The Fe- and Mn-rich intermetallics are of great effect on the mechanical properties of the AA5083 type alloy (Al-5Mg-0.8Mn). The effect of Fe, Si and cooling rate on the formation of the intermetallics were investigated by analyzing the microstructures of the alloys with different Fe and Si contents. The results indicated that increasing the Fe content resulted in the increase of the Al6(Fe,Mn) phase in both amount and size. In the alloys with high Fe content, the platelet-like Al6(Fe,Mn) compounds lined up and formed a band. Increasing the content of Si resulted in the increase of the Mg2Si phase which formed a network structure. Increasing the cooling rate significantly refined the intermetallics. However, increasing the cooling rate did not change the characteristic of the intermetallic compounds in the alloys with low or medium Si contents. For the alloys with high contents of Fe and Si under the condition of near-rapid cooling, the Fe- and Mn-rich intermetallic compound changed to the quaternary Al15(Fe,Mn)3Si phase and displayed a fine fish bone or Chinese script structure. The refinement of the intermetallics would allow higher tolerance of Fe and Si contents in the AA5083 alloy produced, for instance, via the continuous strip casting process.
The AA5083 alloy has many great advantages, such as good weldability, ductility, toughness, formability and corrosion resistance, and has been widely used in the automotive, marine, packaging and construction industries. The demand for a high quality alloy has led to extensive research on improving the AA5083 alloy, with emphasis on microstructures and properties[1, 2, 3], superplasticity[4, 5 , 6], ultrafine grain[7, 8 , 9] and adding element[10, 11 , 12]. Little attempt has been made to improve the quality of the alloy through controlling the formation of the Fe- and Mn-rich intermetallic compounds.
AA5083 alloy is an Al-Mg based aluminum alloy with an intermediate (4%-5%) Mg content. Fe is one of the impurities in aluminum alloys, which has a very low solubility in solid Al (approximately 0.05 wt% at eutectic temperature)[13] and forms the Fe-rich intermetallics during solidification. The Fe-rich intermetallics usually display platelet-like morphologies and have been considered most detrimental to the mechanical properties of the alloy due to its brittle features and stress concentration caused by the needle-like morphology[14, 15 , 16]. To neutralize the harmful effect of Fe, Mn is added to the Al-Mg based alloys as a neutralization element[17 , 18]. Mn modifies the platelet-like morphology into Chinese script morphology, which is expected to minimize their harmful influence on the mechanical properties of the alloy. Mn also contributes to the strength of the alloy through solid solution strengthening. Therefore, relatively high content of Mn is usually employed. In the AA5083 alloy, the nominal content of Mn is 0.4-1.0 wt%, which is much higher than the required content to compensate for the negative effect of Fe. The excess of Mn in the alloy forms Mn-rich intermetallic compounds. Research on Al-Si alloys indicated that the Fe-rich intermetallics usually form polyhedral morphology at high Mn contents[19], Chinese script phase Al8Fe2Si at low Mn content and high cooling rate, but Al15(Fe, Mn)3Si2 phase at high Mn content and low cooling rate[20]. The intermetallics formed during solidification are also a function of the Fe/Mn and Fe/Si ratios. The morphology, size and distribution of the intermetallics can be controlled by properly adjusting the contents of Fe, Si and Mn. But little literature can be found regarding the formation mechanism of the Fe- and Mn-rich intermetallics in the Al-Mg-Mn alloys. It is assumed that their formation mechanism is different from that in the other alloy systems. A systematic research on the effect of Fe and Si on the formation of Fe- and Mn-rich intermetallics is important and necessary.
Cooling rate is another important factor that influences the precipitation of the Fe-rich intermetallics during solidification. Research on the AA2618 alloy indicated that under the condition of near-rapid cooling, the formation of some coarse Fe-rich intermetallics could be inhibited[21]. Therefore, it is hypothesized that by increasing the cooling rate to the level of near-rapid, the coarse Fe- and Mn-rich intermetallics can be refined, even inhibited. Thus, higher tolerance of Fe can be allowed for the alloy and more recycled aluminum with higher contents of Fe can be used without significantly deteriorating its mechanical properties. This will result in production cost savings.
The continuous casting (CC) process for aluminum alloy sheets is of the advantage of high productivity and low production cost and has recently received more and more attention from manufacturers and end users[22]. Al-Mg-Mn aluminum alloy sheet materials are often produced using this process and lots of research has been carried out to study the continuous strip cast materials[23, 24]. However, little attention has been paid to investigate the formation process of the intermetallics during continuous strip casting. The CC process for aluminum alloy sheets is a near-rapid cooling with a cooling rate of 101-102 K s-1 (°C s-1). Conducting a research on the formation of the Fe- and Mn-rich intermetallic compounds under near-rapid cooling is of great theoretical and commercial interest.
To better understand the influence of Fe, Si and cooling rate on the microstructures and optimize the mechanical properties of the AA5083 alloy, a research has been designed to study the influences of the individual and combined additions of Fe and Si as well as the cooling rate on the formation of Fe-rich intermetallic compounds in the Al-5Mg-0.8Mn alloy, which is a simplified form of the AA5083 alloy.
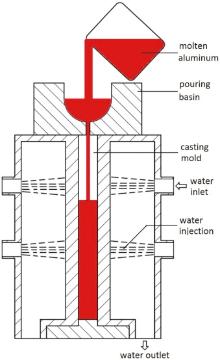
Five Al-5Mg-0.8Mn alloys were designed with the Fe and Si contents varying from less than 0.1 to 0.5 wt%. High grade commercially pure aluminum ingots (< 0.10 wt% Fe and < 0.04 wt% Si), magnesium ingot (99.9 wt%), Al-10Fe and Al-30Si master alloys were used to prepare the experimental alloys. Two kilograms of raw materials for each designed alloy were melted in a graphite-clay crucible in an electric resistance furnace. The molten aluminum was degassed by injecting Ar into the pool, refined by adding Al-5Ti-B grain refiner, then cast in a slab mold which is a double-side water cooled casting apparatus used to simulate the cooling in the CC process, as schematically shown inFig. 1. The cooling rate of the double-side water cooled casting apparatus was approximately 20.0 K s-1 (°C s-1). The chemical compositions of the cast slabs were analyzed by emission spectroscopy; the results are listed in Table 1.
| Table 1 Chemical composition of the alloys (wt%) |
To achieve different cooling rates, some materials cut from the cast labs were re-melted in alumina crucibles in the electric resistance furnace and solidified in the crucibles in furnace and air, respectively. Thus, cooling rates of approximately 0.065 K s-1 (°C s-1) (furnace cooling) and 2.0 K s-1 (°C s-1) (air cooling) were achieved. The cooling rates were measured by recording temperature evolution with time. The average cooling rates before the start of solidification were calculated by using the formula dT/dt and were computed from the approximate straight line portion of the cooling curve. The intermetallic compounds formed during solidification were analyzed by metallographic examination with optical microscopy (OM), electronic scanning microscopy (SEM) with energy dispersive X-ray (EDX) analysis and X-ray diffraction (XRD).
The samples for metallographic examinations were prepared using standard metallographic procedures. The samples were electro-polished at a voltage of 27 V D.C. for 15 s using 60 mL HClO4 + 140 mL H2O + 800 mL C2H5OH. The microstructures of the alloys were examined using an OLYMPUS GX71 optical microscope. A Zeiss scanning electron microscope with OXFORD energy dispersive X-ray (EDX) analyzer and a PANalytical X'pert Pro X-ray diffractometer were employed to identify the intermetallic compounds.
3.1.1. Intermetallics in low Fe and Si containing alloys
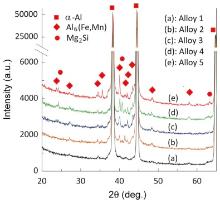
Fig. 2 shows the typical solidification structure of the Al-5Mg-0.8Mn alloy with low Fe (0.09 wt%) and Si (0.04 wt%) contents solidified at a cooling rate of 0.065 K s-1 (°C s-1). The α-Al forms the matrix and a very small amount of intermetallics scatters on the matrix. They are located within the grain boundary triple junctions or inter-dendrites. Two types of intermetallics are observed, as marked in Fig. 2. The type I intermetallic appears to be quite faceted with a blocky morphology. The type II intermetallic shows irregular morphologies and is smaller than the type I intermetallic in both size and amount. The backscattered electron image (Fig. 1(b) and (c)) shows that the type I intermetallic is in light gray and the type II intermetallic is in dark gray. At high magnification, the dark gray phase looks like a tangled string. To identify and confirm the eutectic phases, both the XRD and SEM/EDX were used. It is found that the type I intermetallic is Mn and Fe containing phase. The type II intermetallic mainly contains Si and Mg. Table 2 lists some typical measurements on the eutectic phases. It can be seen that the Mn and Fe containing phase has a composition close to Al6(Fe, Mn). The composition of the Si and Mg containing phase deviates from the Mg2Si phase with a small amount of Al. Fig. 3 provides the XRD spectra of the alloy. The XRD analysis indicates that the solidification structures consist of α-Al and Al6(Fe, Mn), but the spectra of the Si and Mg containing phase were not detected. According to the phase diagram, morphologies, compositions and XRD spectra of the intermetallic compounds, it is most likely that the type I intermetallic is Al6(Fe, Mn), while the type II intermetallic is Mg2Si.
 | Fig. 2. Intermetallic compounds formed in Alloy 1: (a) OM image; (b) backscattered electron image of Al6(Fe, Mn); (c) backscattered electron image of Mg2Si. |
| Table 2 EDX analysis results in various alloys (at.%) |
3.1.2.Intermetallics in medium and high Fe and Si containing alloys In the alloys with medium and high contents of Fe and Si, two types of intermetallics also appear and are marked as I and II, respectively (Fig. 4). The SEM/EDX analyses indicate that, similar to Alloy 1, the type I intermetallics are Fe and Mn containing phase, and the type II intermetallics are Si and Mg containing phase.
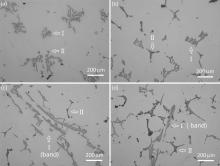 | Fig. 4. Intermetallic compounds formed in: (a) Alloy 2; (b) Alloy 3; (c) Alloy 4; (d) Alloy 5 solidified at 0.065 K s-1 (°C s-1). |
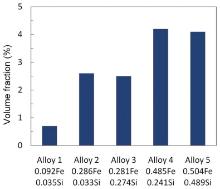
The contents of Fe and Si were of significant influence on the solidification microstructures of the alloys. With increasing Fe and Si contents, both the type I and type II intermetallics increase significantly in both amount and size and change in distribution and morphology. Fig. 5 shows the volume fraction of the type I intermetallic phase on the metallographic surfaces in all the alloys measured by an image analysis system. It can be used to describe the volume percentages of the Mn- and Fe-rich intermetallics vs the Fe contents in the alloys. The amount of the intermetallics formed is highly correlated to the content of Fe.
At medium Fe content (0.29 wt% in Alloy 2), the type I intermetallics are still isolated within grain boundary triple junctions or inter-dendrites. Their morphologies are complicated with flower-like shapes (Fig. 4(a)). When increasing the Si content to medium level (0.27 wt% Si in Alloy 3), both type I and type II intermetallics become major phases which are distinguished by color and morphology, as shown in Fig. 4(b). The type II phase shows the Chinese script morphology. Fig. 6 shows the high magnification images of the constituents. The Al6(Fe, Mn) phase displays a flower-like structure. The image of the deep etched Mg2Si phase looks like a tree branch. In Alloy 4 with high Fe content and medium Si content (0.48 wt% Fe and 0.24 wt% Si), some of the type I phase appear to display a platelet-like feature and line up to form a band. The band of intermetallics extends several millimeters (Fig. 4(c)). In addition, the type I phase displays several different morphologies. Other than the platelet-like structures seen in Fig. 4(c), massive blocky, platelet, strips, Chinese script, etc. are also observed (Fig. 7). At high contents of Fe and Si (0.5 wt% Fe and 0.49 wt% Si in Alloy 5), the type II phase increases significantly in both amount and size. They are located at the grain boundaries and form integrated networks. The type I phase is almost the same as that in Alloy 4. Some bands of the intermetallic phases are also observed, but the amount and size of the bands are inferior to that in Alloy 4 (Fig. 4(d)).
 | Fig. 6. (a) Backscattered electron image of the Al6(Fe, Mn); (b) secondary electron image of the Mg2Si in Alloy 3. |
XRD and SEM/EDX analyses were performed on the alloys with different Fe and Si contents. Fig. 3 also shows the XRD spectra of Alloys 2 to 5. Alloy 2 also consists of α-Al and Al6(Fe, Mn), and no other phase was detected. The spectra of Al6(Fe, Mn) phase are stronger than that in Alloy 1. This is consistent with the OM observation in which the amount of type I eutectic phases significantly increases in Alloy 2. In Alloys 3 to 5, other than the α-Al and intermetallic Al6(Fe, Mn), another intermetallic Mg2Si was detected. These results confirmed the abovementioned observation that in Alloys 3 to 5, other than the type I phases, a large amount of type II phase appeared. The intensity of the spectra of the Mg2Si phase in Alloy 5 was higher.
Some typical EDX measurements are listed in Table 2. It is found that in the alloy with medium and high Fe contents, the composition of Fe in the type I (Fe- and Mn-rich) phase greatly increases compared to Alloy 1. The compositions of Fe and Mn in the phase change slightly among the alloys. The Fe and Mn ratio in the phases also varies with the change of the morphologies, as seen in Table 3 which lists the results of EDX analyses on the intermetallics shown in Fig. 7. However, overall, the composition ratio of aluminum to Fe plus Mn is always close to 6:1. Based on the XRD and EDX analysis, the type I phase should also be the intermetallic compound Al6(Fe, Mn).
 | Fig. 7. Various morphologies of the phase Al6(Fe, Mn) in Alloy 4: (a) blocky; (b) Chinese script; (c) strip-like; (d) platelet-like. |
| Table 3 EDX analysis results of phase Al6(Fe, Mn) in Fig. 7 (at.%) |
In Alloys 3 to 5 with medium and high Si contents, the compositions of the type II (Si- and Mg-rich) phase are still different from that of the Mg2Si phase. But considering the fact that the Mg2Si spectrum was detected by XRD and no other AlMgSi phase existed in the Al-Mg-Si system, it was reasonable to define this phase as Mg2Si.
Cooling rate played a critical role in the control of the solidification structures. Fig. 8 shows the solidification structures of Alloy 4 solidified under air cooling (~2 K s-1 (°C s-1)) and double-side water cooled iron mold cooling (~20 K s-1 (°C s-1)), which was a near-rapid cooling. By comparing the solidification structure of Alloy 4 solidified under furnace cooling (Fig. 4(c)), it can be seen that the coarse intermetallics are refined to a significant extent by increasing the cooling rate. Under the condition of air cooling, the size of the Al6(Fe, Mn) platelets was about 250 µm in length which was about half of the size of the intermetallic found in the sample solidified under furnace cooling. Further increasing the cooling rate to 20 K s-1 (°C s-1), the length of the platelet structures was reduced to less than 100 µm. No bands of Al6(Fe, Mn) were observed.
 | Fig. 8. Solidification structures of Alloy 4 solidified under: (a) air cooling (~2 °C s-1) and (b) water cooled iron mold cooling (~20 °C s-1). |
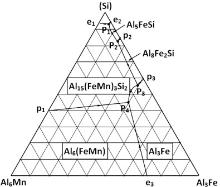
Fig. 9 shows the solidification structures of Alloy 2 and Alloy 5 solidified under the condition of double-side water-cooled iron mold cooling (20 K s-1 (°C s-1)). The intermetallics increase in amount and significantly reduce in size. The solidification structure of Alloy 5 changes significantly. No blocky or platelet like structures were observed. All the Fe- and Mn-rich phases display fine fish bone or Chinese script structures (Fig. 10(a) and (b)). EDX analysis indicates that the phase contains high Si content (Table 4). The composition of the phase apparently met the stoichiometric composition of Al15(Fe, Mn)3Si. The intermetallic Al6(Fe, Mn) observed in the previous alloys was not observed in Alloy 5. The Fe- and Mn-rich intermetallic changed to the quaternary phase Al15(Fe, Mn)3Si. However, according to the phase diagram of Al-Mn-Fe-Si system[25] (Fig. 11), the quaternary phase is Al15(Fe, Mn)3Si2. Most likely, the phase in fish bone or Chinese script shapes is a metastable phase. The content of Si at the interface front is not high enough to form an equilibrium quaternary phase. Fig. 10(c) shows the structure of the Mg2Si phase, which is also significantly refined by the fast cooling.
| Table 4 EDX analysis results of the phases in Fig. 10 (at.%) |
The studied alloys were Al-Mg based alloys. Mg and Mn were the main alloying elements. According to the Al-Mg phase diagram[26], the solubility of Mg in solid aluminum is relatively high (~17.1%), while the temperature of binary eutectic reaction (L→ α-Al + Al8Mg5) is very low (about 723 K (450 °C)). Alloys with low Mg content would solidify with an as-cast microstructure of almost a single phase α-Al[27]. The concentration of Mg in the final solidification zone was not high enough to form Mg-rich eutectic phase. Backerud et al. observed the eutectic phase Al8Mg5 in the AA5182 alloy (Al-4.74Mg-0.34Mn) solidified at a cooling rate of 0.3 K s-1 (°C s-1)[18]. The concentration of Mg in Alloy 1 was similar to that alloy (AA5182), but no eutectic phase Al8Mg5 was observed. It was assumed that the even lower cooling rate retarded the enrichment of Mg in the final solidification zone and inhibited the formation of the eutectic phase Al8Mg5. From the Al-Mn binary diagram, the segregation tendency of Mn is quite small. Only small amounts of Mn segregated to the final solidified zone during solidification and combined with Fe to form the eutectic constituent Al6(Fe, Mn). Therefore, the solidification microstructure of Alloy 1 was very simple.
Fe and Si exist in many Al alloy systems and have a strong tendency of forming Chinese script α-AlFeSi or plate-like β-AlFeSi, depending on the Fe/Si ratio. The latter is much more harmful to the mechanical property of alloys than the former due to its platelet-like morphology. Adding element Mn to modify the platelet-like morphology of the β-AlFeSi phase to Chinese script morphology of α-AlFeSi phase is a common practice[28 , 29]. In this research, neither β-AlFeSi phase nor α-AlFeSi was observed. Fe combined with Mn to form the Fe- and Mn-rich intermetallic Al6(Fe, Mn) and Si combined with Mg to form the Mg2Si phase. The characteristic of the solidification structure was related to not only the contents of Fe and Si, but also Mg and Mn. In the Mg containing alloy, Si tends to combine with Mg to form Mg2Si. In the AA5182 alloy (Al-4.74Mg-0.34Mn-0.28Fe-0.10Si), the AlFeSi phase was not observed[18], and Mg took priority over Fe to combine with Si to form Mg2Si.
Increasing the content of Fe significantly increased the amount of the eutectic constituent Al6(Fe, Mn). This phenomenon was attributed to the strong segregation tendency of Fe. Since the solid solubility of Fe in aluminum was approximately 0.05% at eutectic temperature[13], almost all of Fe segregated to the interface front during solidification, which increased the local concentration of (Fe + Mn) and contributed to the formation of Al6(Mn, Fe). The increase of Fe from 0.09 wt% in Alloy 1 to 0.28 wt% in Alloy 2 caused the volume of Al6(Fe, Mn) to increase from 0.7% to 2.6%. On the other hand, the composition of Al6(Mn, Fe) was also influenced by the Fe content of the bulk alloy. Increasing the Fe content caused a decrease in the Mn concentration in Al6(Mn, Fe). The composition of the intermetallic Al6(Mn, Fe) was determined by the composition of the local liquid from which the compound formed. With the increase of Fe in the alloy, the Fe atoms, which segregated into the final solidification zone, increased, which resulted in the increase of the Fe concentration in the final solidification zone. As a result, the concentration of Fe in the intermetallics increased.
With the further increase of the Fe content in the alloy, the amount of the Al6(Fe, Mn) increased further. The morphology of the Al6(Fe, Mn) changed to coarse platelets. The phenomenon of intermetallics forming bands was observed in high Mn containing alloys[30]. In this research, bands of intermetallic compounds formed in the alloys with high Fe contents. The high content of Fe made the Fe composition of the interface front high enough to form the Al6(Fe, Mn) bands. In the Al6(Fe, Mn) phase, the sum of Fe and Mn had to remain roughly constant, but the individual compositions of Fe and Mn could vary. Fe played the same role as Mn in the formation of the intermetallic Al6(Fe, Mn).
Silicon combined with Mg to form Mg2Si and did not take part in the formation of the intermetallic Al6(Fe, Mn). But comparing the morphologies of the Al6(Fe, Mn) in different alloys, it was found that the composition of Si had an effect. Comparing Alloys 2 to 3 and Alloys 4 to 5, respectively, increasing Si resulted in the refinement of the Al6(Fe, Mn) phase and retarded the formation of the Al6(Fe, Mn) bands. These phenomena were also observed in the Al-5Fe alloy in which the Al3Fe phase was greatly refined by adding Si[31]. It was most likely that Si formed a barrier in front of Al6(Fe, Mn) and prevented it from massively growing.
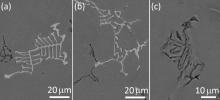 | Fig. 10. (a) and (b) Different morphologies of the phase AlFeMnSi; (c) morphology of phase Mg2Si in Alloy 5 solidified under water cooled iron mold cooling (~20 °C s-1). |
The formation of Fe- and Mn-rich intermetallic compound was affected by both the cooling rate and the contents of Fe and Si in the alloy. Fast cooling significantly refined the intermetallic. However, in the alloys with low Si content (Alloys 1 to 4), increasing the cooling rate did not result in the change of the characteristic of the intermetallic. It was still Al6(Fe, Mn). For Alloy 5 with high Si content, the intermetallic changed to Al15(Fe, Mn)3Si. The content of Si played a critical role in the selection of the intermetallic compound. According to the polythermal projection of the solidification surfaces of the Al-Fe-Mn-Si system[25] (Fig. 11), high Si content favored the formation of the AlFeMnSi phase.
The concentration of Si at the interface front was also affected by two factors: the content of Si in the alloy and the cooling rate. With increasing Si content and cooling rate during casting, the enrichment of Si at the interface front increased. For Alloy 5, under the condition of near-rapid cooling, the concentration of Si in the final solidification zone reached the threshold of forming the AlFeMnSi quaternary phase. Thus, the quaternary phase formed only in Alloy 5.
The Fe- and Mn-rich intermetallic compounds were refined by fast cooling in all the alloys. Under the condition of near-rapid solidification, the grain size and arm space of the dendrite were reduced, which gave rise to the increase in the amount of final solidification zones in the grain boundaries and inter-dendrite regions. As a result, the concentration of solutes in the final solidification zones was thinned. The intermetallics usually formed within the grain boundary triple junctions and inter-dendrites. Therefore, the intermetallics formed during solidification increased in amount due to the increase in the amount of the final solidification zones, but reduced in size due to the reduction of the solute concentration in the final solidification zones. Moreover, in Alloy 5, the morphology of the intermetallic compound changed to fish bone and Chinese script morphologies. This morphology was thought to have less harmful effect on the mechanical properties of alloys[16]. This meant that the higher tolerance of Fe and Si content could be allowed under the condition of near-rapid cooling. This result is of interest for the production of the AA5083 alloy via the continuous strip casting process.
The intermetallics' ability to deteriorate the mechanical properties of the alloys depended on their amount, size, morphology and distribution in the alloys. All these factors were affected by both the contents of Fe, Mn and Si and the cooling rate during solidification. High levels of Fe and Mn promoted the formation of coarse and harmful Al6(Fe, Mn) intermetallic. On the other hand, near-rapid cooling refined the Mn- and Fe-rich intermetallics. Therefore, under the condition of near-rapid cooling, the tolerance of iron in the alloys could be higher without significantly deteriorating the mechanical properties of the alloys. From a production point of view, the characteristic of near-rapid cooling in the continuous strip casting process could be used to produce Al-Mg based alloys with higher Fe and Si content. In other words, for the Al-Mg based alloy sheets which were planned to be produced via the CC process, most likely, the limits of Fe and Si could be higher.
(1)In Al-5Mg-0.8Mn alloy, the content of Fe plays an important role in the formation of the Fe- and Mn-rich intermetallics. In the low Fe alloy, the intermetallic phase Al6(Fe, Mn) is a minor phase with small blocky structure and Fe is a minor element in the phase. With the increase of the Fe content in the alloy, the Al6(Fe, Mn) phase increases significantly in both size and amount. Fe replaces Mn as the major element in the phase. The high Fe containing Al6(Fe, Mn) phase develops as the dominant phase and displays mass blocks with different shapes including platelet, flower-like, etc. The platelet Al6(Fe, Mn) phase lines up and forms a band-like structure.
(2)Si combines with Mg to form Mg2Si phase with network structures, instead of combining with Fe to form AlFeSi or AlFeMnSi phase. Increasing Si gives rise to the refinement of the Al6(Fe, Mn) phase and diminution of the Al6(Fe, Mn) bands.
(3)The cooling rate affects the Fe- and Mn-rich intermetallics in two aspects. The intermetallic phases are significantly refined in the samples solidified under near-rapid cooling. On the other hand, in the alloy with high contents of Fe and Si, the near-rapid cooling process causes the Fe- and Mn-rich intermetallic phase to transform from Al6(Fe, Mn) to the quaternary phase Al15(Fe, Mn)3Si that displays a fine fish bone or Chinese script structure.
The authors are grateful to the Research Foundation of Shenyang Aerospace University for its financial support.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|