The flexible transparent conductive films (FTCFs) of silver nanowire-polyethylene terephthalate (AgNW-PET) were prepared by a facile method including vacuum filtration and mold transferring. The effect of silver nanowire weight density on the optical and electrical properties of films, as well as the electrical percolation was investigated. The obtained typical AgNW-PET film exhibited high figure of merit of 31.3 × 10-3 Ω-1 with low sheet resistance of 4.95 Ω sq-1 and high transparency at 550 nm of 83.0% (excluding PET substrate). The resulting FTCFs based on PET substrate with high transmittance and low sheet resistance have a great potential in the application of high-performance flexible electronics and photovoltaic devices.
Flexible transparent conductive films (FTCFs), which incorporate advantages such as lighter weight, higher shock resistance, flexible stock, and roll-to-roll processing, have shown great promise in flexible electronics such as solar cells, organic light-emitting diodes, and flexible displays[1, 2, 3, 4]. As most of the substrates used in FTCFs are polymer materials and cannot withstand the high temperature compared to rigid glass substrate, it is difficult to deposit high quality commonly used doped metal oxide. Many alternative transparent conductive materials, such as carbon nanotubes, graphenes, and metal nanowires, have been widely studied[5, 6, 7]. Among these materials, silver nanowires (AgNWs) have attracted increasing interest due to their high electrical conductivity and unique optical property[8, 9]. Recently, many works have been done to fabricate silver nanowire to FTCF with different flexible substrate to obtain high conductivity and transparency[10, 11, 12, 13].
Herein, we report fabrication of FTCFs with silver nanowires by vacuum filtration and PET mold transfer. The optical and electrical properties of FTCFs with different weight densities of silver nanowires were investigated. The FTCF possessing high figure of merit (Φ = 31.3 × 10-3 Ω -1) with a low sheet resistance (Rs = 4.95 Ω sq-1) and high transparency (T = 83.0% at λ = 550 nm) was obtained. The electrical percolation of AgNW films on PET substrate was studied as well.
Silver nitrate (AgNO3), poly (vinylpyrrolidone) (PVP, K-30), sodium chloride (NaCl), ethylene glycol (EG) and acetone were purchased from Sinopharm Chemical Reagent Co., Ltd. All reagents were of analytical grade and used without further purification.
AgNWs were synthesized by a modified polyol reduction process[14]. In a typical procedure, 150 ml of 0.15 mol/l PVP EG solution in a three-neck flask was heated to 165 ° C for 1 h under continuous magnetic stirring. Then, 120 µ l of 0.1 mol/l NaCl EG solution was added to the flask. After 10 min, 150 ml of 0.1 mol/l AgNO3 EG solution was injected to the flask at a rate of 5 ml/min. The obtained solution was cooled naturally to room temperature after 20 min, filtered through 0.8 µ m pore-size filter membrane, and diluted with deionized water (3 times by volume). The AgNWs collected on filter membrane were redispersed in deionized water by sonication. Finally, the silver nanowire solution with a concentration of 0.04 mg/ml in deionized water was obtained.
Fig.1(a) shows the fabrication process of FTCFs with AgNWs on PET substrate. The AgNW film was obtained by vacuum filtration using 0.8 µ m average pore size filter membrane, and then transferred to a flexible substrate by direct PET molding. The PET film was heated at 100 ° C for about 4 h while the filter membrane with the AgNWs was placed on the surface. After cooling to room temperature, the filter membrane was removed forming a silver nanowire film on the PET surface. It can be seen from photograph of FTCFs with different silver nanowire contents in Fig.1(b) that the fabrication process produced optically uniform and transparent, flexible PET with the area of AgNWs coverage defined by the filter membrane diameter (~4 cm). The weight density of the AgNW networks (weight of AgNWs per square meter) on PET films was controlled by adjusting the volume of AgNWs solution during filtering.
The surface morphology of AgNW-PET films was examined by a field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Japan). The optical transmittance of the AgNW/MC films was obtained using a UV-Vis-NIR spectrophotometer (Perkin-Elmer, Lambda 950, USA) with an integrating sphere. The sheet resistance was measured by a four-point probe system (Napson Corp. Cresbox).
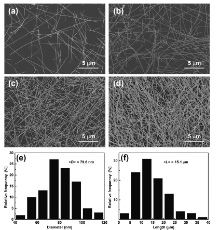
As shown in the photograph of Fig.1(b), the films were transparent and the logo of NIMTE was clearly visible when the density was below 60 mg m-2. Contrarily, the films were opaque and the logo of NIMTE was vague when the density of AgNW was above 200 mg m-2. Fig.2(a-d) shows the microstructure of FTCFs surface using SEM analysis of AgNW film on PET substrate. The AgNWs were distributed on the PET film uniformly. As the density of AgNWs increased, the AgNW-uncovered space of PET film became narrower, which led to the reducing of the transparence of FTCFs. Fig.2(e, f) shows the distributions of diameter and length by measuring 100 nanowires on the substrate. The silver nanowires present a relatively high aspect ratio (approximately 190) with an average length of 15.1 µ m and average diameter of 79.6 nm.
The transmission spectra of FTCFs with different AgNW weight densities are shown inFig.3. The absorption near 380 nm exhibited a surface plasmon resonance of silver nanowires[15, 16]. As the reflecting and scattering light by the AgNWs, the transmittance of films decreased with increasing AgNW weight density. Fig.3(b) shows the difference of the transmittance at a fixed wavelength (550 nm) with AgNW weight density. With the weight density of 16 mg m-2, the transmittance of the FTCF is slightly reduced from 87.5% (for pure PET film) to 82.3%. As the AgNW weight density increased to 80 mg m-2, the film became semi-transparent, with the transmittance of 65.6%. The inverse linear relation between transmittance of films and the AgNW weight density is quite similar to the results discussed elsewhere[17].
Fig.4 shows the electrical sheet resistance of the FTCFs as a function of the nanowire weight density at room temperature. With the AgNW increased from 16 to 60 mg m-2, the sheet resistance of FTCF was reduced from 177.3 to 4.95 Ω sq-1 quickly. As the AgNW weight density increased from 100 mg m-2 to 400 mg m-2, the sheet resistance of the film decreased from 1.9 to 0.56 Ω sq-1 slowly.
 | Fig.4 Sheet resistance of FTCF with various AgNW weight densities. Inset: fit of the experimental data to the percolation equation. |
The relationship between the experimentally determined sheet resistivity Rs and the area fraction of AgNW Af in films can be expressed as[18]:
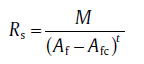 (1)
(1)
where Afc is the critical area fraction of silver nanowires in FTCF, t is the conductivity exponent, and M is a constant. The area fraction of AgNW Af can be calculated based on the following equation[19]:
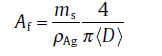 (2)
(2)
where ms is the weight density of AgNWs and ρ Ag is the density of silver. < D> is the average diameter of AgNWs, which was confirmed to be 79.6 nm in this report with SEM analysis. As shown in Fig.4 (Inset), the experiment data were fitted well with R2 of 0.999 and Pearson's r of -0.999. The critical area fraction Afc was found to be 1.90%, which was in good agreement with the theoretical value (1.95%) from Excluded Volume Model[20]. The conductivity exponent was extracted as t = 1.37, which was in agreement with theoretical value of 1.3 for the two-dimensional nanowires network [19].
The figure of merit (Φ ) is usually used to describe the overall performance of transparent conductive film, which can be expressed as [16]:
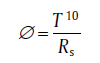 (3)
(3)
where T is the transmittance excluding PET at a wavelength of 550 nm and Rs is the sheet resistance of the film. According to Table 1, AgNWs played a leading role in the electrical property of the film when the weight density of AgNWs is lower, which improved the figure of merit of our FTCFs. As the weight density of AgNWs became much higher, the transmittance of the films decreased rapidly, which resulted in a poor figure of merit. The maximum figure of merit was obtained to be 31.3 × 10-3 Ω -1 when AgNW weight density was 60 mg m-2 with T550nm (excluding PET substrate) of 83.0% and sheet resistance of 4.95 Ω sq-1. This result was higher than what previous literature reported[21, 22].
| Table.1 Comparison of the electrical and optical properties of FTCF with different AgNW weight densities |
In summary, we fabricated flexible transparent conductive PET film with silver nanowires using vacuum filtration and mold transfer. The effect of silver nanowire weight density to the optical and electrical properties of films was investigated. The obtained percolation threshold and conductivity exponent demonstrated the TFCFs in our experiment possess two-dimension nanowires network electrical percolation property. By adjusting the weight density of the AgNWs, a typical TFCF with an optical transmittance at 550 nm of 83.0% (excluding PET substrate), a sheet resistance of 4.95 Ω sq-1 and a figure of merit of 31.3 × 10-3 Ω -1 was obtained. The TFCF based on PET substrate with high transmittance and low sheet resistance described here has a great potential in the application of high-performance flexible electronics and photovoltaic devices.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|