The effect of Ru addition on solidification behavior, microstructure and hardness of Re-free Ni-based equiaxed superalloys with high Cr content has been investigated. With the increase of Ru, the solidus temperature of the alloys and the γ′ solvus temperature decreased, respectively. However, the liquidus temperatures of the alloys had no obvious change. The microstructure of the as-cast alloys was mainly composed of γ, γ′, γ/γ′ eutectic and MC carbides. The γ/γ′ eutectic was completely dissolved after the heat treatment. The morphology of γ′ was more cuboidal in heat-treated alloys with increasing Ru. Furthermore, the volume fraction of γ′ in the as-cast and heat-treated alloys diminished upon the increase of Ru. It was noted that Ru addition changed the segregation behaviors of Cr and Mo in the alloys from positive segregation element to negative segregation element and promotes the segregation degree of W. As the Ru content increased, the magnitude of segregation of the positive segregation elements Ta and Ti increased accordingly. Meanwhile, the magnitude of Al segregation decreased and Ru tended to segregate in the dendrite core. In addition, the hardness of the alloys improved and their porosity reduced with increasing amount of Ru.
Due to the demand for increasing material temperature capabilities, the development of Ni-based superalloys has led to continuous addition of new refractory elements such as Re, Ru, Pt, Os and Ir[1, 2, 3, 4, 5, 6, 7, 8, 9]. In particular, Ru as a crucial element in the development of Ni-based superalloys has become a symbol element in the fourth and fifth generations of single crystal (SC) Ni-based superalloys like MC-NG, EPM102 and TMS138[2, 10, 11, 12, 13, 14, 15, 16, 17]. Thus, the effect of Ru on superalloys has become a hot topic. Numerous literature of Ru addition has been reported to increase the microstructural stability during thermal exposure and suppress the formation of topologically close-packed (TCP) phases[18, 19, 20] and thus improves the creep strength. It is revealed that Ru serves as dual roles in enhancement of both microstructure stability and solid solution strengthening[2, 19]. There are different viewpoints about the effect of Ru on solidification behavior and segregation characteristic of alloying element in Ni-based superalloys[3, 21]. Feng et al. claimed that the addition of Ru can improve the liquidus temperature of Ni-based superalloys[3], and Shi et al. hold an opposite viewpoint[21]. Some studies approved that the extent of element segregation decreased with the addition of Ru in Ni-based SC superalloys[21, 22], which appeared to be beneficial to suppressing TCP phase formation. The key mechanism of this effect was attributed to the so-called reverse partitioning of refractory elements[18, 23, 24, 25]. However, this approach remains open questions since evidence for reverse partitioning is not consistently found for each Ni-based superalloy system, indicating a dependence on the alloy composition[8, 26, 27, 28, 29]. In short, the effect of Ru on the segregation characteristic of alloying element and solidification behavior is still controversial.
In the past, investigation of segregation characteristic and solidification behavior with correlation of Ru normally focused on directionally solidification (DS)[30] and SC superalloys containing Re[21], but few articles reported the progress on Ru effect of Ni-based equiaxed superalloys without Re[31, 32]. In this study, Re-free Ni-based equiaxed superalloys with high Cr content was selected as a template material to study the effect of Ru addition on solidification behavior, microstructure and hardness of the alloy. Furthermore, the volume fraction and morphology of γ ′ of the alloys were detected.
Re-free Ni-based equiaxed superalloys with different Ru contents were prepared in a vacuum induction furnace (VIM-F25). Nominal composition of the experimental alloy is 0.05 C, 12 Cr, 8.5 Co, 1 Mo, 5 W, 5.2 Al, 5 Ta, 0.5 Ti, 0.008 B (various contents: 0, 1, 3 and 6) Ru and balanced Ni in weight percent. According to various Ru contents, four alloys are named as 0Ru, 1Ru, 3Ru and 6Ru, respectively. The analyzed compositions of experimental alloys are listed in Table 1. All alloys were investigated in detail by microstructure analysis with the cut samples from the top half of the cast ingot. The as-cast alloys were subjected to solution and aging heat treatments in a heat treatment furnace by using the same heat treatment process (1270 ° C/2 h + 1290 ° C/2 h, air cooling (AC) + 1120 ° C/2 h, AC + 900 ° C/2 h, AC). The solidification process of the samples (sample size ϕ 3 mm × 4 mm) was detected by differential scanning calorimetry (DSC). The heating and cooling rates are both 5 ° C/min under a 30 mL/min flowing argon atmosphere. The instrumental measurement tolerance was less than ± 2 ° C. The microstructures of the as-cast and heat-treated samples were analyzed by optical microscopy (OM), scanning electron microscopy (SEM) and electron probe micro analyzer (EPMA). Samples for microstructure examination were prepared by standard metallographic procedures with an etchant of 5 g of CuSO4, 20 mL of HCl and 20 mL of H2O and an electrode etchant of 10% HNO3 solution. The voltage and time of electrochemical etching are 5 V and 10 s, respectively. The volume fraction and the size of γ ′ were measured using a standard image analysis software (Image-Pro Plus 6.0). The area fraction of γ ′ can be considered to be equivalent to the volume fraction in this case since the extent of γ ′ across the depth can be reasonably assumed to be equal to that across the field of view used for measurement[33]. The densities of samples were detected using the ‘ Archimedes-method’ in deionized water at 23.5 ° C with an analytical balance (Mettler Toledo XS150). The Vickers hardness of samples was measured with an automatic micro-indentation hardness testing system (LECO AMH 43) with 500 g load and keeping 15 s. The porosity was measured by image analysis of the polished cross-sections of samples.
| Table 1. Analyzed composition of experimental alloys (wt%) |
Fig. 1 provides typical optical micrographs of the as-cast alloys. The disorder distribution of dendritic microstructure exhibits a typical equiaxed grain microstructure due to three-dimensional temperature field heating during the solidification. The dendrite core (DC), second dendrite arm (SDA) and interdendritic region (IR) are clearly observed. DC and SDA show higher lightness and IR displays lower lightness. By using an energy dispersive X-ray spectrometer (EDS) of SEM, it is confirmed that refractory elements such as W and Mo were enriched in DC. The magnitude of segregation of alloying element between DC and IR can be better characterized by average segregation coefficient (ks). To ensure statistically significant mean values, an adequate number of measurements (at least ten) were taken within DC and IR. The average segregation coefficient (ks) can be defined in Eq. (1). The ks > 1 or ks < 1 represents the element partition preferably to DC or IR, namely negative segregation or positive segregation. This method is straightforward and is generally accepted[34, 35, 36].
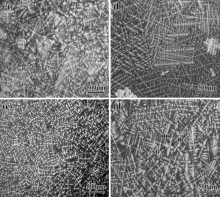 | Fig. 1. Dendrite morphology of as-cast alloys: (a) 0Ru alloy; (b) 1Ru alloy; (c) 3Ru alloy; (d) 6Ru alloy. |
ks=CDC/CIR(1)
where ks is the average segregation coefficient; CDC is the chemical composition in the dendrite core area; and CIR is the chemical composition in the interdendritic region.
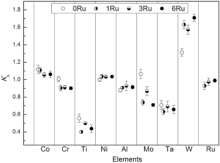
Fig. 2 highlights the average segregation coefficient of alloying elements in the as-cast alloys. Fig. 2 shows that Co, Mo and W tend to segregate toward the dendrite core region during solidification in Ru-free alloy and display a negative segregation character. In other words, some phases rich in Co, Mo and W elements solidified first within the cores during the casting process. In contrast, Al, Ti and Ta elements present a positive segregation character in Ru-free alloy. These elements existed in greater quantity in the last liquid phase to solidify and result in large eutectic γ /γ ′ phases within the interdendritic regions, as shown in Fig. 3. The ks values of Cr and Ni elements in 0Ru alloy are close to unity.
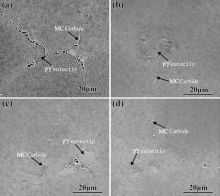 | Fig. 3. SEM images of the as-cast alloys: (a) 0Ru alloy; (b) 1Ru alloy; (c) 3Ru alloy; (d) 6Ru alloy. |
With the addition of Ru, the segregation characteristic of Mo has changed from negative segregation element to positive segregation element and that of Cr becomes an obvious positive segregation characteristic. And the segregation characteristic of other alloying elements is unchanged. Ru represents a positive segregation characteristic, which is contrary to recent investigations[21, 37]. But Li et al. had the idea that Ru was mainly enriched in interdendritic region during the solidification process of Ni3Al single crystal alloy[38]. With increasing Ru, ks of W obviously increases and that of Al element slightly increases, which is not in agreement with the result of some reports that in the case of the Ru addition, only little or contrary influence on the segregation distribution coefficient of W or Al[3, 31, 39, 40]. For an example, Liu et al. reported that the tendency for Al segregation to IR increases with the increase of Ru in bearing higher Re SC superalloys[40]. The reason is possibly due to an addition of 0.5Ti wt% in our test alloys. In terms of alloy materials, it is worth noting that Ti probably plays an important role in controlling the partitioning behavior of Re and W[31, 41]. Addition of Ti might be useful to reduce the nominal overall W concentration while maintaining a similar W content in the γ matrix. Furthermore, Kearsey et al. noted that Ru additions effectively reduced the severity of micro-segregation that occurs due to heavy addition of Re and high total refractory levels[22]. To our experimental alloys, without Re, the lower total refractory elements (W + Ta + Co = 18.5 (wt%)) and high content Cr (12wt%) are of an inherent difference.
The degree of segregation of Co, Al and Ru decreased and that of Ti, Mo, Ta and W increased with the increase of Ru content. It demonstrates that Ru has a strong effect on the segregation behavior of other alloying elements in experimental alloys during solidification. But several researchers approved that Ru did not induce remarkable effect on the segregation behavior of other alloying elements in superalloys and it is important to note that these alloying investigations considered a relatively limited range of composition, with alloys typically containing lower Cr and higher Re[26, 29, 30, 31]. Furthermore, the variation degree of segregation of Co, Cr and Ni is similar since the atomic radii of those elements are nearly equivalent[42].
All these results suggest that segregation behaviors of alloying elements are very complex in multicomponent Ni-based superalloys over a broad range of compositions. As a result, the influence of Ru on segregation behavior of alloying elements in high Cr Re-free equiaxed Ni-based superalloys exhibits distinct characteristics.
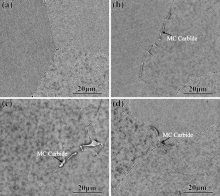
Fig. 3 exhibits typical microstructure of as-cast alloys by SEM. The microstructures of four as-cast alloys are composed of γ , γ ′ , γ /γ ′ eutectic and carbide. The type of carbide is MC type by EDS analysis. The area percentages of carbides of the as-cast alloys are 0.057 ± 0.0041% (6Ru alloy), 0.028 ± 0.0021% (3Ru alloy) and 0.031 ± 0.0074% (1Ru alloy), respectively. The Ru-free alloy contains very little carbide due to serious carbon burning during casting process (as shown in Table 1) and such small quantities could not be easily detected. Addition of Ru has no effect on the phase composes of as-cast alloys. The area fractions of γ /γ ′ eutectic of as-cast alloys are 0.8 ± 0.014% (0Ru), 1.13 ± 0.018% (1Ru), 1.52 ± 0.012 (3Ru) and 2.12 ± 0.016% (6Ru), respectively. The so-called eutectic fraction displays the remaining liquid at the last stage of solidification[31]. Without Ru addition, the amount of remaining liquid at the end of solidification is marginally lower. With the increase of Ru, the area fraction of γ /γ ′ eutectic increases, which is different from the result of Shi et al.[21] but similar to recent studies[31, 40]. Although with addition of Ru, Al concentration in interdendritic region is slightly less, that of Ti and Ta strongly tends to enrich in this area (as shown in Fig. 2). As a consequence, an increase in the amount of eutectic occurs.
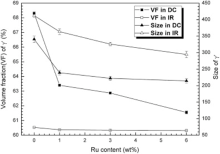
Fig. 4 is the γ ′ morphology of as-cast alloys. The primary γ ′ phase of as-cast samples shows butterfly and the size of γ ′ in IR is larger than that in DC. The influence of Ru on the size and the volume fraction of primary γ ′ of as-cast alloys is illustrated in Fig. 5. In light of the image analysis results, the γ ′ size and its volume fraction in DC and IR decrease, the γ ′ shape is more regular and the distribution of γ ′ particles in DC is more uniform with increasing Ru (as shown in Fig. 4), which is different from the reported results in the literature[43] and [44] and consistent with the study of Liu et al. [45, 46]. This effect may not result from modified alloying element fractions since both elements were added at the expense of Ni and all other alloying elements were kept as constant (as shown in Table 1 ). Thus, the decreasing volume fraction and size of γ ′ can be an effect of the Ru-addition itself. One possible suggestion is the reduction in the γ ′ volume fraction by addition of Ru, accompanied by decreased γ supersaturation due to a higher γ volume fraction[25].
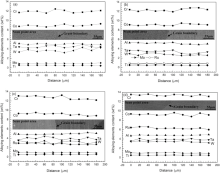
Fig. 6 displays morphologies of heat-treated alloys. After heat treatment, the primary γ ′ and γ /γ ′ eutectic dissolved completely. It is very difficult to distinguish DC and IR, which presents the uneven distribution of all elements between DC and IR after heat treatment. The phase of four heated alloys is composed of γ , γ ′ and carbide. The type of carbide has no change. Fig. 7 illustrates alloying element distribution plotted vs distance between the adjacent grains of the heat-treated alloys. Cr, Co and W elements display relatively large variation and Ta shows intermediate variation as well as Al, Mo, Ti and Ru nearly keep constant. It reveals that most of alloying elements present very uniform distribution after heat treatment. It is interesting to note that the content of Cr and Co is higher than their average content and that of W and Ta is lower than their average value. It can be attributed to a discrepancy of standard samples of EPMA.
Fig. 8 compares the γ ′ morphology of the heat-treated alloys. The morphology of γ ′ has been noticed to be more cuboidal in Ru bearing alloy as compared to Ru-free alloy and the degree of cubic of γ ′ with the evolution of Ru addition. When the content of Ru reaches 6 wt%, the cuboidal degree of γ ′ becomes the optimum. It can be observed that smaller γ ′ exists between larger γ ′ phases and small quantity of uneven planes can be also found due to spalling of partial larger γ ′ . Thus, using electrode chemical etched samples to evaluate the volume fraction of γ ′ easily leads to bigger discrepancy. In this study, we analyzed the volume fraction of γ ′ using chemical etched samples due to the accuracy of the method.
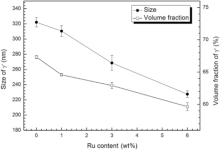
Fig. 9 reveals the variation of the size and the volume fraction of γ ′ in the heat-treated alloys with the content of Ru. With the addition of Ru, the volume fraction and size of γ ′ in heat-treated alloys decrease and the case is the same as that in the as-cast alloys. The morphology of γ ′ particles depends on both the interfacial energy and elastic strain energy, which are related to the interfacial area and volume of precipitates, respectively[47]. It is well accepted that the composition of γ and γ ′ phases controls the sign and magnitude of the lattice misfit, which influences the morphology of γ ′ precipitates[48]. The difference in the γ /γ ′ partitioning coefficients could give a reasonable explanation for why there is a decreasing γ ′ volume fraction with increasing Ru content. Thus, it is very important to understand the γ /γ ′ partitioning coefficient of all alloying elements and the γ /γ ′ lattice misfit δ of alloys. Normally, the γ /γ ′ partitioning coefficient of alloying elements can be detected by three dimensional atomic probing (3DAP) or using EPMA to measure the chemical composition of γ and γ ′ after growing treatment (the size of γ /γ ′ is at least 1 µ m, which can minimize the matrix effect [49]). As to δ , it can be calculated with different model equations or detected by TEM or XRD that concludes synchrotron radiation [50]. It has been reported that Ru partitioned preferentially to the matrix (γ ) resulting in more negative lattice misfit (δ ) between γ ′ and γ [10, 51, 52, 53], an effect that can be attributed to the low diffusion coefficient of Ru and the increased γ /γ ′ lattice misfit[54, 55, 56, 57]. It can be seen from Fig. 8 that with increasing Ru content, the morphology of γ ′ particles evolved from spherical in 0Ru alloy and 1Ru alloy to intermediately shaped in 3Ru alloy, to cuboidal in the 6Ru alloy, which reveals that the addition of Ru prompts the lattice misfit becoming more negative. In the Ru-free alloy, γ ′ precipitates as sphere, so the lattice elastic energy is deduced to be negligible, which gives a small value of the lattice misfit between γ ′ and γ . With the increase of Ru content, the lattice parameter of γ would increase due to larger atom radius of Ru incorporated into γ phase[2, 58]; as a consequence, the lattice misfit would become bigger, resulting in the increase of lattice elastic energy.
For the alloy with smaller magnitude of lattice misfit, interfacial energy predominates and spherical γ ′ particles are shown due to their minimum surface area with the same volume. Inversely, for the alloy with larger magnitude of lattice misfit, elastic strain energy induced by lattice misfit predominates and cuboidal γ ′ particles are exhibited. The cuboidal degree of γ ′ increases with the increase of lattice misfit. Furthermore, in the characteristics of cuboidal γ ′ precipitation, the degree of parallelism of γ /γ ′ interfaces is improved with the increase of magnitude of lattice misfit (Fig. 8). Consequently, the distribution of γ ′ is the most uniform in 6Ru alloy.
In accordance with Heckl et al.[44], the γ ′ -solubility of the typical γ ′ forming elements (Al, Ti, Ta and Ni) is lowered by the addition of Ru and the γ solubility is increased, which illustrates that the tendency for supersaturation and precipitation of γ ′ in the matrix is lowered. Thus, with the addition of Ru, decreasing the available amount of Ni for the γ ′ -formation in alloy leads to a lower volume fraction of γ ′ in alloys.
The effect of Ru addition on thermo-physical properties and high-temperature transformations was investigated by DSC instrument. The DSC heating curves of four alloys are displayed in Fig. 10. The initial γ ′ solvus temperature of alloy (Tγ ′ solvus) is defined as the first endothermic peak. The γ /γ ′ eutectic transformation temperature of alloy (Tγ /γ ′ eutectic) is identified as the second endothermic reaction. The liquidus temperature of alloy (TLiquidus) is named as the peak of the maximum temperature difference. The solidus temperature of alloy (TSolidus) is described by extrapolation of the onset temperature of the liquidus. The range of melting temperature (Δ T) has a difference between TLiquidus and TSolidus. Above defined temperatures are marked in Fig. 10.
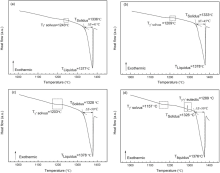 | Fig. 10 DSC heating curves of as-cast alloys: (a) 0Ru; (b) 1Ru; (c) 3Ru; (d) 6Ru. Tγ ′ — The γ ′ solvus temperature, Tγ /γ ′ — The γ /γ ′ eutectic transformation temperature, TL— The liquidus temperature, TS— The solidus temperature, Δ T— The range of melting temperature. |
It can be seen from the heating curves of the alloys that Ru addition decreases the initial γ ′ solvus temperature and solidus of alloys, but has a negligible effect on the liquidus temperature of alloy. This result is different from the results of some other reports[3, 32, 59]. Feng et al. and Chen et al. found that addition of Ru enhanced the solidus and liquidus temperatures of Ni-based SC superalloys[3, 59], and Cui et al. found that Ru only increased the liquidus temperature in Ni-based disc alloys[32]. On the contrary, Shi et al. observed that addition Ru can reduce the solidus and liquidus temperatures[21]. Furthermore, Zheng et al. investigated the solidification behavior of Ru-containing Ni-based Re-free superalloy with lower Cr high W and found that addition of Ru decreased the precipitation temperature of γ /γ ′ eutectic and secondary γ ′ in alloy and had no effect on the temperature of solidus and liquidus[60], and Ai et al. maintained that the addition of Ru tended to lower the solidus and liquidus temperatures and to increase freezing range, but the influence of Ru was negligible[39]. The difference between γ ′ solvus and the solidus is considered to be the solution treatment “ window” [55]. As seen in Fig. 10, these alloys typically have at least a 90 ° C solution treatment window and the window increases with the increase of Ru. Microscopy analysis confirmed that all alloys were completely homogenized by the chosen heat treatments.
The Δ T of the alloy with Ru addition is wider than that of Ru-free alloy, which is in agreement with other experimental data [31]. In particular, from Fig. 10(d), an obvious γ /γ ′ eutectic endothermic peak can be seen and there are no findings in other heating curves (as shown in Fig. 10(a-c)). It indicates that the quantity of γ /γ ′ eutectic in 6Ru alloy is much more than that of other alloys. Compared with our experimental alloys, most studies have investigated the Re-containing SC superalloys[3, 39, 59]. Different results can be attributed to the multicomponent of superalloys. It reveals that different superalloy systems show different solidification characteristic.
Fig. 11 represents DSC cooling curves of four alloys. During cooling, the temperature of initial solidification (Ti) is defined as the end temperature of overall liquid. The temperature of final solidification (Tf) is represented as the onset temperature of overall solid. The temperature of forming γ /γ ′ eutectic (Tp γ /γ ′ eutectic) is determined as an endothermic reaction near final solidification region and the starting point of the eutectic solidification was set as the first appearance of the eutectic γ ′ phase. The eutectic temperature and eutectic fraction were taken from the solidification curve at this point. In previous works[31, 61, 62], the eutectic fraction was evaluated by calculating the eutectic solidification enthalpy in proportional to the overall released enthalpy from DSC cooling curves of alloys. The temperature of precipitating γ ′ from solid (Tp γ ′ ) is named as the temperature of last endothermic peak. The range freezing temperature (Δ T′ ) is a difference between Ti and Tf. All defined temperatures are illustrated in Fig. 11. It can be seen from Fig. 11 that Ru has a negligible effect on Ti but has pronounced influence on Tf, Tp γ ′ , Tp γ /γ ′ eutectic and Δ T′ . With the addition of Ru, Tf and Tp γ ′ are lowered and Δ T′ is widened. For Ru-free alloy, there is no finding of any γ /γ ′ eutectic endothermic reaction due to tiny γ /γ ′ eutectic phase quantity in the alloy. With the increase of Ru, the value of Tp γ /γ ′ eutectic changes initially from 1324 ° C (1Ru alloy) to 1321 ° C (3Ru alloy) and then increases to 1326 ° C (6Ru alloy). It is interesting to note that the reaction of precipitating carbide can be seen in the DSC cooling curve of 1Ru alloy, and the temperature of precipitating carbide (TCarbide) is approximately 1338 ° C, which is not presented in other alloys. It is possible that the DSC sample of 1Ru contains relative high carbon content. According to previous works[31, 63], the concentration CDC or CIC of the first solid formation can be assumed to be controlled by the liquid diffusion coefficient. An increase in the freezing range with the addition of Ru provokes more time for liquid diffusion, which in turn leads to an increase in W microsegregation into the dendrite core due to its solubility limit. As a result, the value ks of W element increases with the addition of Ru. The calculated eutectic fractions are 0% (0Ru), 9.54% (1Ru), 10.36% (3Ru) and 12.54% (6Ru), respectively. The agreement between the calculated and experimentally derived values of eutectic volume fraction is rather poor. It is believed that there are other low-melting-point phases in equiaxed superalloys besides γ /γ ′ eutectic, for example boride. But, with the addition of Ru, the change of eutectic fraction shows similar trend. Table 2 concludes the results of DSC.
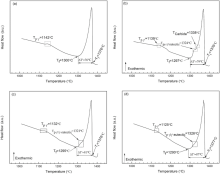 | Fig. 11 DSC cooling curves of as-cast alloys: (a) 0Ru; (b) 1Ru; (c) 3Ru; (d) 6Ru. Ti— The temperature of initial solidification, Tf— The temperature of final solidification, Tp γ /γ ′ eutectic— The temperature of forming γ /γ ′ eutectic, Tp γ ′ — The temperature of precipitating γ ′ from solid, Δ T′ — The range freezing temperature, TCarbide— The temperature of precipitating carbide. |
| Table 2. Results of DSC heating and cooling curves of as-cast alloys (° C) |
The densities of as-cast and heat-treated alloys were determined using the ‘ Archimedes-method’ in deionized water at 23.5 ° C. Fig. 12 shows the densities of four alloys under different conditions. It can be seen from Fig. 12 that the density of alloys increases with the increase of Ru. One reason is that the density of Ru element (12.45 g/cm3) is larger than that of Ni (8.88 g/cm3).
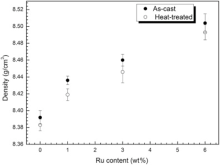 | Fig. 12 Densities of alloys vs Ru content. |
As we know, the quantity of defects in alloys has a closer relationship with the density of alloy. Fig. 13 displays the area fraction of porosity of as-cast and heat-treated alloys. It exhibits that the area fraction of porosity decreases with the addition of Ru, which suggests that Ru can reduce the formation of porosity in alloys. This result is in agreement with several investigations[15, 22, 64]. Thus, the other reason of improving density by Ru addition is that Ru addition is effective in suppressing the tendency of grain defect formation. The size of porosities of the as-cast and heat-treated alloys is presented in Fig. 14. The size of porosities in the as-cast alloys is not more than 2 µ m and decreased with the increase of Ru. After heat treatment, the size of porosities is larger than that in the as-cast alloys and the change trend with increasing Ru is the same as that in the as-cast alloys. It reveals that Ru addition suppresses the formation of porosity and the reason is that Ru strongly affects the segregation behavior of refractory alloying elements, which possibly lowers the density inversion between the solute and bulk liquid in mushy zone[63] and thus reduces the driving force for grain defect formation. Although the value ks of W in Fig. 2 increases with the addition of Ru, Liu et al. found that the partition coefficients from point matrix scanning technique are quite different from the directional solidification followed quenching (DSQ) technique due to solid backdiffusion[63]. They found that the partition coefficient of W slightly decreased from 1.23 (Ru-free alloy) to 1.22 (Ru-containing alloy) using DSQ technique and clearly increased from 1.26 (Ru-free alloy) to 1.37 (Ru-containing alloy) using point matrix scanning technique[63]. It is a future work to use DSQ technique to measure test alloys.
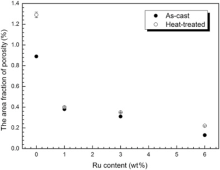 | Fig. 13 Area fraction of porosity vs Ru content. |
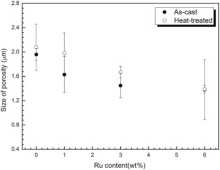 | Fig. 14 Size of porosity in as-cast and heat-treated alloys vs Ru content. |
Furthermore, it is interestingly noted that the area fraction and size of porosity in the as-cast alloys is lower than that of heat-treated alloys. The reason could be dissolving of γ /γ ′ eutectic after solution treatment, which leads to form much micro pore. As a result, the density of the as-cast alloy is higher than that of heat-treated alloys as shown in Fig. 12.
This is confirmed by the hardness of four alloys at different conditions in Fig. 15.With increasing Ru, the hardness of alloys increases. Several literatures presented similar result that the hardness of γ and γ ′ phases increased with the addition of Ru[45]. They thought that the Ru additions appear to provide a certain amount of solid solution strengthening to both the γ and γ ′ phases[48, 49, 54]. Since the element Ru also has a hexagonal close-packed structure (HCP) structure, its addition would be expected to decrease the stacking fault energy of the matrix[2]. The a/2[110] dislocation motion in the matrix channel leaves interfacial dislocations around cuboidal precipitates and forms homogeneous γ /γ ′ interfacial dislocation networks [65, 66]; the addition of Ru extends the width between the partial dislocations in the matrix[1] and would inhibit the cross slip of dislocation at this temperature. Addition of Ru reduced the volume fraction of γ ′ as shown in Fig. 5 and Fig. 9 and a low γ ′ volume fraction also widens the width of the γ channels between the γ ′ precipitates. Intrinsic reason could be the fact that Ru addition promotes larger lattice distortion, and then the hardness of alloy increases[67]. As mentioned above, Ru addition improves the hardness of experimental alloys. For the same alloy, the hardness of the as-cast alloy is bigger than that of heat-treated alloy, so, the degree supersaturation of the as-cast alloy is larger than that of heat-treated alloy.
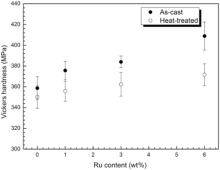 | Fig. 15 Vickers hardness vs Ru content. |
(1)With the increase of Ru, the segregation characteristics of Cr and Mo in alloys changed from positive segregation element to negative segregation element. The segregation degree of Ta, Ti and W increased and that of Al decreased, while Ru tended to segregate in the dendritic core with increasing Ru.
(2)The volume fraction of γ ′ in the as-cast and heat treated alloys was reduced and the morphology of γ ′ was more cuboidal upon the addition of Ru.
(3)The solidus temperature of the alloys was suppressed by Ru addition; at the same time, the freezing range of the alloy was enhanced by the γ ′ solvus temperature variation. However, the liquidus temperature of the alloys was maintained unchanged throughout the process. The density and hardness of the alloys were greatly improved by Ru addition as well as less porosity formation in Ni-based equiaxed superalloys.
The authors acknowledged the support of Fei-xue Yang for SEM analysis and Shuang Qu for analysis of DSC data.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|