Birnessite-type MnO2 (δ-MnO2) nano-sheets were successfully synthesized by an interfacial synthesis method in this work. The properties and electrochemical performance of the as-prepared δ-MnO2 were analyzed and evaluated by scanning electron microscopy (SEM), X-ray diffraction (XRD), nitrogen adsorption measurement and electrochemical tests. This facile synthesis method enables δ-MnO2 nano-sheets to show a large specific surface area (257.5 m2 g-1). The electrochemical test results show that the specific capacitance is 272 F g-1 and the specific capacitance retention is over 96.7% after 1000 cycles at a scan rate of 10 mV s-1. All results demonstrate that δ-MnO2 has a great potential application in high-performance electrochemical capacitors, and this interfacial synthesis method will be a very promising method to synthesize highly active MnO2 materials in a large scale.
Electrochemical capacitors (ECs), also known as super-capacitors, are among the most promising energy storage systems for their high power density, fast charging/discharging rate and excellent long cycle-life[1]. ECs are commonly based on either large-surface-area carbon-based materials exploiting electric double-layer capacitance (EDLC) or redox pseudo-capacitive materials taking advantage of the fast and reversible electrochemical reactions[2]. At present, carbon materials have been commercially applied as electrodes for ECs; however, their electrochemical performance cannot meet the need of rapid increase of high power density and energy density in practical use. As potential alternatives to carbon materials, many metal oxides/hydroxides have been studied and demonstrated excellent electrochemical capacitance due to their typical pseudo-capacitance charge mechanism[3, 4, 5]. Among the investigated electrode materials, tetravalent manganese oxide (MnO2) has been taken into intensive investigations as one of the most attractive electro-active materials for ECs because of its easy preparation, good environmental benign nature, low cost, and favorable pseudo-capacitive characteristics in a neutral electrolyte system[6, 7]. MnO2 possesses several different crystallographic forms, such as α -, β -, γ -, δ -, λ - and ε -type, made of MnO6 octahedron with different connectivity [8, 9, 10, 11]. Among them, δ -MnO2 has attracted great interest due to its good electrochemical capacitance behavior[8]. Moreover, it is believed generally that pseudo-capacitors store charges in the first few nanometers on the surface of electrode materials, and decreasing the particle size will increase usage of active materials. Therefore, ultrathin porous nanostructures with thickness below 10 nm and their three-dimensional assemblies have been regarded as ideal structures for improving the performance of super-capacitors[9]. By a microwave-hydrothermal method, Ming et al. synthesized δ -MnO2 with a specific area of 213.6 m2 g-1, and the as-prepared δ -MnO2shows a good capacitance performance as high as 210 F g-1[9]. Hu et al. prepared nanoporous layered δ -MnO2 thin film on stainless steel (SS) plate. The thin δ -MnO2/SS film plays a high capacitance of 447 F g-1 at 2 mV s-1, and the capacitance retention ratio of 87% is obtained after 1000 CV cycles[11]. All the above results illustrated that δ -MnO2can show a good capacitance performance and long-term electrochemical stability.
For improving the energy storage performance of MnO2 materials, δ -MnO2 nano-sheets with lamellar structure and a large surface area were synthesized by an interfacial synthesis method in this work. This is a simple one-step process without using any template or surfactant. The physical characteristics and electrochemical properties of the as-prepared δ -MnO2 were investigated thoroughly via X-ray diffraction (XRD), scanning electron microscopy (SEM), nitrogen adsorption and electrochemical measurements. Insights about the energy storage mechanism of this material are also discussed in this work.
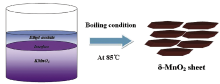
The 2D δ -MnO2 was prepared by an interfacial synthesis method, which was schematically shown in Scheme 1. For the synthesis of δ -MnO2 nano-sheets, KMnO4 and ethyl acetate were employed as raw materials. As shown in Scheme 1, an aqueous solution of KMnO4(350 mL and 20 mmol L-1) and 200 mL of ethyl acetate were put together into 1 L capacity flat-bottom flask. Thus a biphasic mixture was formed and kept on a water bath under refluxing condition at 85 ° C with continuous stirring. After complete disappearance of the pink color of KMnO4, brown MnO2 precipitate was precipitated out at the bottom of the round-bottom flask. The brown product with colloidal nature was separated from the upper part of ethyl acetate by a separatory funnel, washed with distilled water and dried at 80 ° C for 24 h in air.
The structure of δ -MnO2 nano-sheets was examined by X-ray diffraction (XRD, X'Pert-PRO MPD) with Cu Kα radiation. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observations were conducted using a JEOL JSM-6700F and JEOL JEM-2011, respectively. The specific surface area was measured using the Brunauer-Emmett-Teller (BET) method based on the nitrogen adsorption-desorption isotherm at 77 K on a Micromeritics ASAP2020 sorption analyzer.
The working electrode was prepared by mixing the electro-active material (δ -MnO2, 75 wt%), acetylene black (15 wt%), and polyvinylidene fluoride (10 wt%). The mixture was then pressed onto nickel foam (about 1 cm2) and dried at 80 ° C for 12 h. The electrochemical performance of the material was evaluated on a CHI760D workstation by using a three-electrode cell with Pt sheet as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. Cyclic voltammogram (CV) was measured between -0.2 and 1.0 V in aqueous Na2SO4 electrolyte (1 mol L-1), and galvanic charging-discharging (GC) tests were carried out between -0.2 and 0.6 V with different current densities. All the capacitances were calculated according to the active material of working electrode. The reproducibility of all presented data was generally checked by triplicate measurements. All experiments were carried out at ambient temperature.
The XRD pattern (Fig.1(a)) of the as-prepared material shows a reflection characteristic of the hexagonal birnessite-type manganese oxide (δ -MnO2, JCPDS 80-1098)[10]. The diffraction peaks at about 12.3° , 24.6° and 37.1° indicate the (001), (002) and (110) crystal plane of δ -MnO2. These diffraction peaks also correspond to a basal spacing of 0.72 nm[5]. The broadening and low intensity of the diffraction peaks indicate that the prepared δ -MnO2 has a weak crystalline. δ -MnO2 has a lamellar or sheet structure, which is constructed by [MnO6] octahedron (shown as Fig.2(a)) in a 2-dimensional array as shown in Fig.2(b). It is very suitable for charge storage in this lamellar or sheet structure due to its large specific surface area and high lattice imperfection density. As shown inFig.2(c), there are two charge storage mechanisms for this layered or sheet material: 1) at the surface of this kind of materials, the alkali cations in electrolyte would be adsorbed or desorbed by the electrostatic effect; 2) the alkali cations also could be inserted or extracted to/from the interlayer domain of the lamellar structure. SEM image (Fig.1(b)) shows a sheet morphology character, but the sheet structure is irregular, and a serious agglomerate and wrinkle behavior is observed in comparison with the regular plate-like particles. Furthermore, the TEM image of the prepared δ -MnO2 sample in Fig.1(c)demonstrates that the prepared δ -MnO2 has an ultrathin sheet structure with a typical curved/wrinkled appearance.
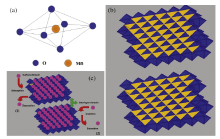 | Fig.2 (a) [MnO6]-basic structure of MnO2 materials; (b) crystallographic structure of birnessiteδ -MnO2; (c) energy storage mechanisms for MnO2 materials. |
The N2 adsorption and desorption isotherm, as shown in Fig.3(a), clearly indicates the presence of mesopores in the prepared δ -MnO2, classified as type IV as defined by the International Union of Pure and Applied Chemistry (IUPAC). A hysteretic loop between the adsorption and desorption branches can be considered type H4, indicative of slit-like pores. The sample shows a Brunauer-Emmett-Teller (BET) surface area of 257.5 m2 g-1, which is much larger than that reported in the previous work [5, 6, 11]. As shown in Fig.3(b), the pore size distributions from the adsorption branch of the isotherm using the Barrett-Joyner-Halenda (BJH) method reveals that the as-prepared δ -MnO2 sample has a meso-macroporous hierarchical structure from 2 to 170 nm.
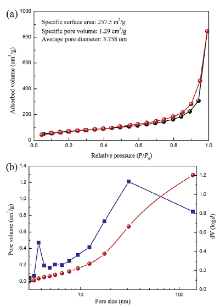 | Fig.3 (a) N2 adsorption-desorption isotherm of the as-prepared δ -MnO2; (b) pore size distribution of birnessiteδ -MnO2. |
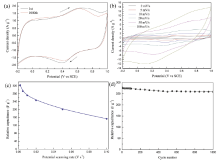
Electrochemical properties of the as-prepared sample are studied by CV and GC techniques. The CV results in 1.0 mol/L Na2SO4 electrolyte at a scan rate of 10 mV/s are shown in Fig.4(a). All these CV curves exhibit a rectangular-like shape with broad redox current peaks, indicating an ideal faradic capacitive behavior. Electrochemical properties are further elucidated by CVs at different potential scan rates ranging from 2 to 100 mV s-1, which are shown in Fig.4(b). At lower scan rates, δ -MnO2 shows an ideal pseudo-capacitive behavior, which is indicated by the rectangular-like shape CV curves. As the scan rate increases, the deviation of CV curves from rectangularity becomes obvious, which can be ascribed to the change of Na+ diffusion behaviors. As shown in Fig.4(c), the specific capacitance of δ -MnO2 decreases with increasing scan rate. As known, the capacitive behavior of δ -MnO2 depends on the adsorption/desorption or insertion/extraction of Na+ from electrode materials [7, 11, 12, 13, 14]. At slower scan rates, Na+ from the electrolyte could gain access to almost all available pores of the electrode, leading to a complete adsorption or insertion reaction and hence a reverse process. Therefore, a much larger specific capacitance is obtained. With a larger scan rate, Na+ can only reach the outer surface of the active material and not diffuse into the interior area; the effective interaction between the ion and the active material is greatly reduced, resulting in a declining capacitance [8, 15, 16, 17, 18, 19]. The specific capacitance values calculated from the 1st and the 1000th CV curves (scan rate: 10 mV s-1) are found to be 272 and 263 F g-1, respectively. The variation of the specific capacitance as a function of cycle number shows that the specific capacitance slightly decreases with increasing cycle number, as shown in Fig.4(d). After 1000 cycles, the electrode maintains 96.7% of the initial value, indicating the excellent cycling stability of δ -MnO2, which is attributed to its lamellar structure.
Fig.5(a) displays the representative GC curves of δ -MnO2 electrodes at different current densities. The good symmetry and linear slopes of these charging and discharging curves indicate good capacitive behavior of δ -MnO2. The resulting dependence of the specific capacitance for δ -MnO2 electrodes on current densities is plotted in Fig.5(b). It can be seen that the capacitance decreases with increasing applied current densities. In the energy storage process, there are two proposed storage mechanisms for MnO2 materials, which are shown in Fig.2(c): (1) the first one is based on the surface adsorption/desorption of alkali cations from electrolyte; (2) the second mechanism involves the insertion/extraction of alkali cations into/from the interlayer position of with concomitant redox between Mn3+ and Mn4+[7]. The as-prepared samples in this study have large specific surface area, revealing that the storage mechanism mainly comes from the first mechanism. Still, the lamellar structure (0.7 nm) of birnessite can also accommodate hydrated Na+ (0.358 nm) to yield capacitance based on the second mechanism. At lower current densities, Na+ can diffuse sufficiently into the interior zone of electrodes, resulting in more available surface for effective utilization of MnO2 and thus a higher specific capacitance. The reduced capacitance at larger current densities can be resulted from the limited diffusion time for Na+ diffusing into the interior surfaces. The specific capacitance values of the as-prepared sample at 0.2 A g-1 is calculated to be 183 F g-1. Notably, when the current density increases by an order of magnitude (2 A g-1), the as-prepared sample still delivers a specific capacitance of 125 F g-1 (68% of that at 0.2 A g-1), revealing its better rate capability. The electrochemical stability of the as-prepared sample is also examined by GC cycling test at 1 A g-1. The GC curves of the 1st, 2nd, 99th and 100th cycle are shown in Fig.6. There is no obvious change in the GC curves, showing good cycling performance of δ -MnO2.
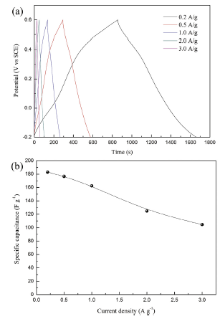 | Fig.5 (a) GC curves of δ -MnO2 electrodes at different current densities and (b) dependence of the specific capacitance for δ -MnO2 electrodes on current densities. |
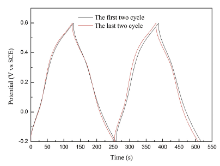 | Fig.6. Comparison of the GC curves of δ -MnO2 electrode in the cycling test (1st, 2nd and 99th, 100th cycle). |
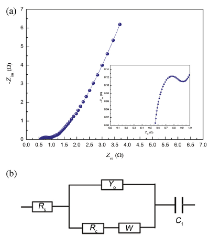
Electrochemical impedance spectrum (EIS) was also measured and fitted using the electrical equivalent circuit given in Fig.7 to investigate the charging kinetics of δ -MnO2. In the circuit, Rs is the sum of resistance of electrolyte, electrode material and the contact resistance at the interface of the active material/current collector. Y0 represents the constant phase element, which models the double layer capacitance (Cdl). Rc is the charge transfer resistance, W is the Warburg impedance and C1 is the limited capacitance. Rc value is 0.33 Ω , which is smaller than that reported in previous works[14], indicating that the δ -MnO2 electrode has much faster kinetics than that in previous works, which benefits to the capacitive performance of composite materials, particularly at high charge/discharge current densities[20].
δ -MnO2 nano-sheets have been successfully synthesized by interfacial synthesis method. The facile technique enables δ -MnO2 nano-sheets to show large specific surface area (257.5 m2 g-1). The sample has an ideal pseudo-capacitive performance, and exhibits a higher specific capacitance of 272 F g-1 with good rate capability and stable cycling performance. The enhanced electrochemical properties of δ -MnO2 electrode can be rationalized by its unique 2D lamellar structure. All the results demonstrate that δ -MnO2holds great potential in high-performance electrochemical capacitors, and this interfacial synthesis will be a very promising method to synthesize MnO2 materials.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|