Zinc oxide, which has photocatalytic activity, is used as white pigment for cosmetics. A certain degree of sebum on the skin is decomposed by ultraviolet radiation in sunlight. In this work, zinc oxide was shaken with phosphoric acid to synthesize a white pigment for cosmetics. Zinc oxide was set with 0.1 mol/L of phosphoric acid at P/Zn = 1/1 and 1/2, and then shaking in hot water for 1 h. The chemical composition, powder properties, photocatalytic activity, color phase, and smoothness of the obtained powder were studied. The P/Zn ratio in preparation had an effect on the reaction between phosphoric acid and zinc oxide. The photocatalytic activity of zinc oxide was inhibited by phosphoric acid treatment. The obtained samples had enough high reflectance at the visible light region.
As a white pigment, zinc oxide is used for cosmetic applications[1]. This oxide is well known to have photocatalytic activity[2]. Therefore, a certain degree of sebum on the skin is decomposed by ultraviolet radiation in sunlight. To repress this effect, technical processes of several kinds have been investigated and used. For example, composite particles with silicon dioxide have been used[3]. However, these particle materials are too hard to use on a human face. Mild materials are required for use as a white pigment on a human face. In addition, microfine zinc oxide is adsorbed through the skin[4]. A white pigment that is not adsorbed must be used.
Phosphates have been used for ceramic materials, catalysts, adsorbent, fluorescent materials, dielectric substances, biomaterials, for metal surface treatment, as fertilizer, detergents, food additives, in fuel cells, pigments, and in other applications[5, 6]. Phosphate materials are well known to have high affinity for living organisms[7]. Therefore, as a white pigment, phosphates are expected to be useful as cosmetics.
When being used as a cosmetic pigment, the particle shape and size distribution of the phosphate particles are important. Homogeneous spherical particles are expected to spread well on the skin[8]. However, overly small particles are unsuitable because the pigments might enter the pores of the skin. Generally, sub-micrometer size pigments are used. The standard size of white pigment particles used in cosmetics is difficult to determine because the pore sizes of the skin are affected by such factors as age, gender, and climate[9]. Furthermore, overly large particles are inappropriate owing to a cracking of their coating on the skin[10]. It is therefore important to control the particle sizes of the pigment. In our earlier studies[11, 12], we prepared zinc phosphate pigments without catalytic activity. However, zinc phosphates had particle sizes larger than 1 µ m.
Because these particles were too large, the novel process was required to produce smaller particles as a white pigment. The zinc phosphate without photocatalytic activity had too many large particles; on the other hand, zinc oxide with small particle size had a photocatalytic activity. In this work, we suggest the zinc oxide particles with zinc phosphate coating. The target particles have the core-shell structure, where core part is zinc oxide and shell part is zinc phosphate without photocatalytic activity. Our purpose in this work was to obtain the white pigment in sub-micrometer size without photocatalytic activity.
In this work, zinc oxide was shaken with phosphoric acid under various conditions. The chemical compositions, powder properties, photocatalytic activity, color phases, and smoothness of the obtained samples and their thermal products were studied for application in cosmetics.
Zinc oxide was set with phosphoric acid (0.1 mol/L, 50 mL) at molar ratios of P/Zn = 1/1 and 1/2 in a glass tube, and then this glass tube was shaken in hot water at 60, 70, and 80 ° C for 1 h (rate of shaking; 100 times/min), respectively[13]. The solutions were decantated off, and the powder samples were washed with water, and then dried at 50 ° C for 3 days. All chemicals were of commercial purity from Wako Chemical Industries Ltd. (Osaka Japan) and used without further purification.
The crystalline phase composition of these materials was analyzed by X-ray diffraction (XRD). The XRD patterns were recorded on an X-ray diffractometer (MiniFlex; Rigaku Corp.) using monochromated CuKα radiation. The samples were heated at 100 ° C for 1 h in air conditions. These thermal products were also analyzed according to their XRD patterns. The IR spectra were recorded on a HORIBA FT-IR 720 (Horiba Ltd.) using the KBr disk method. A part of the samples was dissolved in a hydrochloric acid solution. The ratios of phosphorus and zinc in the precipitates were also calculated based on the inductively coupled plasma (ICP) results of these solutions using an SPS1500VR from Seiko Instruments, Inc.
The particle shapes and sizes of the precipitates, as well as their thermal products at 100 ° C, were estimated based on scanning electron microscopy (SEM) images and particle size distributions. The SEM images of the sample powders were observed under a scanning electron microscope (JSM-5510LV; JEOL). The particle size distributions of these materials were measured using a centrifugal precipitation particle-size distribution analyzer (SA-CP3L, Shimadzu Corp.).
The cosmetic properties were estimated according to the photocatalytic activity, the color phase, and the smoothness. The photocatalytic activity of the samples was estimated with the decomposition of methylene blue by 365 nm radiation[14, 15]. The 0.01 g of the sample was placed in 4 mL of methylene blue solution (1.0 × 10-5 mol/L), and then this solution was radiated. The decrease of the absorption at about 660 nm was estimated for 120 min.
The color of phosphate pigments was estimated using ultraviolet-visible (UV-Vis) reflectance spectra with a spectrometer (UV2100; Shimadzu Corp.) (reference compound: BaSO4). The whiteness was also estimated in L* a* b* color space with a TES135 plus color analyser (TES Electrical Electronic Corp) (average of 5 times).
The particle smoothness was measured on artificial leathers with KES-SE objective evaluation of surface friction property (Kato Tech Co. Ltd.) (average of 5 times). The sample powders were spread on the leather. Then a sensor was run over these powders. The values of MIU and MMD respectively represent the slipping resistance (average value in a distance of 20 mm) and roughness of powders (fluctuation of average frictional coefficient). The values of MIU and MMD have no unit because these values are related with coefficient of kinetic friction and their scattering, respectively.
Fig. 1 represents XRD patterns of the samples prepared with P/Zn = 1/1 at various shaking temperatures, and then heated at 100 ° C for 1 h. The peaks of zinc phosphate appeared in XRD patterns of the samples in spite of shaking temperatures. The samples treated at 70 and 80 ° C had stronger peaks of zinc phosphate than that treated at 60 ° C. On the other hand, the peaks of zinc oxide became weak by phosphoric acid treatment. Therefore, a part of zinc oxide was confirmed to react with zinc phosphate in this phosphoric acid treatment.
Fig. 2 shows IR spectra of the samples prepared with P/Zn = 1/1 at various shaking temperatures, and then heated at 100 ° C for 1 h. Original zinc oxide had no absorption at 1110-940 cm-1 while the samples treated with phosphoric acid had several peaks at this region due to the existence of zinc phosphate. Besides, the strong absorption peak located at 400-550 cm-1 for zinc oxide was missing for all samples treated with phosphoric acid, further confirming the formation of zinc phosphate.
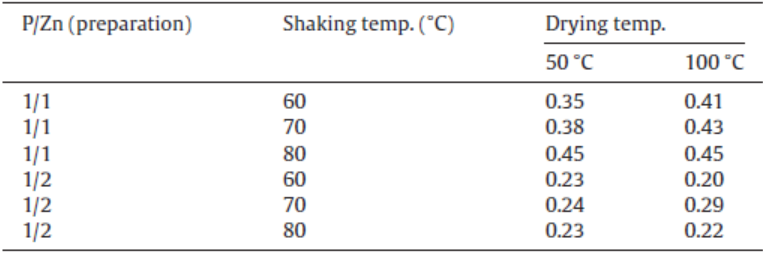
Table 1 shows the calculated P/Zn ratio based on ICP results for the samples prepared under various conditions. The P/Zn ratios of starting zinc oxide and zinc phosphate, Zn3(PO4)2, are 0 and 2/3, respectively. The calculated P/Zn ratio for the samples treated at P/Zn = 1/1 after drying at 100 ° C was about 0.4, while that for the samples treated at P/Zn = 1/2 was about 0.2. The high ratio of phosphoric acid means that the samples contained high ratio of zinc phosphate. Shaking temperature had less influence on the P/Zn ratio in the samples. When the sample was a mixture of zinc oxide and phosphate in the molar ratio of ZnO / Zn3(PO4)2 = 2/1, the P/Zn ratio became 2/5. The mixture of zinc oxide and phosphate in the molar ratio of ZnO / Zn3(PO4)2 = 7/1 was P/Zn = 2/10. The molar ratio of ZnO / Zn3(PO4)2 was much affected from P/Zn ratio in preparation process.
| Table 1. P/Zn ratio in the samples prepared under various conditions |
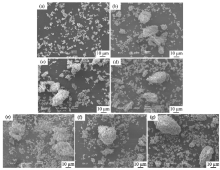
From the viewpoint of particle shape, spherical particles are suitable for cosmetic applications[16, 17]. Fig. 3 portrays SEM images of the samples prepared in P/Zn = 1/1 and 1/2 at various shaking temperatures, and then heated at 100 ° C. All samples had no specified shape in particles and large aggregates. No obvious morphological change can be observed when varying the P/Zn ratio (P/Zn = 1/1 and 1/2) and shaking temperatures (60, 70 and 80 ° C).
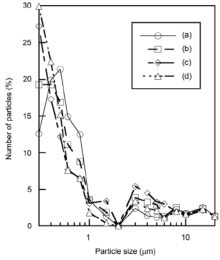
Fig. 4 presents the particle size distribution of the samples prepared in P/Zn = 1/1 at various shaking temperatures, and then heated at 100 ° C. The untreated zinc oxide had a high ratio of particles smaller than 1 µ m. The ratio larger than 1 µ m became higher by phosphoric acid treatment. Because zinc phosphate had larger particle size than zinc oxide[11, 12], the formation of zinc phosphate produced large particle size. Fig. 5 shows the particle size distribution of the samples prepared in P/Zn = 1/2 at various shaking temperatures, and then heated at 100 ° C. The samples prepared in P/Zn = 1/2 had smaller particles than those in P/Zn = 1/1, because the calculated P/Zn ratios in the sample prepared in P/Zn = 1/2 were low, as shown in Table 1.
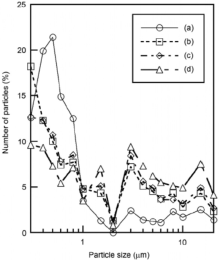 | Fig. 4. Particle size distribution of the samples prepared with P/Zn = 1/1 at various shaking temperatures and then heated at 100 ° C: (a) original ZnO, (b) 60 ° C, (c) 70 ° C, (d) 80 ° C. |
Fig. 6 shows the photocatalytic activity of the samples prepared with shaking at various temperatures, and then heated at 100 ° C. Methylene blue was decomposed with zinc oxide using UV radiation (curve (b) in Fig. 6). The photocatalytic activity of zinc oxide was inhibited by the phosphoric acid treatment (curves (c), (d) and (e) in Fig. 6). Because zinc phosphate, expected as a product on the surface of zinc oxide particles, had no photocatalytic activity[11, 12], the inhibition of photocatalytic activity was related with the reaction between zinc oxide and phosphoric acid. The sample prepared at 60 ° C indicated the weakest photocatalytic activity. The influence of shaking temperature was not clear on the photocatalytic activity of the samples.
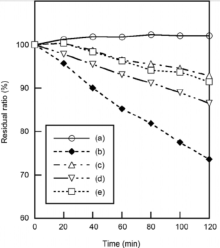 | Fig. 6. Photocatalytic activity of the samples prepared in P/Zn = 1/1 at various shaking temperatures and then heated at 100 ° C: (a) blank, (b) original ZnO, (c) 60 ° C, (d) 70 ° C, (e) 80 ° C. |
Fig. 7 shows UV-Vis reflectance spectra of the samples prepared in P/Zn = 1/1 at various shaking temperatures, and then heated at 100 ° C. All samples indicated high reflectance at visible light region. Because zinc phosphate had the high reflectance at ultraviolet region[6], the samples treated in the present work indicated higher reflectance than zinc oxide at this region. The sample treated at 80 ° C indicated higher reflectance than others. From the above ICP results, the molar ratio of ZnO / Zn3(PO4)2 was 2/1 in the samples prepared in P/Zn = 1/1. However, the reflectance at ultraviolet region was weak, not corresponding to the P/Zn ratio from ICP results.
 | Fig. 7. UV-Vis. reflectance spectra of the samples prepared with P/Zn = 1/1 at various shaking temperatures and then heated at 100 ° C: (a) original ZnO, (b) 60 ° C, (c) 70 ° C, (d) 80 ° C. |
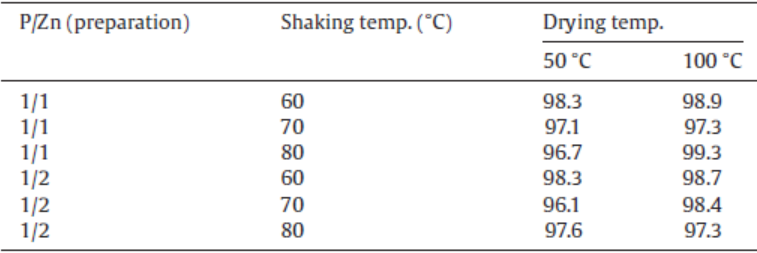
The color of the sample powder was also estimated by L* a* b* color space. Table 2 shows the whiteness of the samples prepared in various conditions. These values were L* value in L* a* b* color space. Because all samples were indicated over 95, the obtained samples were suitable as a white pigment. These results were corresponding to the UV-Vis reflectance spectra of the samples.
| Table 2. Whiteness of the samples prepared under various conditions |
As described above, pigment with high smoothness spreads well on the skin. The powder smoothness is also important for cosmetics[18]. Table 3 shows the smoothness of the samples prepared under various conditions. Generally, for a cosmetic application, the suitable MIU and MMD values are smaller than 0.6 and smaller than 0.04, respectively. All samples prepared in this work indicated enough low MIU and MMD values.
| Table 3. Smoothness of the samples prepared under various conditions |
Zinc oxide was shaken in phosphoric acid under various conditions. A part of zinc oxide reacted to form zinc phosphate in this process. The P/Zn ratio in preparation had influence on the progression of this reaction. The treated samples had a certain degree of larger particles than 1 µ m and were easy to aggregate. The photocatalytic activity of zinc oxide was inhibited by this phosphoric acid treatment. The obtained samples had enough high reflectance at the visible light region. This phosphoric acid treatment is the effective method to obtain a novel white pigment for cosmetics.
The authors are grateful to Dr. Takeshi Toyama, Nihon University, Japan, for smoothness measurements.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|