biomedical Ti2448 alloy by anodic oxidation in neutral electrolyte. Similar to oxide nanotubes fabricated on pure titanium and its alloys, the as-grown nanotubes on Ti2448 also exhibit gradually changing chemical distribution along the direction of tube growth. Furthermore, several kinds of oxides with different valence states ( MxO y) are formed simultaneously for each alloying element M, while their volume fractions vary gradually along the tube-growth direction. The findings of this study would provide insight into the effect of valence states on the desired nanotube properties and help develop ways to enhance the properties of the preferred oxide.
Oxide nanotubes can be easily fabricated by electrochemical oxidation on pure metals and their alloys[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. Because they have potential applications in many fields, the formation of highly ordered and self-organized nanotubes on titanium and its alloys has been investigated extensively[22, 23, 24, 25, 26, 27].
The morphology of oxide nanotubes grown on pure Ti is generally different from that of nanotubes grown on its alloys. The self-organized nanostructure on pure Ti has a uniform size or a continuous size distribution[1]. On its alloys, the nanostructure dimensions, e.g., tube diameter, length, and wall thickness can be controlled by varying the solution chemistry, applied potential, anodization time, and solution temperature[5, 7, 11]. Thus, the morphology can be customized to enhance some desired nanotube properties[6, 10, 11, 28].
Besides the above morphological contribution, alloying also has a high impact on amorphous-crystalline transitions, the types of oxides formed, and their high-temperature stability. The thermal stability of nanotubes and the transformation temperature of TiO2from the anatase to rutile crystal structure can also be enhanced by alloying[5]. Adding large amounts of Nb can prevent the nucleation and growth of anatase crystallite from the initial amorphous titania nanotubes, resulting in a higher transition temperature[29]. Zirconium titanate (Zr1-xTixO2), with excess TiO2 and ZrO2, is formed on the Ti-Zr binary alloy whereas Nb addition (to form a Ti-Zr-Nb ternary alloy) results in TiO2 and three kinds of complex ternary oxides [7, 10]. Such a significant difference in the types of oxides seems impossible for titanium alloys containing large amounts of Nb. For example, the oxide nanotube layer mainly consists of TiO2 and Nb2O5 in the Ti-Nb binary alloy and extra Ta2O5 in the Ti-Nb-Ta-Zr quaternary alloys [5, 12].
The Ti2448 alloy (Ti-24Nb-4Zr-8Sn, wt%) is a kind of multifunctional β -type biomedical titanium alloy, which has a high strength and an ultralow elastic modulus that is similar to that of human bone[30, 31, 32]. In our study, oxide nanotubes with different tube diameters and lengths were fabricated on Ti2448 by anodic oxidation in a neutral electrolyte. The kinds of oxides, their thermal stability, and their impact on the morphology and composition along the tube growth direction were studied.
All samples with a diameter of 10 mm and thickness of 1 mm were prepared from the hot-forged Ti2448 alloy with a single β phase[31]. They were polished using SiC water-proof papers of up to #1200 grit; ultrasonically cleaned in acetone, ethanol, and deionized water, successively, for 10 min each; and then dried in air. Anodic oxidation was performed at room temperature in a neutral electrolyte containing 1 mol/L (NH4)2SO4 and 0.15 mol/L NH4F (pH = 6.7) prepared from analytical-grade chemicals and deionized water. A direct current (DC) power supply was used to keep the potential at a constant value for several cycles after the potential was swept from the open-circuit potential to the desired final potential at a sweep rate of 0.5 V/s. A two-electrode system consisting of a stainless steel cathode and the sample as the anode was used to fabricate the nanotube oxide layer without stirring. All samples were cleaned using deionized water after anodization. Some samples were annealed in air at a temperature between 550 ° C and 750 ° C for 2 h to investigate the effect of annealing temperature on the morphology and phase transformation of nanotube oxide layers. The morphology and microstructure of the nanotube layer were characterized using a field-emission scanning electron microscope (FESEM; LEO SUPRA35, Zeiss, Germany) and a transmission electron microscope (TEM) equipped with an energy-dispersive X-ray (EDX) detector (Tecnai G2 F30, FEI, USA). The diameter and length of the nanotubes were measured from the SEM images, and more than 50 tubes were measured to obtain the experimental accuracy. The phase compositions were analyzed with a glancing angle X-ray diffractometer (GAXRD; D/max2400, Rigaku, Japan) using a CuKα radiation source with an accelerating voltage of 40 kV and a current of 250 mA. The samples were also subjected to X-ray photoelectron spectroscopy (XPS; Escalab250, Thermo Fisher Scientific, USA) analysis in a vacuum chamber at a base pressure of ~3.5 × 10-8 Pa. To analyze the chemical distribution along the nanotube, the oxide layers were removed by sputtering with Ar+ ions (2 kV, 2 µ A) at an angle of ~45° .
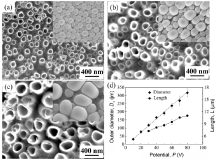
Highly ordered and vertically oriented nanotubes were successfully fabricated on the Ti2448 alloy (Fig.1 and Fig.2)). The insets in Fig.1(a-c) show that the nanotubes were conical, as evidenced by their diameters at the bottom, which were larger than those at the top. Similar to nanotubes grown on pure Ti in aqueous electrolytes[33, 34], both the tube diameter and length varied linearly with the applied potential that ranged from 10 to 80 V (Fig.1(d)). The influence of anodization time on the tube length can be fitted to a curve, which represents a typical behavior of such kind of chemical process. At a potential of 40 V, for example, the nanotubes grew rapidly in an early stage (up to 2 h from the beginning), following a linear relation; the growth then proceeded slowly with further increase in the anodization time (Fig.2(d)). An additional study showed that the anodization time had no obvious effect on the tube diameter. These results can be explained by the growth mechanism of nanotubes that was based on the competition between oxide growth at the metal-oxide interface and oxide dissolution at the tube-bottom-electrolyte interface[1, 34].
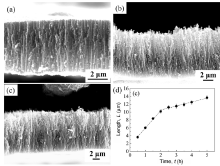 | Fig.2 SEM images showing the nanotube layer formed on Ti2448 at 40 V after different anodizing times (a) 1 h; (b) 3 h; (c) 5 h, and (d) variations in tube length with anodization time at 40 V. |
The GAXRD pattern (Fig.3(a)) shows that an amorphous layer characterized by a halo at 2θ of 20° -35° was formed on Ti2448 after anodization. This is further confirmed by the selected area diffraction (SAD) pattern with a continuous halo (inset in Fig.2(b)). Widely spaced ridges on the tubes' outside walls, which formed during nanotube growth, were also observed by TEM (Fig.3(b)).
High temperature annealing for 2 h at 550 ° C and 650 ° C resulted in the transition from the amorphous phase to anatase (Fig.3(a)). With the increase in annealing temperature up to 750 ° C, small amounts of both rutile and Nb2O5 were detected in addition to anatase, which remained a major phase in the tube layer (Fig.3(a)). This shows that the anatase-rutile transformation temperature of Ti2448 was ~750 ° C, which is much higher than that of pure Ti (~430 ° C[35]) and slightly higher than that of the Ti-Nb binary alloy (~650 ° C[5]). This suggests that alloying elements in titanium alloys hinder the nucleation and growth of rutile from anatase because of the barrier effect on the diffusion of Ti and O atoms. The alloying also had a significant effect on the nanotube thermal stability. TEM images inFig.3(c) show that the tubular structure was still stable when being annealed at temperatures ≤ 750 ° C, which is much higher than the annealing temperature of ~580 ° C for pure Ti[35]. Furthermore, the ridges (Fig.3(b)) almost disappeared after the annealing treatment at 750 ° C for 2 h (Fig.3(c)).
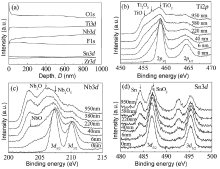
XPS results (Fig.4(a)) show that the as-grown oxide layer contained mainly Ti, Nb, Zr, Sn, and O, and a small amount of F. The qualitative data demonstrate slight variations in chemical composition along the tube growth direction: the amounts of Ti, Nb, and Zr increased but the amounts of O, Sn, and F decreased. Such a phenomenon has been observed in as-grown tubes of pure titanium and its alloys; here, it is ascribed to the sluggish chemical dissolution rate of the oxides in the electrolyte solution[12].
XPS spectra of the as-grown surface layer without removal by Ar+ ions sputtering (at a depth of 0 nm in Fig.4(b-d)) clearly show large amounts of TiO2, Nb2O5, and SnO2 and a small amount of ZrO2, as evidenced by the binding energies of 458.8, 207.4, 486.9, and 182.5 eV for Ti2p, Nb3d, Sn3d, and Zr3d, respectively (XPS spectra of Zr3d are not shown here). Since the increase in Ti, Nb, and Zr contents and the decrease in the O content along the tube-growth direction would have resulted in a high metal content and oxygen deficiency, new oxides of individual components with lower valence states would have been formed and their volume fractions would have increased during tube growth.
The XPS spectra in Fig.4(b-d) clearly indicate that the spectral peaks contributed by Ti2p, Nb3d, and Sn3d are gradually broadened and separated with increasing tube depth from the outer surface, while the intensity of these new peaks increased along the tube-growth direction up to a depth of ~1 µ m. Since these new peaks' valence states could be identified from the database of binding energy in Ref. [36], the nanotube growth can be summarized by the following trends (Fig.4(b-d)): three of the most stable oxides— TiO2, Nb2O5, and SnO2— formed on the outer surface while their volume fractions decreased along the tube-growth direction; oxides with lower valence states— Ti2O3 and TiO instead of TiO2, Nb2O, and NbO instead of Nb2O5, pure Sn instead of SnO2— formed in the oxide layer and their volume fractions increased gradually. These results show that the alloying components Ti, Nb, Zr, and Sn were oxidized with different valence states while their volume fractions along the growth direction continued to increase for oxides with lower valence states and decrease for oxides with higher valence states.
The above evidence of gradual variations of chemical compositions with the tube growth depth suggests that ion diffusion in the solid phase plays an important role in determining the distribution of oxides with different valence states. According to the point defect model proposed by Macdonald[37], the oxide growth during anodic oxidation is closely related to the movement of cation and oxygen anion in oxide. The cation transition between the metal and the oxide layer is governed by the following equation:
M→ Mx++VM+xe′ (1)
where M is the metal atom in metal phase and VM is a vacancy in the metal substrate. The transmission of metal cation and oxygen anion results in oxide growth by the following way:
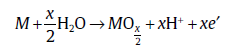 (2)
(2)
As time goes by the thickness of oxide layer increases and the anodic current density decreased slightly due to the diffusion of ionic species in the electrolyte[8], which will influence the movement of the metal cation in oxide layer and then the formation of oxide layer. Some oxides with lower valence form in the layers because of metal excess and oxygen deficiency.
Nanotubes with different diameters and lengths were fabricated on the biomedical Ti2448 alloy by electrochemical anodic oxidation in a neutral electrolyte. The tubular structure was much more stable than that formed on pure Ti metal, maintaining chemical and structural integrity at high temperatures of up to 750 ° C. Furthermore, several kinds of oxides with different valence states for each component of the Ti2448 alloy were found and their volume fractions varied gradually along the tube-growth direction. Such phenomenon is also expected for nanotubes grown on pure titanium and its alloys because they follow the identical tendency of concentrations of metallic cations and oxygen anions varying gradually along the tube-growth direction. As oxides with different valence states have different electron kinetics and photocatalytic properties[38, 39, 40], the findings of this study would provide insight into the effect of valence states on the desired nanotube properties and help develop ways to enhance the properties of the preferred oxide.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|