ZnSO4-Zn(CH3COO)2, Zn(NO3)2-Zn(CH3COO)2, ZnSO4-Zn(NO3)2, ZnSO4, Zn(NO3)2 or Zn(CH3COO)2 have been used as zinc sources to prepare ZnS thin films by chemical bath deposition and co-deposition methods. Zn(NO3)2 or/and Zn(CH3COO)2 is/are favorable for cluster by cluster deposition process while ZnSO4 favors ion by ion deposition process regardless of concentration ratios of ZnSO4. However, Zn(NO3)2 affects the nucleation density of ZnS nuclei on the substrate. ZnS thin films deposited from ZnSO4-Zn(CH3COO)2 are not only more homogeneous and compact, but also have higher growth rate and adhesion on to the glass substrate. The cubic ZnS films are obtained after only single deposition. The average transmission of films from S6, S7, S8, S9 and S1 for 2 and 2.5 h is greater than 85% in visible region. Compared with the film from S6 (112 nm), the film from S7 is not only thicker (125 nm), but also more transparent. The band gaps of the films deposited from S6, S7, S8, S9 and S1 for 2 and 2.5 h range from 3.88 to 3.98 eV. The effects of anions from different zinc salts are discussed in detail.
Thin film solar cells (TFSCs), such as CuInS2, CuInSe2 (CIS), Cu(In, Ga)Se2 (CIGS), Cu(In, Ga)(SSe)2 (CIGSSe) and Cu(In, Ga)(SeS)2 (CIGSS)[1, 2, 3], have attracted much attention as potential non-silicon absorber materials. A CdS/CIGS solar cell, in which CdS buffer layer has been employed by chemical bath deposition (CBD) [4], has exhibited record 20.8% conversion efficiency. However, the use of cadmium is toxic and causes serious environmental problems. Meanwhile, the quantum efficiency drops at short wavelengths ranging from 350 to 550 nm due to optical absorption losses from the CdS layer[5, 6, 7]. ZnS and ZnSe are the promising candidates due to their non-toxicity, economical price and low conduction band offsets[8, 9]. However, compared to Ksp of ZnS (10-24.7) [8], Ksp of ZnSe (10-31) [10] is very low, which leads to the nonuniformity of ZnSe film and has to choose appropriate complexing agent to obtain uniform ZnSe film, such as hydrazine hydrate[11, 12, 13]. On the contrary, Ksp of ZnS is high, the uniform ZnS film can be easily obtained. At the same time, ZnS is an important II-VI semiconducting material with a high direct band gap of 3.7 eV [14, 15]. The high band gap can eliminate the absorption loss at short wavelengths and enhance the blue response of the cells, which have a vast potential application on TFSCs. As a Cd-free buffer layer, CBD-ZnS/Cu(In, Ga)Se2 (CIGS) TFSCs have higher efficiency of 18.6%[16]than that of CBD-ZnSe/Cu(In, Ga)Se2 (CIGS) TFSCs (15.7%)[17].
Over the past two decades, various compositions of chemical bath have been used to deposit CBD-ZnS thin films, such as zinc salt/NH3/SC(NH2)2[7, 16, 18, 19, 20, 21, 22, 23], ZnCl2/NH4NO3/SC(NH2)2/KOH[24], zinc salt/NH3/(NH2)2/SC(NH2)2[25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38] (the compositions consist of the addition of complementary agents), ZnCl2/NH3/HONH3Cl/SC(NH2)2[39], ZnCl2/NH3/C6H15NO3 (triethanolamine, TEA)/SC(NH2)2[40], ZnCl2/urea/CH3CSNH2 (thioacetamide, TAA)/HCl[41], Zn(CH3COO)2/NH3/(NH2)2/COOH(CHOH)2COOH/SC(NH2)2[42], Zn(CH3COO)2/Na3C6H5O7/SC(NH2)2[43], Zn(CH3COO)2/C6H15NO3/Na3C6H5O7/SC(NH2)2/NH3/NH4Cl[44, 45], ZnSO4/CH3CS-NH2[46], Zn(CH3COO)2 or ZnSO4/Na3C6H5O7/SC(NH2)2/NH3[47, 48, 49, 50, 51, 52, 53, 54], ZnCl2/N(CH2CO-OH)3 (nitrilotriacetic acid, NTA)/CH3CSNH2/NaOH[55], Zn(CH3COO)2/COOH-(CHOH)2COOH/Na3C6H5O7/SC(NH2)2/NH3[56], Zn(CH3COO)2/H2NCH2CH2NH2 (ethylenediamine, En)/CH3CSNH2/HCl[57], ZnSO4/CH3CSNH2/HCl/CH3COOH[58], Zn(CH3COO)2/C10H14N2O8Na2 (tetra-acetate disodium salt, Na2EDTA)/CH3CSNH2/NaOH[59], Zn(CH3COO)2/C10H14N2O8Na2 or EDTA/Na3C6H5O7/SC(NH2)2/NH4OH[8, 60], Zn(CH3COO)2/CH3CSNH2/C10H14N2O8Na2/C6H12N4 (hexamethylenetetramine, HMTA)/HCl[61], ZnSO4/NH3/CH3CSNH2[62], zinc salts (different zinc salts: Zn(CH3COO)2, ZnSO4, ZnCl2, or Zn(NO3)2)/urea/CH3CSNH2/NaOH/HCl[63], and so on. All the reports are based on the use of single zinc salt. Zn(CH3COO)2, ZnSO4 and ZnCl2, and SC(NH2)2 and CH3CSNH2 are the common choices as Zn2+ and S2- sources, respectively. NH3, N2H4, and Na3C6H5O7 (tri-sodium citrate) are popular choices as the complexing agents. In addition, it is obvious that there are many compositions of chemical bath employed to prepare ZnS thin films. At the same time, many variables, such as concentrations of various reagents[22, 24, 25, 32, 33], pH values[28, 29, 31, 34, 61, 63], complexing agents[25, 40, 42, 43, 49, 56, 59, 64], deposition temperature[21, 35, 57, 65] and time[35, 36], annealing treatment[21, 27, 42, 59], stirring speeds[7, 25, 35, 55, 66], and substrates[39, 57], co-deposition of alloy compounds[26, 37, 52, 67], ammonium salts or buffer solutions[28, 32, 38] have been investigated. However, there are few reports about the effect of anions from different zinc salts and the understanding level of the effect of anions on the growth and properties of ZnS thin film is lower. Cao et al.[63] and our group[36] studied the effects of four zinc salts (ZnSO4, Zn(NO3)2, ZnCl2 or Zn(CH3COO)2) on the structural and optical properties of CBD-ZnS thin films, respectively. The results were different due to the difference from the acidic and basic reaction baths, respectively. We have demonstrated[36] that the anion from zinc salt can affect growth mechanism of ZnS in a chemical bath with the compositions of single zinc salt/NH3/(NH2)2/SC(NH2)2 significantly. It means that the anion from zinc salt is also a very important effect factor, which needs to be further investigated.
It is well known that hydrazine hydrate, as a complementary complexing agent, improves the homogeneity and specularity of ZnS thin films in a reaction bath containing ZnSO4/NH3/(NH2)2/SC(NH2)2[25]. However, Oladeji and Chow[38] reported that the ZnS growth in NH3/N2H4 medium leads to less adherent film. Meanwhile, the excessive homogeneous reaction minimizes the heterogeneous reaction and film thickness. To improve the quality of ZnS films, Oladeji and Chow[37] introduced potassium nitrilotriacetate (KNTA) in the reaction bath as an additional complexing agent. Doñ a and Herrero[25] and Vidal et al.[27] reported that the thickness of the films is lower than 90 nm and the films are amorphous. Our group[36] increases the growth rate and improves the structural and optical properties of ZnS thin films without stirring during the deposition. In order to further increase ZnS growth rate, improve physical properties and obtain high quality ZnS thin films, two zinc salts were used to co-deposit ZnS thin films without stirring during the deposition (the compositions of chemical bath is Zn(CH3COO)2/ZnSO4/NH3/(NH2)2/SC(NH2)2). The results show that ZnS thin films are not only more homogeneous and compact, but also have higher growth rate and adhesion on to the glass substrate.
In this work, it is the first time that Zn(NO3)2-Zn(CH3COO)2 or ZnSO4-Zn(CH3COO)2, or ZnSO4-Zn(NO3)2 were used as zinc ion source to co-deposit ZnS film simultaneously. The effects of different concentration ratios of zinc salts on the growth, morphology, structure, mechanical and optical properties of ZnS thin films prepared without stirring during deposition were investigated in detail. In addition, the mechanism of anions was also discussed.
ZnS thin films were grown on the soda lime glass substrate at 80 ° C by chemical bath deposition or co-deposition method. The solutions consist of analytical-grade reagents with zinc salts (Zn(NO3)2, Zn(CH3COO)2, and/or ZnSO4⋅ 7H2O), and thiourea, ammonium hydroxide and hydrazine hydrate. The concentrations of reagents are given in Table 1. The sum concentration of Zn(NO3)2-Zn(CH3COO)2, ZnSO4-Zn(CH3COO)2, and Zn(NO3)2-ZnSO4 is 0.068 mol/L ([Zn2+] = [Zn(NO3)2] + [Zn(CH3COO)2] = 0.068 mol/L, [Zn2+] = [Zn(CH3COO)2] + [ZnSO4] = 0.068 mol/L, and [Zn2+] = [Zn(NO3)2] + [ZnSO4] = 0.068 mol/L). The concentration ratios, X1 = [Zn(NO3)2]/([Zn(NO3)2] + [Zn(CH3COO)2]), X2 = [Zn(CH3COO)2]/([ZnSO4] + [Zn(CH3COO)2]), and X3 = [Zn(NO3)2]/([Zn(NO3)2] + [ZnSO4]) vary from 0 to 1, from 0 to 0.67, and from 0.33 to 0.67, respectively, as shown in Table 2, Table 3 and Table 4. The calculated amount of reagent was put into a 250 mL beaker and the rest was completed with deionized water to make the total volume of the solution 150 mL. The chemical bath was not stirred and was maintained at 80 ° C during the deposition. The glass substrates were cleaned with detergent, degreased with ethanol in an ultrasonic cleaner, washed thoroughly with deionized water, and dried with N2. The cleaned substrates were vertically inserted into the reaction bath. After the deposition, the films were taken out of the bath, rinsed with deionized water and dried with N2.
| Table 1. Concentrations of various reagents from the deposition compositions of Zn(NO3)2-Zn(CH3COO)2, ZnSO4-Zn(CH3COO)2, and ZnSO4-Zn(NO3)2 (mol/L) |
| Table 2. Concentration ratios: X1 = [Zn(NO3)2]/([Zn(NO3)2] + [Zn(CH3COO)2]) |
| Table 3. Concentration ratios: X2 = [Zn(CH3COO)2]/([ZnSO4] + [Zn(CH3COO)2]) |
| Table 4. Concentration ratios: X3 = [Zn(NO3)2]/([ZnSO4] + [Zn(NO3)2]) |
The crystalline structures of films were analyzed using an X-ray diffractometer with a wavelength of 0.154 nm from the Cu Kα line (Japan, XRD6100). Optical measurements were obtained employing an UV-spectrophotometer (Unico, 2802PCS). The layer thickness of ZnS films was estimated by surface profiler (Ambios, XP-2) and the weight method simultaneously. The surface morphology was investigated by field emission scanning electron microscopy (FESEM, Zeiss, Sigma) and atomic force microscopy (AFM, AJ-III). The critical load was measured by multi-functional tester for material surface (China, MFT-4000).
Comparing with ZnS films deposited from S1, S2, S3, S4, S5, S10, S11 and S12 for 2 and 2.5 h, the films obtained from S6, S7, S8 and S9 for 2 and 2.5 h are more uniform, better reflecting and transparent, and have higher adhesion on to the glass substrate.
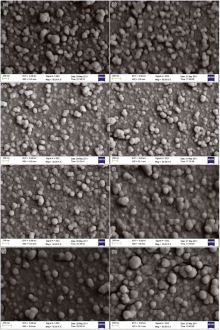
Fig. 1 and Fig. 2 show the FESEM micrographs of ZnS films deposited from S1, S2, S3, S4 and S5 for 2 and 2.5 h, respectively. ZnS films deposited from S1, S2, S3, S4 and S5 correspond to concentration ratios (X1) of [Zn(NO3)2]/([Zn(NO3)2] + [Zn(CH3COO)2]) ranging from 0 to 1. It is obvious that all the films are formed via cluster by cluster deposition. When X1 ranges from 0 to 0.5, a lot of small clusters are adsorbed on the film surface. When X1 ranges from 0.67 to 1, ZnS thin film with larger grains becomes compact and has some cracks. It means that when [Zn(CH3COO)2] is larger than [Zn(NO3)2], the clusters is smaller and less dense, and the formation of ZnS film is more favorable for homogeneous precipitation.
 | Fig. 1. FESEM micrographs of ZnS films deposited from S2 for (a) 2 h, (b) 2.5 h, (c) S3 2 h, (d) 2.5 h, (e) S4 2 h, (f) 2.5 h, (g) S5 2 h, (h) 2.5 h. |
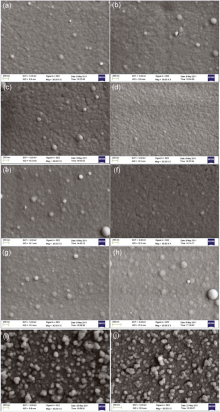 | Fig. 2. FESEM micrographs of ZnS films deposited from (a) S6 2 h, (b) 2.5 h, (c) S7 2 h, (d) 2.5 h, (e) S8 2 h, (f) 2.5 h, (g) S9 2 h, (h) 2.5 h, (i) S1 2 h, (j) 2.5 h. |
Fig. 2(a-j) shows the FESEM micrographs of ZnS films deposited from S6, S7, S8, S9 and S1 for 2 and 2.5 h, respectively. ZnS films deposited from S6, S7, S8, S9 and S1 correspond to concentration ratios (X2) of [Zn(CH3COO)2]/([ZnSO4] + [Zn(CH3COO)2]) ranging from 0 to 1. It is clear that the films deposited from S6, S7, S8 and S9 for 2 h and 2.5 h are formed via ion by ion deposition. Except ZnS thin films deposited from S7 for 2 h, the others are more uniform than those deposited from S6 and S1. There are a few white dots on the film surface, which are the colloids adherent to ZnS films. It is noteworthy that the white dots of ZnS films deposited from S7, S8 and S9 disappear when deposition time increases from 2 h to 2.5 h. It means that the film surface can be improved with increasing deposition time. Combining the FESEM micrographs of Fig. 1(a-h) with Fig. 2(a-j), ZnS films can be formed via ion by ion deposition when SO42- is present in the reaction bath regardless of the concentration ratio of SO42-, while ZnS films are formed via the cluster by cluster deposition when SO42- is not present. On the contrary, the existence of CH3COO- or/and NO3- is favorable for homogeneous deposition.
Fig. 3(a-c) shows the FESEM micrographs of ZnS films deposited from S10 (a), S11 (b), and S12 (c) for 2.5 h. ZnS films deposited from S10, S11 and S12 correspond to concentration ratios (X3) of [Zn(NO3)2]/([Zn(NO3)2] + [ZnSO4]) ranging from 0.33 to 0.67. Based on high transparency, small particles, low root-mean-square roughness (RMS) (following discussion), and good adherence to the substrate, we concluded that the deposition mechanism is ion by ion deposition process, which agrees with the literature[54]. However, when X3 ranges from 0.33 to 0.5, there is no continuous film on the substrate and a few voids are present in the films. It means that the addition of Zn(NO3)2 decreases the number of heterogeneous nucleation on the substrate surface. When X3 is 0.67, the film covers the substrate completely, and the nucleation density of ZnS nuclei on the substrate increases. From the above discussion, we summarized the growth mechanism of ZnS thin film deposited from different zinc salts in Table 5.
| Table 5. Growth mechanism of deposition compositions from different zinc salts |
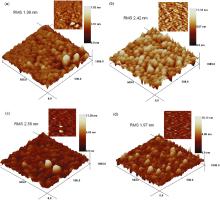
Fig. 4(a-d) shows the AFM micrographs of ZnS films deposited from S6, S7, S8 and S9 for 2.5 h. ZnS films deposited from S7, S8 and S9 are more uniform than those from S1 and S6, which agree well with FESEM micrographs. The RMS of films deposited from S6, S7, S8 and S9 are 1.98, 2.42, 2.50, and 1.97 nm, respectively. The roughness of the film deposited from S1 is much larger than that of the films deposited from S6, S7, S8 and S9 due to the different deposition mechanisms. In addition, the average grain sizes of films deposited from S6, S7, S8 and S9 are 54, 30, 60 and 22 nm, respectively.
Fig. 5(a-d)shows the AFM micrographs of ZnS films deposited from S3, S10, S11, and S12 for 2.5 h. The root-mean-square roughness (RMS) of the film deposited from S3 is 8.29 nm, and that of the films deposited from S10, S11, and S12 is 3.85, 3.25, and 4.60 nm, respectively, which is larger than that of all the films deposited from ZnSO4-Zn(CH3COO)2. The average grain size of the films deposited from S3, S10, S11, and S12 is 32, 29, 28, and 102 nm, respectively.
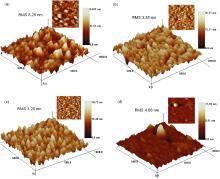 | Fig. 5. AFM micrographs of ZnS films deposited for 2.5 h from (a) S3, (b) S10, (c) S11, and (d) S12 for 2.5 h. |
From the discussion above, we investigated the effect of different anions from zinc salts on the surface morphology of ZnS film in detail, which can help us to clarify the growth mechanism of ZnS film and the role that anions play during the deposition. At the same time, because homogeneous deposition and voids in ZnS film lead to bad quality ZnS film, in this case, we mainly investigated the effect of the anions from ZnSO4-Zn(CH3COO)2 in the following studies.
In addition, in order to investigate the uniformity of ZnS thin film and the compositions of samples in and out of the spherical microstructures without stirring during the deposition, the EDS analysis of S3 for 2.5 h was carried out, as shown in Table 6. The results show that there is a slight change of the composition of S3 from in and out of the spherical grains, and surface-scanning from three different regions. Because the nanocrystalline ZnS film with small grain is thinner and easily absorb O2 on the film surface due to nanograins, we think that the film is relatively uniform without stirring during the deposition.
| Table 6. Zn/S atom ratios of films deposited from S3 for 2.5 h |
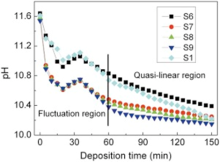
Literature[24] indicates that the optimum pH values to produce good quality CBD-ZnS films were found to vary from 10 to 12. In our case, pH varies from 11.7 to 10 during the deposition, which agrees with the report. Fig. 6 shows the change of solution pH from S6, S7, S8, S9 and S1 during the deposition. Two regions of film growth from S6, S7, S8, S9 and S1 are displayed: an initial fluctuation region and a finally quasi-linear region. It is noteworthy that the pH values from S7, S8 and S9 are much lower than those from S6 and S1 in the fluctuation region, which shows that the simultaneous presence of ZnSO4 and Zn(CH3COO)2 can decrease the pH of solution rapidly. In the quasi-linear region, when deposition time increases from 75 to 150 min, the pH values from S7, S8 and S9 decrease from 10.41, 10.37 and 10.28 to 10.25, 10.21 and 10.15, respectively. The difference of pH is only 0.16, 0.16 and 0.13, respectively. It means that pH values are relatively stable and easily controlled in the quasi-linear region. In the deposition of CBD-ZnS thin film, controlling solution pH value is a common and effective technique for ZnS growth. For example, Antony et al.[28] adjusted solution pH values to 10, 10.3 and 10.6, Ben Nasr et al.[29] controlled pH at 11.5, 10.99, 10.31 and 10, and Lekiket and Aida[34] adjusted pH to 11, 10.66, 10, and 9. In our case, it is possible for our next work to deposit ZnS thin film when deposition time is larger than 75 min, due to the stability of pH values in the reaction bath.
Fig. 7(a-e) displays the thickness of ZnS thin films deposited from S6, S7, S8, S9 and S1. It is observed that the thickness of ZnS thin films from S6, S7, S8, and S9 determined by the surface profiler agrees with that of ZnS thin films determined by the weight method during the deposition, which shows that the films are compact. Meanwhile, two regions of film growth from S6, S7, S8, S9 and S1 are displayed: an initial linear region and a final saturation one, which agree well with the literature[25, 26, 27, 35]. According to the linear fit, as shown in Fig. 7(f), the growth rates of ZnS thin films from S6, S7, S8, S9 and S1 are 1.09, 1.44, 2.13, 1.49 and 0.89 nm/min, respectively. The growth rate of ZnS films deposited from S7, S8 and S9 is much larger than that of ZnS films deposited from S6 and S1. It means that the codeposition from Zn(CH3COO)2-ZnSO4 improves the growth rate of films compared to the deposition from single zinc salt.
 | Fig. 7. Film thickness deposited from S6, S7, S8, S9 and S1 for 2.5 h as a function of deposition time. |
Combining pH value with the growth rate of ZnS films, the linear region of ZnS growth corresponds to the fluctuation region of solution pH. The co-deposition from Zn(CH3COO)2-ZnSO4 leads to the rapid decrease of pH, which favors the increase of the growth rate of ZnS films. Ben Nasr et al.[29] reported that low pH values produce thicker films in NH3/N2H4 mediums, which agrees with our results.
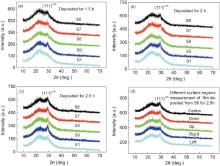
ZnS crystals exist in cubic and hexagonal forms generally. The cubic form is stable at room temperature, and the hexagonal form is stable above 1020 ° C at atmospheric pressure[68]. However, some groups have observed the hexagonal structure for CBD-ZnS films[43, 44, 53]. Fig. 8(a-c) shows the X-ray diffraction (XRD) patterns of ZnS thin films deposited from S6, S7, S8, S9 and S1 for 1.5, 2 and 2.5 h. The films deposited from S6, S7, S8, and S9 for 1.5, 2 and 2.5 h show one weak peak at 2θ ranging from 29.10° to 29.35° . The film deposited from S1 for 1.5 h is amorphous, and with increasing deposition time, the crystallinity of films is improved. The broadening of the diffraction peaks is an obvious characteristic of nanosized materials. According to our previous work[35, 36], it is obvious that the ZnS films have cubic structure and the characteristic peak can be assigned to the (111)cub plane of ZnS. At the same time, in order to investigate the uniformity of ZnS thin film without stirring during the deposition, the structure from five different points of S6 surface (up, down, right, left, and center) for 2.5 h was measured by XRD. The result is similar, as shown in Fig. 8(d), indicating that the film is relatively uniform.
To investigate the mechanical properties of ZnS films deposited from S6, S7, S8, S9 and S1 for 2.5 h, the testing results for bonding force between the substrate and film are shown in Fig. 9. The adhesion strengths of ZnS films deposited from single zinc salt, ZnSO4 (S6) or Zn(CH3COO)2 (S1) are about 18.0 and 15.4 N, respectively. However, the adhesion strengths of ZnS films deposited from S7, S8 and S9 are about 28.9, 30.6 and 29.5 N, respectively. It means that the co-deposition from Zn(CH3COO)2-ZnSO4 improves the adhesion strengths of ZnS films significantly, which agrees well with the FESEM micrographs.
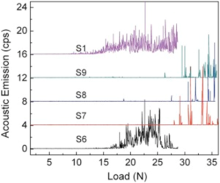 | Fig. 9. Scratch testing curves for measuring interface bonding force of ZnS thin films deposited from S6, S7, S8, S9 and S1 for 2.5 h. |
Fig. 10 shows the average pH value during the deposition, RMS, growth rate and adhesion strength of films as a function of X2. The growth rate of films deposited from S6, S7, S8, S9 and S1 is proportional to their adhesion strength. The RMS of films is proportional to the growth rate and adhesion strength of films when X2 is between 0 and 0.67. However, the RMS is inversely proportional to the growth rate and adhesion strength of films and becomes large when X2 is 1, due to different growth mechanisms. The average pH value is inversely proportional to the growth rate and adhesion strength of films except the film from S8.
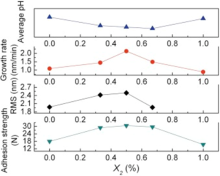 | Fig. 10. Average solution pH during the deposition, RMS, growth rate, and adhesion strength deposited |
Fig. 11(a) and (b) shows the optical transmission of ZnS films deposited from S6, S7, S8, S9 and S1 for 2 and 2.5 h. The average transmission of films deposited from S6 and S8 for 2 h is the largest when the wavelengths are lower than 550 nm. In the wavelengths ranging from 550 to 1100 nm, the average transmission of films deposited from S7, S8, S9 and S1 is larger than that of the film deposited from S6 (> 85%). With increasing deposition time from 2 to 2.5 h, the transmission of the film deposited from S7 is improved significantly in short wavelength, and enhanced slightly in the visible region. It is noteworthy that though the thickness of films from S6 to S9 for 2.5 h increases from 112 to 130 nm (determined by surface profiler), the transmission of films from S7, S8 and S9 is also improved, and the transmission of the thicker film from S7 (125 nm) is even larger than that of the film from S6 (112 nm). It is believed that the ratio of transmission/thickness is one of the important parameters which can determine the quality of ZnS thin film. The high optical transmission and blue response of the films can be potentially used as the buffer layer of CIGS solar cells.
 | Fig. 11. Transmission spectra of ZnS films deposited from S6, S7, S8, S9 and S1 for (a) 2 and (b) 2.5 h. |
The band gap of semiconductor could be obtained from the optical data. For a direct band gap material, the absorption coefficient is related to the incident photon energy hv by the relation:
α 2=A(hν -Eg)α 2=A(hν -Eg)(1)
where α is the absorption coefficient, A is the constant, hv is the photon energy (eV), and the band gap value (Eg) is the separation between valence and conduction bands. Eg is determined from the intercept of the straight-line portion of α 2 against hv graph on the hv-axis using a linear extrapolation technique. Fig. 12(a-c) shows α 2 versus hν plot of ZnS films deposited from S6, S7, S8, S9 and S1 for 2 and 2.5 h. The ZnS films from S7, S8 and S9 has a steeper absorption feature, indicating good homogeneity in the shape and size of the grains and lower defect density near the band edge [47]. In other words, the quality of ZnS films was improved when co-deposition method was used. The band gaps of films deposited from S6, S7, S8, S9 and S1 for 2 or 2.5 h range from 3.88 to 3.98 eV or from 3.88 to 3.95 eV, respectively, which is consistent with the literature[28, 44, 53, 69]. In addition, as shown in Fig. 11(c), when X2 varies from 0.33 to 0.5, the change trend of Eg from S7 is close to that of Eg from S8 with deposition time increasing from 2 to 2.5 h. When X2 ranges from 0.67 to 1, the change trend of Eg from S9 is close to that of Eg from S1 with increasing deposition time from 2 to 2.5 h.
 | Fig. 12. Plot of α 2 versus hν for ZnS thin films deposited from S6, S7, S8, S9 and S1 for (a) 2 and (b) 2.5 h, (c) Eg of ZnS thin films deposited for 2 and 2.5 h. |
According to our previous[36] and this work (as shown in Table 5 and Fig. 13), the films deposited from Zn(CH3COO)2-Zn(NO3)2 are formed via cluster by cluster deposition process, and the films deposited from Zn(CH3COO)2-ZnSO4 or Zn(NO3)2-ZnSO4 are formed via ion by ion deposition. However, the films deposited from Zn(NO3)2-ZnSO4 cannot cover the substrate completely when X3 ranges from 0.33 to 0.5. It means that the anion from zinc salt strongly influences ZnS growth mechanism and results in the difference of surface morphologies of CBD-ZnS film.
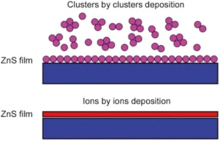 | Fig. 13. Schematic for cluster by cluster and ion by ion deposition of ZnS thin film. |
The reasons are as follows: the anions from Zn(CH3COO)2, Zn(NO3)2, and ZnSO4 have two, three and four oxygen atoms, respectively, which lead to the difference in shape, charge density, polarity and symmetry of the anions. When these anions are present in reaction bath, they can form different solution effects and bonds between reaction and non-reaction ions, such as intermolecular hydrogen bond between N-H from complex precursor ion (reaction ion) and O from NO3-, CH3COO- and SO42- (non-reaction ion). At the same time, because the direction and intensity of the interaction between the anion and complex precursor ion, diffusion rate and direction, and adsorption of complex precursor ion are different, these factors strongly influence the diffusion/adsorption of complex precursor ion toward/on substrate, which lead to different deposition mechanisms and growth rates. According to the results of the experiment, the addition of Zn(NO3)2 or/and Zn(CH3COO)2 (especially X1 ranging from 0 to 0.5) is favorable for homogeneous deposition, excessive clusters are easily adsorbed on the substrate, and consequently heterogeneous reaction is impeded significantly. However, when SO42- is present in reaction bath, heterogeneous deposition takes place regardless of the concentration ratios of SO42-. In other words, NO3- and CH3COO- are not favorable for the adsorption of complex precursor ion onto the substrate, and NO3- affects the number of heterogeneous nucleation on the substrate surface. It should be pointed out that though CH3COO- is not favorable for the adsorption of complex precursor ion onto the substrate, but favors the diffusion of complex precursor ion, and leads to the increase of the growth rate of the films. Meanwhile, CH3COO- can improve adhesion strengths between ZnS films and substrate when CH3COO- and SO42- co-exist in reaction bath. It is obvious that SO42- is favorable for the adsorption of complex precursor ion onto the substrate. It means that SO42- could potentially act as a bridging anion between complex precursor ion and substrate, and favors surface binding of complex precursor ion onto substrate, which leads to the ion by ion deposition.
From the discussion above, when two anions were added to the reaction bath simultaneously, they can play different roles in the deposition. Meanwhile, the deposition mechanism, pH, growth rate and physical properties of ZnS thin films are efficiently controlled by using different zinc salts or adjusting the concentration ratios of different zinc salts. In other words, the addition of different anions is a research line in the deposition of CBD-ZnS.
The optimum growth conditions of ZnS thin film deposited from single zinc salt have been investigated in our previous work. In this case, two zinc salts used to co-deposit ZnS thin film can further improve the quality of ZnS film. Like the concentrations of reagents, pH values, complexing agents, deposition temperature and time, and annealing treatment, the anion from zinc salt plays a key role in the ZnS growth and governs deposition mechanism. For example, when ZnSO4 is present in the reaction bath, the growth mechanism of ZnS thin films is ion by ion deposition regardless of concentration ratios of ZnSO4. On the contrary, the growth mechanism of ZnS films is cluster by cluster deposition when ZnSO4 is not present. ZnS thin films deposited from Zn(CH3COO)2-ZnSO4 are more homogeneous and compact, and the growth rate and adhesion strengths of ZnS films increase from 0.88 to 2.13 nm/min and from 13.3 to 30.6 N, respectively. The average transmission of the films from Zn(CH3COO)2-ZnSO4 is greater than 85% in the visible region. The band gaps of films deposited from S6, S7, S8, S9 and S1 for 2 and 2.5 h range from 3.88 to 3.98 eV. In this work, the deposition mechanism, growth rate and physical properties of ZnS thin films are efficiently controlled by employing different zinc salts or adjusting the concentration ratios of different zinc salts, and the addition of different anions is a research line in studies.
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 50963003), the Natural Science Foundation of Jiangxi Province (No. 2010GZC0044), the Foundation of Jiangxi Educational Commission (No. GJJ14558), and the Project of Jiangxi Youth Scientist (No. 20122BCB23031).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|