted to 10 mL by adding appropriate quantity of ultrapure water. This stock solution of antibiotic adsorbed metal nanoparticles was further used for the antibacterial test. Antibiotic content and antibiotic loading efficiency of tetracycline/kanamycin on nanoparticle surface was determined by UV-visible spectroscopy[20]. Antibiotic contents and loading efficiencies are reported in Table 1.
| Table 1 Antibiotic content and antibiotic loading efficiency of antibiotics on metal nanoparticles |
Investigations of metal and antibiotic adsorbed metal nanostructures were performed by UV-visible spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM) and photon correlation spectroscopy (PCS). UV-visible spectra of colloidal SNPs and CNPs were recorded on a Hitachi U-3900H double beam UV-visible spectrophotometer. Structural characterization of nanostructures was carried out by recording their powder X-ray diffraction patterns. The XRD patterns of powdered SNPs and CNPs were recorded on a PANalytical X'Pert Pro diffractometer operated at 45 kV and 40 mA at 25 ° C with monochromatic CuKα radiation of wavelength 0.15406 nm. TEM micrographs were recorded on a Philips CM200 transmission electron microscope at an accelerating voltage of 200 kV. Samples for TEM were prepared by placing a drop of the colloidal dispersion of SNPs/CNPs onto amorphous carbon-coated copper grids. The solvent was allowed to evaporate slowly at 25 ° C by keeping the grid under IR lamp. Hydrodynamic size and polydispersity were measured by PCS. It was performed on a Brookhaven 90Plus Particle Size Analyser.
Six sets of 10 mL nutrient broth medium containing nanoparticles with effective metal concentration from 0 to 100 g/mL were prepared. Each set was inoculated aseptically with 108 CFU/mL of the respective bacterial suspension. The inoculated sets were incubated at 37 ° C for 24 h. Each experiment was carried out in triplicate. Turbidity of the media is a measure of bacterial growth. The minimum inhibitory concentration (MIC) is determined to evaluate effectiveness of antibacterial agent. MIC is defined as the lowest concentration of antibacterial agent that inhibits the bacterial growth[21]. It was evaluated by measuring the turbidity of bacterial strains exposed to different nanoparticles' concentrations. Control experiments were also run parallel to investigate the antibacterial activities of nutrient broth medium and capping agents.
32 mL Mueller Hinton Agar media was poured into the disposable Petri dishes and allowed to solidify. The bacterial strain (108 CFU/mL) was inoculated onto entire surface of Mueller-Hinton agar plate with the help of sterile cotton-tipped swab to form an even lawn. The sample disk/well containing 30 g of antibacterial agent (SNPs/tetracycline/kanamycin/tetracycline adsorbed SNPs/kanamycin adsorbed SNPs) was put on every plate. Each experiment was carried out in triplicate, and results were statically averaged. Each set was incubated at 37 ° C for 24 h. Zone of inhibition (ZIH) was measured after the incubation period, which was the area on the agar plate where the colony formation was inhibited due to the leaching of the antibacterial agent[21].
As-synthesized SNPs and CNPs were characterized by UV-visible spectroscopy. UV-visible spectra of SNPs and CNPs are shown in Fig. 2. For both the nanoparticles, a single plasmon band is observed, which is centered at 404 nm for SNPs and 564 nm for CNPs. Plasmonic characteristics are observable only when the particle size of metal nanoparticles is below its excitonic radius. Presence of plasmon band in the UV-visible spectra of SNPs and CNPs indicates that the synthesized nanoparticles are of very small size. XRD was used to confirm the crystallographic phase of nanoparticles. XRD patterns of SNPs and CNPs are shown in Fig. 3. Both SNPs and CNPs crystallize into FCC structure. No evidence of any of the impurity or oxide phases has been observed in either of the diffractograms. Diffraction peak positions in both the diffractograms are in good agreement with the standard diffraction patterns of silver [JCPDS card No. 040783] and copper [JCPDS card No. 040836]. The peak broadening in diffractograms indicates that the crystallite sizes of nanoparticles are small. From the Williamson-Hall analysis[22], the crystallite size of SNPs and CNPs was determined, which is shown in Table 2. The crystallite size of SNPs is 5.5 nm and CNPs is 11.8 nm. The TEM micrographs of SNPs and CNPs are shown in Fig. 4(a) and (b), respectively. Well dispersed, nearly spherical nanoparticles are observed in both the micrographs. Size distribution histograms obtained from these TEM micrographs are also shown in Fig. 4. Each histogram is fitted with lognormal particle size distribution function and from the best fit of the histograms, the mean physical size and polydispersity indices are determined. The mean physical sizes of SNPs and CNPs are 8.07 nm and 19.86 nm, and their polydispersities are 0.17 and 0.18, respectively. The hydrodynamic size is the true size of a nanoparticle in colloidal state. The hydrodynamic particle size distribution obtained from PCS is shown in Fig. 5. Each histogram is fitted with lognormal particle size distribution function and from the best fit; the mean hydrodynamic size and polydispersity indices of SNPs and CNPs are obtained. The mean hydrodynamic sizes of SNPs and CNPs are 10.2 nm and 39.6 nm, respectively for SNPs and CNPs. Their polydispersity indices are 0.12 and 0.14, respectively for SNPs and CNPs. Crystallite size, physical size and hydrodynamic size of SNPs and CNPs and their polydispersity are compared in Table 2.
 | Fig 2. UV-visible absorption spectra of SNPs and CNPs. Characteristic plasmon resonance band of SNPs and CNPs are observed indicating the formation of spherical nanoparticles. |
 | Fig 3. X-ray diffraction patterns of SNPs and CNPs. Each reflection is in good agreement with the FCC structure of both the nanoparticles. |
| Table 2 Size and size distribution of as-synthesized metal nanoparticles |
 | Fig 4. Transmission electron microscopic images of (a) SNPs and (b) CNPs. Size distribution histograms of (c) SNPs and (d) CNPs are fitted with lognormal particle size distribution function. |
 | Fig 5. Hydrodynamic particle size distribution histograms of (a) SNPs and (b) CNPs. Each histogram is fitted with lognormal particle size distribution function. |
To understand the interaction of antibiotics with SNPs and CNPs, UV-visible spectra of SNPs, CNPs, tetracycline, kanamycin, tetracycline adsorbed SNPs, kanamycin adsorbed SNPs, tetracycline adsorbed CNPs, and kanamycin adsorbed CNPs are recorded. Absorption spectra of these samples are shown in Fig. 6. The characteristic surface plasmon resonance (SPR) band of SNPs is observed at 428.5 nm. The UV-visible spectra of tetracycline show three characteristics absorption bands centered at 357 nm, 275 nm, and 250 nm, respectively. These multiplets correspond to the π → π * transitions of C = C. Kanamycin as such does not show any characteristic absorption in the UV-visible region but copper-kanamycin complex has a characteristic absorption maximum at 256 nm. This characteristic band is also observed in the UV-visible spectra of kanamycin-CuCl2 complex in Fig. 6. Multiplets of tetracycline are observed at 374 nm, 272 nm, and 252 nm, respectively, in the case of tetracycline adsorbed SNPs. SPR band originating from SNPs shows a red shift and positioned at 435 nm (Fig. 6(A)). In the case of kanamycin adsorbed SNPs, the characteristic absorption of kanamycin at 256 nm shifted to 252 nm while the SPR band of SNPs shifted from 428 nm to 430 nm. Similar trends are observed in UV-visible spectra of CNPs and their complexes with tetracycline and kanamycin (Fig. 6(B)). Little variation in the spectral signatures of tetracycline and kanamycin after their interactions with SNPs and CNPs confirm that both tetracycline and kanamycin are physisorbed on nanoparticle surface.
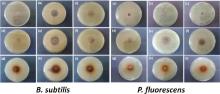
Antimicrobial activities of nanoparticles and antibiotics adsorbed nanoparticles have been studied against biorecycling microbes of B. subtilis and P. fluorescens by micro-dilution and disk diffusion tests. From micro-dilution method, the MIC values of as-synthesized SNPs and CNPs were determined. MIC values lies in the range of 20-75 g/mL. The MIC values in the case of SNPs are 75 ± 5 g/mL and 50 ± 5 g/mL for B. subtilis and P. fluorescens, respectively and for CNPs, the values are 50 ± 5 g/mL and 20 ± 5 g/mL, respectively. These MIC values are low as compared to our previous reports of MICs of SNPs against pathogenic microorganisms [8, 23]. This difference in the MIC values could be ascribed to better colloidal stability of nanostructures in nutrient broth media. The observed MIC values in this study are also comparable with those reported in literature [24, 25, 26]. B. subtilis and P. fluorescens are more sensitive to CNPs as compared to SNPs. To further understand the effectiveness of nanoparticles and antibiotic adsorbed nanoparticles on these microbes, disk diffusion tests were also performed. The results of disk diffusion tests of SNPs, CNPs, antibiotics adsorbed SNPs and antibiotics adsorbed CNPs are shown in Fig. 7 and Fig. 8. As reference, the ZIH results of tetracycline and kanamycin are also reported. Inhibition zone of SNPs was in the range of 6-7 mm and for CNPs, it is between 22 and 28 nm.
Synergistic effect of SNPs and CNPs is clearly evidenced from the increase in ZIH of antibiotic adsorbed nanoparticles (250 ppm/100 ppm) with respect to ZIH for a particular antibiotic. Sharp increase in the antimicrobial activity was observed when antibiotics were adsorbed on the surface of SNPs. For 250 ppm metal nanoparticle concentration, the antibacterial activity increases by 346% when tetracycline adsorbed on SNPs and by 154% when kanamycin adsorbed on SNPs against B. subtilis. In the case of P. fluorescens, antibacterial efficiency increases by 286% and 289%, respectively for tetracycline and kanamycin adsorption on SNPs. Percentage enhancement was determined by applying correction for drug loading efficiency (15.3% for tetracycline and 6.44% for kanamycin) in antibiotic adsorbed SNPs. For 100 ppm SNPs concentration in antibiotic adsorbed nanoparticles, the percentage enhancement in antibacterial activity is lower than that observed for 250 ppm samples. The antibacterial activity increases by 301% when tetracycline adsorbed on SNPs and by 103% when kanamycin adsorbed on SNPs against B. subtilis. In the case of P. fluorescens, antibacterial efficiency increases by 209% and 198%, respectively for tetracycline and kanamycin adsorption on SNPs. Disk diffusion tests clearly show strong antimicrobial activities of antibiotic adsorbed SNPs against non-pathogenic strains of B. subtilis and P. fluorescens even at nanoparticle concentrations that are close to their MICs.
For 250 ppm metal nanoparticle concentration, the biocidal activities of tetracycline adsorbed CNPs increases by 30% against B. subtilis. In the case of P. fluorescens, when tetracycline wasadsorbed on CNPs, no improvement in biocidal activities of tetracycline was observed. For kanamycin adsorbed CNPs, this increment was 20% and 3% for B. subtilis and P. fluorescens, respectively. For 100 ppm CNPs concentration in tetracycline adsorbed CNPs, the biocidal activities increase by 30% against B. subtilis. For kanamycin adsorbed CNPs, this increment was 16% and 3% for B. subtilis and P. fluorescens, respectively. No synergistic effect was observed for tetracycline adsorption on CNPs against P. fluorescens. Percentage enhancement were calculated by applying correction for drug loading efficiency (8.6% for tetracycline and 10.63% for kanamycin) in antibiotic adsorbed CNPs. Synergistic effect of antibiotic adsorption on CNPs is small as compared to antibiotic adsorption on SNPs. This might be because of the adsorption of antibiotic on CNPs at a site, which hinders their activities. For 100 ppm SNP/CNP adsorbed antibiotics, the percentage enhancement in antibacterial activities of antibiotic adsorbed nanoparticles is lower than that observed at 250 ppm. Test results at two different concentrations of SNPs/CNPs in antibiotic adsorbed nanoparticles show that the synergistic effect of antibiotic adsorption on antibacterial activity of silver and copper nanoparticles is persistent. Strong synergy has been observed even at minimum active concentration of metal nanoparticles.
Mechanism of bactericidal action of metal nanoparticles is not well understood. Various modes of antimicrobial action of metal nanoparticles have been proposed[27, 28, 29, 30, 31, 32]. First-step in antimicrobial action of nanoparticles as well as conventional antibiotics is their internalization into bacterial cell. They can diffuse into the cell directly through pores or indirectly by ion channels, transport proteins or by endocytosis. The integrity of cytoplasmic membrane is severely compromised when being exposed to toxic doses of metal nanoparticles. The bacterial cell wall is composed mainly of a thick peptidoglycan layer linked to teichoic acid that gives overall negative charge to the cell wall. The negative charge facilitates the interaction between the cell wall and positively charged metal. Such an interaction causes the loss of membrane integrity. The damaged membrane allows the entry of antibiotics (tetracycline and kanamycin) from the environment, which results into osmotic imbalance[27]. Damaged cell membrane also allows the leakage of minerals and genetic materials. This leakage of cytoplasmic content and consequent rupture of the cell is responsible for its death.
After entering the bacterial cell, antibiotics and metal nanoparticles poison microbial cells by (i) generating reactive oxygen species and antioxidant depletion, (ii) through protein dysfunction, (iii) genotoxicity, and (iv) interference with nutrient assimilation.
Metal nanoparticles can induce toxicity in bacterial cells by ROS mediated cellular damage or through metal catalyzed oxidation reaction that could damage specific proteins or DNA. Increasing ROS level in the cellular environment leads to the rapid degradation of cellular contents that eventually leads to the cell death. Antioxidant depletion in bacterial cell is also responsible for observed metal nanoparticle toxicity. Within microbial cells, metal ions form covalent bonds with sulphur leading to the formation of protein disulphides and depletion of antioxidant reserves, particularly glutathione[27]. Glutathione depletion could leave protein targets vulnerable to attack by metal species. It also prevents repair of oxidized protein-thiols by cellular thiol-disulphide exchange enzymes and thus enhances the possibility of oxidative stress induced cellular damaged in bacteria.
Another possible mode of antimicrobial action of metal nanoparticles is protein dysfunction induced metal nanoparticle poisoning of bacterial cell. One or more amino acid residues in protein are susceptible to metal-catalyzed oxidation[28]. Oxidation of amino acid side chains in proteins may cause loss of catalytic activity and trigger an active process of protein degradation, which is also responsible for toxicity of metal nanoparticles[28].
The genotoxicity induced antimicrobial activity of metal nanoparticles could lead to cell apoptosis. Metal mediated Fenton reaction can catalyze lethal DNA damage in bacteria. For instance, mutations that disrupt metal homeostasis increase the amount of Fenton active metal ions in the cell. This accelerates DNA damage and causes cell death[29]. The exogenous addition of reactive oxygen species also leads to DNA damage and inhibits enzyme activities that are vital for cell growth[30]. Toxic doses of metal nanoparticles can also upregulate genes that are involved in the elimination of ROS[31]. Metal ions released from nanoparticles also interfere with DNA replication. The consequence is DNA loses its replication ability.
The toxicity of metals is also linked to starvation-induced growth arrest. Metal nanoparticles inhibit the uptake of sulphur, deplete intracellular sulphur metabolite pools and induce enzymes of sulphur-sparing response[32]. Supplementation with sulphur and other sulphur sources mitigates toxicity in a concentration dependent manner. It has been suggested that metal nanoparticles starve cells of sulphur by interfering with its sulphur uptake and thus induces the cell apoptosis. These modes of metal nanoparticles toxicity might not be exclusive, and it is believed that the growth inhibition and cellular death are likely to be the result of a combination of more than one of these processes, which are further enhanced in the presence of conventional antibiotics.
Antibacterial activities of CNPs are better than SNPs against biorecycling microbes under investigation. P. fluorescens are more vulnerable to metal nanoparticles than B. subtilis. Antibiotic adsorption on metal nanoparticles has synergistic effect on their biocidal activities even at minimum active concentration of metal nanoparticles. Synergy is strong between antibiotics and SNPs, and it is weak between antibiotics and CNPs. The observed differences in the synergistic effect between antibiotic adsorbed SNPs and CNPs might be because of the difference in the site of adsorption of antibiotics on these nanoparticles surface. In the case of SNPs, where there is large enhancement in antibiotic effect after antibiotic adsorption (i.e. strong synergy) is because the site of antibiotic adsorption on SNPs is such that it does not hinder the activities of SNPs or antibiotics. In the case of CNPs, the synergy is poor. This might be because the sites at which antibiotics are adsorbed on CNPs are the active sites of copper nanoparticles that induce antibacterial effects on strains. The suppression of such sites on CNPs surface minimizes this synergy. The observed synergy could be a potential threat to ecosystem stability if accumulation of nano-waste will not be contained via strict regulations in future.
This work was financially supported by the University Grants Commission, New Delhi (scheme No. F. No. 42-850/2013 (SR)). Authors also acknowledge SAIF, IITB for extending transmission electron microscopy facility and Mr. Rajan Singh for helping in TEM and ICP measurements.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|