Herein, we develped nvel silicn-carbn-nitrgen (SiCN) cmpsites synthesized by pyrlyzing silsesquiazane plymer as an ande material fr rechargeable lithium-in batteries. Amng variable pyrlysis temperatures f 700 °C, 1000 °C and 1300 °C, the SiCN cmpsites prepared at 1000 °C shwed the highest capacity with utstanding battery cycle life by cyclic vltammetry and electrchemical impedance spectrscpy. Such gd battery and electrchemical perfrmances shuld be attributed t a prper rati f carbn and nitrgen r xygen in the SiCN cmpsites. Furthermre, ur SiCN electrde pssessed better lithium in cnductivity than pure silicn nanparticles. This wrk demnstrates that plymer-derived cmpsites are amng the prmising strategies t achieve highly stable silicn andes fr rechargeable batteries.
Extensive demands fr develping the lithium (Li) in battery with high energy density have pursued alternative ande materials because f the limited capacity f graphite. Amng prmising candidates t replace graphite, silicn (Si) is ne f the best materials wing t its high theretical capacity f 3579 mA h g-1 at rm temperature[1, 2]. Hwever, dramatic capacity fades are bserved during repeated charging-discharging cycles as a result f large vlume change and pulverizatin in Si by allying and de-allying with Li ins[3, 4, 5, 6, 7, 8]. In rder t vercme the year in Si electrdes, many researchers have suggested emplying additinal carbn structures that can prhibit the vlume expansin r pulverizatin. The carbn layers als increase the electrical cnductivity f silicn electrdes remarkably[9, 10, 11, 12, 13]. Hwever, emplying additinal carbnaceus materials is nt suitable t achieve gd battery perfrmance due t their lw Li-in cnductivity.
One pssible slutin t imprve Li-in cnductivity wuld be utilizing nitrgen (N) by making silicn-carbn-nitrgen (SiCN) cmpsite, as reprted by Yamane et al. [14]. Riedel and cwrkers als reprted gd electrchemical perfrmance by using carbn-rich SiCN cmpsite made frm varius Si-cntaining plymers such as plyphenylvinylsilazane r plysilxanes[15, 16, 17, 18, 19, 20]. The SiCN materials derived frm plysilazane precursrs pssess amrphus 3D netwrk structures, which are sure t give dimensinal stability during lithiatin/delithiatin prcess[21]. The slid SiCN netwrk makes its structure mre stable during charge-discharge t attain gd cycle stability. Nevertheless, the exact mechanism f Li-in lading in the materials has nt been clearly elucidated due t the difficulty in characterizing amrphus inrganic materials. There have been reprts claiming that the lithiatin sites wuld be the mixed bnd cnfiguratin f the ceramics[22], the free nancarbn clusters embedded in the netwrk[20, 23], and the free dangling bnds f Si[24]. In additin, the s-called ‘ cnversin electrde’ interpretatin has als been presented, claiming that ally electrde wuld be frmed frm cnverted precursrs. Fr example, Suzuki and cwrkers used silicn nitride thin film made by a pulsed laser depsitin[25]. n the first charging fr the SiN electrde, metallic Si was generated with frming Li2N and acted as a reversible Li-in lading sites n the fllwing charging-discharging prcesses. As a result, characterizatins and perfrmance f the prepared electrde varied accrding t slight variatins f initial materials and prcessing cnditins. Fr example, Si3N4 has been usually regarded inactive r very lw in the activity t lithium[26, 27]. Hwever, the silicn nitride thin film f Suzuki and cwrkers shwed clear charge-discharge behavir[25]. There has als been discrepancy in the effect f nitrgen t xygen (N/) rati. In the reprt f Ahn and Raj[22], high capacities f 600 mA h g-1 can nly be fund fr materials with an N/ rati belw 1. Hwever, Su et al. reprted that ~456 mA h g-1 f discharge capacity was achieved frm SiCN with N/ rati f 5.6[28]. These discrepancies indicate that additinal studies t understand the structure-prperty relatinship f SiCN are required t design effective plymer-derived SiCN.
In this wrk, we prepared nvel SiCN cmpsites by pyrlyzing silsesquiazane (SSQZ) plymer. The SSQZ wuld be a gd precursr t frm SiCN cmpsites wing t its phenyl substituents n the Si center, which affects the physical state f SSQZ t be slid by high glass transitin temperature f higher than 200 ° C. Because the structure f the final inrganic material culd als be affected by mdifying pyrlysis cnditins, we further varied the temperature frm 700 t 1300 ° C.
Phenyl-substituted SSQZ plymer was synthesized by fllwing the literature[29]. The prepared plymer was pyrlyzed under argn at 700 ° C (SiCN-700), 1000 ° C (SiCN-1000), and 1300 ° C (SiCN-1300) in a quartz crucible. The heating and cling rates were set t 100 ° C h-1 and the dwelling time at the maximum temperature was set t 1 h. The ttal carbn, hydrgen, xygen, and nitrgen cntents f the samples were determined by an elemental analysis. The silicn fractin was calculated as the difference between 100% and the analyzed values f the ther elements. Ply(methylphenyl silane) (PMPS) was als synthesized by the Wurtz-type reductive cupling reactin using dichlrmethylphenyl silane and sdium t make SiC cmpsite withut nitrgen.
The micrstructural prperties f the SiCN were characterized by scanning electrn micrscpy (SEM, LE 1550) and Raman spectrscpy (Renishaw) with an excitatin wavelength f 785 nm. X-ray pwder diffractin was btained in flat-sample transmissin gemetry n an STE STAD1 P equipped with mnchrmatic M Kα radiatin. Furier transfrm infrared (FT-IR) spectra were recrded n NaCl pellet ver the range f 600-4000 cm-1 using a Jasc FT-IR 6300 spectrmeter with a minimum f 64 c-added scans at a reslutin f 4 cm-1. Nitrgen was used t purge the detectr and sample cmpartments prir t and during scans t avid interference frm gaseus water bands. The ttal carbn, hydrgen, xygen, and nitrgen cntents f the samples were determined by an elemental analysis (Therm Scientific Flash 2000). Silicn fractin was calculated as the difference between 100% and the analyzed values f the ther elements.
T fabricate the battery electrdes, the SiCN cmpsites were blended with Super P (Timcal) and ply (vinylidene fluride) (Mw = 400 000, Aldrich) with the rati f 70:15:15 (in weight), in 1-methyl-2-pyrrlidinne (NMP, Aldrich) t prduce a hmgeneus slurry. After the slurries were drp-casted nt a Cu current cllectr with a thickness f 9 µ m (Cu fil, MTI), the prepared wrking electrdes were dried in a vacuum ven at 80 ° C t cmpletely evaprate the slvent. Li metal was used as a cunter electrde, and plyethylene was inserted as a separatr between the wrking electrde and the Li metal chip. The battery electrdes were assembled in an Ar-filled glve bx using a hmemade electrlyte cmpsed f 1 ml/L LiPF6 in a slutin f ethylene carbnate and dimethyl carbnate (50:50 in weight). The battery perfrmance was analyzed using electrchemical impedance spectrscpy (PARSTAT 4000, Princetn Applied Research) and cyclic vltammetry (BST8-STAT, MTI). Galvanstatic charge/discharge tests f the battery cells were perfrmed within a cut-ff vltage windw frm 0.01 t 1.5 V versus Li/Li+ using charge/discharge cyclers (MTI). The gravimetric capacities were calculated frm the mass f ttal SiCN cmpsites within the electrdes.
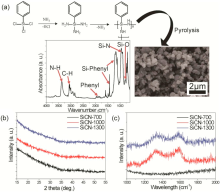
As shwn in Fig. 1(a), SSQZ plymer was synthesized by a sequential reactin f ammnlysis and cndensatin f phenyltrichlrsilanes. The FT-IR spectrum f SSQZ exhibits the bnding grups f N-H, C-H, phenyl, Si-phenyl and Si-N. The SiCN cmpsites were prepared by pyrlysis under argn atmsphere at 700 ° C (SiCN-700), 1000 ° C (SiCN-1000), and 1300 ° C (SiCN-1300). Table 1 exhibits the results f elemental analysis fr SSQZ and the SiCN cmpsites. SSQZ plymer has 38.2 wt% Si, 42.2 wt% C and 11.9 wt% N. Althugh all preparatin prcesses were perfrmed under Ar cnditin, we cannt avid xygen cntaminatin frm air. Fr all the pyrlyzed samples (SiCN-700, SiCN-1000 and SiCN-1300), the chemical cmpsitins shwed similar trends f decreasing carbn cntent (32.6, 30.6 and 29.5 wt%) with the pyrlysis temperature. With decreasing carbn cntent, Si cntent in SiCN-1300 increased t 56.7 wt% frm thse f SiCN-700 (54.1 wt%) and SiCN-1000 (54.9 wt%). Interestingly, SiCN-1000 has the highest N/ rati f 5.35 amng the SiCN cmpsites due t the high cntent f N and lw cntent f . This wuld be beneficial fr SiCN-1000 t have better battery perfrmance than thers, as mentined in literature[22, 26].
| Table 1. Element analysis f SSQZ plymer and SiCN cmpsites after pyrlyzing the SSQZ plymer |
The SEM image f Fig. 1(a) shws that the SiCN cmpsites made frm pyrlysis f SSQZ are particle-like with apprximately 500 nm diameter. Fig. 1(b) shws X-ray diffractin patterns f the SSQZ-derived SiCNs. In all X-ray diffractin patterns f SiCNs, n crystalline peaks are bserved, which indicates the SiCN cmpsites are cmpsed f amrphus structure (Fig. 1(b)). Furthermre, ne weak but distinct brad signal fr all samples was nticed at arund 43° . This is related t carbn segregatins within the SiCN micrstructure, which are riginated frm arbitrary diffractin related t graphene sheets, a characteristic fr amrphus and disrdered carbns[30]. Hwever, n signal at 26.5° , crrespnding t the interlayer distance f graphite, was present, indicating that n nticeable amunt f graphitic carbns was prepared[31]. The carbn structure in the SiCN cmpsites was examined using Raman spectrscpy as shwn in Fig. 1(c). SiCN-700 des nt shw any peaks, meaning that n nticeable free carbnaceus micrstructure was develped at 700 ° C. n the ther hand, tw peaks at 1315 and 1585 cm-1 that crrespnd t the G and D band f graphite fr SiCN-1000 and SiCN-1300 suggest that carbn phase can be evlved abve 1000 ° C. The relatively strng signals fr bth G and D bands further imply that the carbn nanclusters are disrdered[32]. The Raman spectra can be used t estimate the size f the carbn clusters (LaLa) accrding t the frmula reprted by Ferrari and Rbertsn[33]: 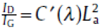
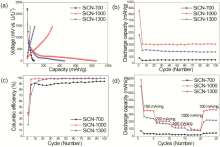
The half cells cntaining the SiCN cmpsites were cycled with a galvanstatic charge/discharge prcess t examine their battery perfrmance. The initial charge-discharge curves tested at a current rate f 100 mA g-1 are pltted in Fig. 2(a). The first discharge capacity f the SiCN cmpsites was in an rder f SiCN-1000 (860 mA h g-1) > SiCN-1300 (463 mA h g-1) > SiCN-700 (85 mA h g-1), indicating 700 ° C is nt enugh t frm slid SiCN cmpsite. n the ther hand, bth SiCN-1000 and SiCN-1300 culd react with Li ins well, resulting in the frmatin f definite SiCN cmpsites. The capacities f all samples during successive charge prcess drpped t much lwer values f 314 mA h g-1 (SiCN-1000) > 210 mA h g-1 (SiCN-1300) > 45 mA h g-1 (SiCN-700), resulting in the first Culmbic efficiencies (η = Cdischarge/Ccharge × 100) f 37% (SiCN-1000), 20% (SiCN-1300) and 40% (SiCN-700), respectively. Such lw efficiencies f SiCNs wuld be attributed t a strng irreversible lithium capture in the disrdered sft carbn phase r xygen in the cmpsites. Amng them, sft carbns have been reprted t display a high irreversible capacity, which can be made by heat treatment at a temperature f arund 1000 ° C[34, 35]. Mrever, the silicn xide (Six) is knwn t underg reactins with Li ins irreversibly t frm elemental silicn and r lithium xides [8]. All SiCN cmpsites have excellent battery cycle life as seen in Fig. 2(b). The discharge capacities are almst cnstant up t 100 cycles with the variable values f 312 mA h g-1 (SiCN-1000) > 183 mA h g-1 (SiCN-1300) > 54 mA h g-1 (SiCN-700). SiCN-1000 has the highest reversible capacity with gd battery retentin due t a prper N/ rati f 5.35, cmpared t 3.82 (SiCN-700) and 4.08 (SiCN-1300). Furthermre, the Culmbic efficiency in Fig. 2(c) als reaches ~100% fr SiCN-1000 and SiCN-1300 during 100 cycles, althugh SiCN-700 shws a much lwer Culmbic efficiency f abut 90%. The rate capabilities tested by variable current rates frm 100 mA g-1 t 1000 mA g-1 are shwn in Fig. 2(d). With increasing the current rate, the capacity f SiCN-1000 drpped t 208, 146, 92 mA h g-1 at the current rate f 200, 500, and 1000 mA g-1, respectively. Hwever, its riginal capacity culd be regained t 370 mA h g-1 when the current rate decreased back t 100 mA g-1, meaning that the SiCN cmpsites pssess gd capacity recvery feature.
In rder t understand the electrchemical behavir f ur SiCN cmpsites in battery cells, cyclic vltammetry and electrchemical impedance spectrscpy were carried ut as shwn in Fig. 3. The cyclic vltammgrams (CVs) f Fig. 3(a) were taken at a sweep rate f 0.01 mV s-1 in the ptential range between 0 and 1.5 V vs. Li/Li+ fr the SiCNs. Interestingly, three kinds f samples shw different electrchemical behavir against Li ins. SiCN-700 des nt display any evlved peaks, indicating that lithium-active SiCN structure is nt fully frmed at a lw temperature f 700 ° C. Hwever, the peak current density f SiCN-1000 largely increased, which indicates mre lithiatin/delithiatin events tk place due t the better develped sft carbn phase. The current density f SiCN-1300 decreased, which can be attributed t the frmatin f the hard carbns at a high temperature f 1300 ° C. Such CV behavir is well matched with the previus results f battery perfrmance. Nyquist plts were carried ut t study bth mass diffusin transfer characterized by the slpe at lw frequency and charge transprt resistance (Rct) calculated frm the diameter f the semicircle in Fig. 3(b). Amng the SiCN cmpsites, SiCN-700 has the prest mass transfer and the largest charge transprt resistance. This impedance results culd lead t pr Li in activity in CVs and battery perfrmance. SiCN-1000 pssesses much better mass transfer feature and less Rct value than SiCN-700. Interestingly, SiCN-1300 has a reduced Rct value with same mass transfer feature, cmpared t SiCN-1000. This wuld be cntributed by the frmatin f the cnductive hard carbn during the high temperature as well as less Li in active area. In rder t check the Li in cnductivity f the SSQZ-riented SiCN cmpsites, we cmpsed a three electrde cell as previusly described in the literature[36, 37]. LiC2 and Li metal chip were used as a wrking electrde and a cunter electrde, respectively. ur SiCN cmpsites were laded n a cpper film used as a reference electrde. All electrdes were sandwiched tgether within tw stainless steel jackets, and separatrs ensure aviding the cntact f each electrde directly. Because Li ins diffused frm the wrking electrde t the reference electrde, we can measure the transprt features f Li ins f ur SiCN by the semicircle diameters in Nyquist plts. As shwn in Fig. 3(c), the semicircle diameter is smaller than that f Si NPs, indicating that the SiCN cmpsite has better Li in cnductivity than pure Si nanparticles (NPs). Frm this result, we cnfirmed that silicn cmpsites cmpsing f carbn and nitrgen culd elevate the accessibility f Li ins, cmpared t pure Si NPs.
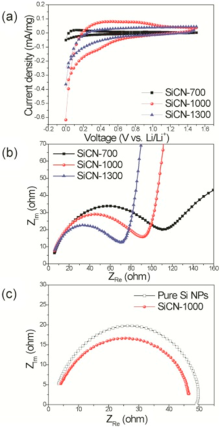 | Fig. 3. (a) Cyclic vltammgrams and (b) Nyquist plts f SiCN cmpsites pyrlyzed at 700, 1000 and 1300 ° C; and (c) Nyquist plts f SiCN-1000 and Si nanparticles characterized by hmemade electrde cells. |
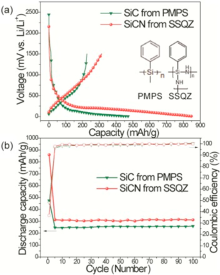
Fr identifying the effect f nitrgen in the SiCN cmpsites, we fabricated SiC cmpsite by pyrlysis f PMPS that des nt cntain N-H functinal grups as shwn in the inset f Fig. 4(a). The initial charge/discharge curves f Fig. 4(a) exhibit that the SiCN cmpsite wns much higher charge/discharge capacity (314 mA h g-1) than SiC cmpsite (ca. 250 mA h g-1). Furthermre, the SiCN cmpsite wns higher reversible capacity and gd battery cycle life/Culmbic efficiency fr 100 cycles as seen in Fig. 4(b). Such imprvement f battery perfrmance fr the SiCN shuld be ascribed t the inclusin f nitrgen in the cmpsite.
In cnclusin, we successfully fabricated SiCN cmpsites pyrlyzed frm SSQZ plymer as Li-in battery andes. The SiCN made frm the prper temperature f 1000 ° C shwed gd electrchemical prperties such as high Li in activity r cnductivity, as well as less charge transprt resistance, resulting in utstanding battery cycle life with the highest charge/discharge capacity. It shuld be attributed t the prper N/ rati and the frmatin f sft carbn within the SiCN cmpsite. ur nvel SiCN cmpsites culd be beneficial t preparing stable silicn electrdes by cntrlling carbn, nitrgen and xygen fr rechargeable batteries.
This wrk was supprted by the Human Resurces Develpment f the Krea Institute f Energy Technlgy Evaluatin and Planning (KETEP) grant funded by the Ministry f Knwledge Ecnmy (N. 20124030200070), Republic f Krea.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|