The influence of active elements C and Hf on the interface reactions and wettability between a Ni3Al-based superalloy and the ceramic mould material was studied by using a sessile drop experiment. The microstructure of the alloy interface was investigated by scanning electron microscopy analysis and the phase identification was performed by X-ray diffraction analysis. The results show that interface reactions occur as C and Hf contents reach a critical value. The critical values for C and Hf to cause interface reactions are 0.12 wt% and 1.17 wt%, respectively. The reaction products contain HfO2 and 9Al2O3·Cr2O3. Adsorptions of Hf and interface reactions improve the wettability obviously.
Superalloys have been used for over 50 years for producing blades in aircrafts and gas turbines in hot machineries. In these applications, the components need to fulfil the condition of high quality intricate shapes, good surface finish as well as thin-walls[1, 2, 3]. Therefore, investment casting is extensively adopted as it allows dimensionally accurate components to be produced. Moreover, investment casting is a cheaper alternative than forging or machining[4, 5]. In investment casting, proper wettability is required for mould-filling of the components. Poor wettability between the liquid alloy and the ceramic mould will lower the mould-filling capability of the alloy melt[6]. However, if the alloy melt well wet the ceramic mould, infiltrations of the alloy melt through the capillaries on the ceramic mould surface will occur[7]. Moreover, interface reactions between the alloy melt and the ceramic material are prone to take place under the condition of good wettability, which produce new compounds at the interface and lead to metal contamination and defect formation on the surface of the components[8, 9]. In order to minimize the formation of surface defects and to improve the surface quality of the castings, knowledge is required about the wettability and interactions between the alloy melt and the ceramic mould materials.
C plays an important role in strengthening grain boundaries by precipitating carbides in superalloys. The addition of Hf in superalloys aims to enlarge the solidification range and to improve the creep properties of the alloy. However, some report on the interface reactions between superalloys and ceramic mould has indicated that C, Hf as well as Cr and Al are the active elements that will cause alloy-mould interactions[10, 11, 12, 13]. Li et al.[10] has reported that Cr and Hf accelerate interface reactions at high temperature and the reaction products are mainly composed of HfO2 and Al2O3. Zheng et al.[11]pointed out that Cr, Al and Ti in superalloys reacted with silicon oxide, forming Cr2O3 and inducing some metallic nodular protrusions on the surface of alloy. Liu et al.[12] has also reported that Cr could react with SiO2 to form Cr2O3 on the alloy surface. However, HfO2and Cr2O3 are not found in our former experiments with minor contents of Hf and C. On the other hand, information is not available on the effect of interfacial reactions on the wettability between superalloy and ceramic mould. Valenza et al.[13] has studied the wettability and interface reactions of three superalloys on different ceramic substrates (sapphire, polycrystalline alumina, zirconia and mullite). They found that the compositions of the alloy and the ceramic both have an influence on the wettability. There is hardly any other relevant work on how interface reactions influence the wettability between superalloy melt and the ceramic mould material.
The aim of the present work is to investigate the interface reactions between ceramic mould material and a typical intermetallic superalloy by taking into account the effect of C and Hf contents. Particular attention is paid to figure out critical values of C and Hf contents that would cause interface reactions and to clarify the reaction mechanism. The effect of the interface reactions on the wettability is also discussed.
Nine superalloys (Alloys 1-9) with different contents of C and Hf were prepared by an induction furnace. The major elements of the alloy (mass fraction, %) were Cr 4.37, Co 8.92, W 7.5, Mo 2.02, Al 5.72, Nb 1.05, Ta 6.7, Re 2.02 and Ni in balance. C and Hf contents in Alloys 1-9 are listed in Table 1. Experimental alloys were cut into 5 mm × 5 mm × 5 mm cubic samples, which were polished to remove the oxides on the surface.
| Table.1 Contents of C and Hf in the alloys (wt%) |
Ceramic shells were made by the standard procedure for preparation of ceramic moulds in the investment casting. Firstly, a wax pattern was prepared by injecting molten wax into a metallic ‘ master’ mould. Secondly, the wax pattern was dipped into the primary slurry, sprinkled with refractory stuccos and dried. The primary slurry was prepared from a liquid binder and fine mesh refractory filler. The liquid binder was colloidal silica with 30 wt% SiO2. The refractory filler consisted of 95 wt% Al2O3 powder and 5 wt% SiO2 powder. Alumina (Al2O3) sand was used as stuccos. When the primary coat was air dried to finish gelling of binder, the assembly was systematically dipped into the secondary slurry and stuccoed. The dipping-drying process continued for four times until the thickness of the ceramic increased to about 6 mm. Thirdly, the wax pattern was melted out by using a steam autoclave, leaving a hollow shell and then fired. Ceramic substrates with dimensions of 20 mm in length, 20 mm in width and 6 mm in thickness were cut from the ceramic shells. Before experiments, the alloy samples and the ceramic substrates were ultrasonically cleaned in acetone.
Sessile drop experiments were carried out in a vacuum induction directional solidification furnace. The ceramic substrate was placed in the middle of the furnace and adjusted to a horizontal position. The alloy was then placed on the ceramic substrate. Subsequently, the furnace was evacuated to about 0.01 Pa and then heated at a rate of 20 K min-1 to 1550 ° C to melt the alloy. After a dwell time of 20 min at 1550 ° C, the alloy-ceramic couples were drawn from the furnace and cooled down to room temperature. The alloy melt formed regular droplets after solidification. The wetting angle was then calculated according to the geometric formula θ = 2arctan(2h/d) with an accuracy of ± 2° , where h is the drop height and d is the base diameter of the alloy drop.
The alloy drops were photographed to show the residues at the bottom. Afterwards, they were cut into two parts perpendicular to the interface. One part was embedded by resin and polished for microstructure observation using a scanning electron microscope (SEM, JMS-6301F, Japan) equipped with energy dispersive spectroscopy (EDS) analysis. Another part was used for phase identification by X-ray diffraction (XRD, D/Max 2500PC, Japan).
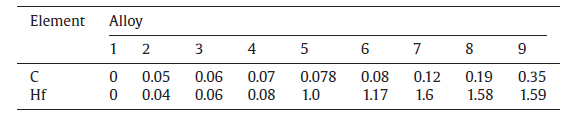
Fig.1 shows the under view images of each solidified alloy drop. There are no sand adhesion or reaction products on the bottom of alloys without C and Hf or with minor C and Hf contents (Fig.1(a and b)). With increasing contents of C and Hf up to 0.06 wt%, sand adhesions take place, just as seen in Fig.1(c-e). When Hf content exceeds 1.17 wt%, some white products are observed on the bottom of the alloys, as seen in Fig.1(f-i). In addition, when C contents exceed 0.12 wt%, purple reaction products are observed, as shown in Fig.1(g-i). These results indicate that sand adhesion and interface reactions are closely related to C and Hf contents in the alloy.
 | Fig.1 Under view images of solidified alloy drops 1-9: (a) Alloy 1, (b) Alloy 2, (c) Alloy 3, (d) Alloy 4, (e) Alloy 5, (f) Alloy 6, (g) Alloy 7, (h) Alloy 8 and (i) Alloy 9. |
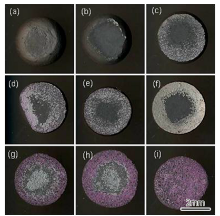
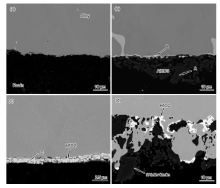
Fig.2 shows the typical SEM images of the interface for alloys with distinct characteristics. It can be seen that the interface is flat and there are no sand adhesion and reaction layers for the alloys without C and Hf addition (Fig.2(a)). But an approximately 20-µ m-thick sand adhesion layer appears at the interface for the alloy with 0.078% C and 1% Hf (Fig.2(b)). EDS analysis indicates that Al and O are the two dominant elements, which suggests that the sand adhesion layer is Al2O3 stripped from the ceramic surface. Besides the sand adhesion layer, a thin white layer is also observed at the interface and this layer is rich in Al, O, Ni, Hf, Cr and Co. For the sample with 0.08% C and 1.17% Hf, a continuous layer of white product is formed at the interface (Fig.2(c)). EDS analysis shows that this layer mainly corresponds to HfO2, and XRD analysis further confirms it (Fig.3(a)). Besides HfO2, the remained peaks correspond to Ni in the alloy matrix. For the sample with 0.12 wt% C and 1.6 wt% Hf, a double layer of reaction products are formed at the interface (Fig.2(d)). The inner layer is also HfO2, and XRD analysis shows that the main constituents of the outer layer are 9Al2O3· Cr2O3, Al2O3 and 3Al2O3· 2SiO2 (Fig.3(b)). 3Al2O3· 2SiO2 is the reaction product between Al2O3 and SiO2 in the substrate. As there is no Cr2O3 in the ceramic substrate, the formation of Cr2O3 may be correlated with the interface reactions between the ceramic substrate and the alloy melt.
 | Fig.2 Typical interface images of alloy drops with different contents of C and Hf (wt%): (a) 0 C and 0 Hf, (b) 0.078 C and 1.0 Hf, (c) 0.08 C and 1.17 Hf and (d) 0.12 C and 1.6 Hf. |
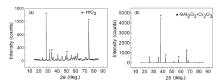 | Fig.3 XRD patterns for the interface reaction products obtained on the bottom: (a) Alloy 6, (b) Alloy 7. |
From the above results, it is obvious that the contents of C and Hf in the alloys play an important role in the interface reactions. When C and Hf contents are minor, no sand adhesion is observed. With increasing contents of C and Hf (Alloys 3-5), a sand adhesion layer appears on the alloy bottom but no interface reaction products are observed. It has been reported that during the cooling procedure in investment casting, the mismatch of thermal expansion coefficient between the melt alloy and the ceramic will induce stress[12, 14] and the ceramic surface materials therefore fall off and adhere to the alloy surface, forming the sand adhesion layer[12]. Moreover, there is always some dissolution of the ceramic oxides at the alloy/ceramic interface. The dissolved ceramic will adhere to the alloy or react with the liquid alloy[15]. These two mechanisms are both likely to be the main reasons for sand adhesion taking place while C and Hf contents are in a proper range.
In the case of interface reactions, it should be noted that the interface reactions occur when the contents of C and Hf reach a critical level. Meanwhile, it can be seen that HfO2and 9Al2O3⋅ Cr2O3 are the main reaction products at the interface. The formation of these oxides is correlated with the substitution reactions at the interface. Hf is reported to react with SiO2 following the equation Hf + SiO2 (s) → HfO2 (s) + (Si)[10]. Thermodynamically, the change in the Gibbs free energy for the reaction could be written as[16, 17]
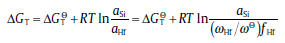 (1)
(1)
where
| Table.2 Activities of C and Hf in the alloys at 1550 ° C |
| Table.3 Wetting angle (deg.) of the alloys on the ceramic substrates |
According to previous works[19, 20], the addition of active elements such as Ti, Cr, Zr, Nb and Hf in the alloy will improve the wettability. In our experiments, Hf is the main element that improves wettability in the non-reactive wetting systems. There are many capillaries on the ceramic mould surface and the capillary force P is written as
In summary, the effect of C and Hf contents on the interface reactions and wettability between a Ni3Al-based superalloy and ceramic mould materials was investigated in detail. The activities of C and Hf in the alloys were calculated and the critical values of C and Hf contents that will cause interface reactions are proposed. The main conclusions are as follows:
(1)The critical values of C and Hf that will cause interface reactions are 0.12 wt% and 1.17 wt%, respectively.
(2)When the content of active element is lower than the critical value, sand adhesion takes place on the alloy surface.
(3)HfO2 layers form at the interface when Hf content exceeds 1.17 wt% and 9Al2O3· Cr2O3 forms as C content reaches 0.12 wt%.(4)The wetting angle decreases from 133° to 87° when C content increases from 0 to 0.35 wt% and Hf content increases from 0 to 1.59 wt%.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|