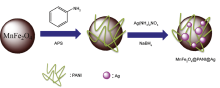
This paper reports a stable heterogeneous nanoparticles catalyst MnFe2O4@PANI@Ag for the degradation of azo dyes. In this synthesizing method, MnFe2O4 is used as magnetic core and polyaniline (PANI) a linker to stabilize the Ag nanoparticles (NPs) on the surface of catalyst. The method has a high ability to prevent Ag NPs from aggregation on the PANI surface, thus resulting in small size and highly dispersed Ag NPs. The composition and nano-structural features of polycrystalline sample were studied by X-ray powder diffractometry, Fourier transform infrared spectroscopy, and scanning electron microscopy. Vibrating sample magnetometer measurements proved the super-paramagnetic property of the catalyst, and UV results demonstrated that MnFe2O4@PANI@Ag has a high ability to reduce the azo dyes, which come from industrial wastes in the form of pollutant. The nanocomposites could be readily separated by magnet and reused for the next four reductions with high generation efficiency.
The development of noble metal nanoparticles (MNPs) has become an important issue for worldwide scientist and researchers. Few decades, MNPs have reported remarkable potential for several applications in the field of electronic, biological chemical, and catalytic due to their strange and excellent properties, when compared to their bulk counterparts[1, 2, 3]. Specifically, the transition metal nanoparticles like silver are synthesized and exploited extensively due to their unique physicochemical-catalytic properties[4] and their size-induced quantum effects. Additionally, they are used for various applications in fabrication process of electronic devices, photochemical catalysts, optical data storage, nonlinear optical materials and sensors[4, 5, 6]. Similarly the combination of inorganic-polymer (hybrid nanocomposites) is used to demonstrate the excellent properties and could be applied in diverse application such as drug delivery system, catalysis and biological diagnostics[7] [8].
Each day the demand on natural and synthetic dye products is increasing, making our world spectacular. Due to the large application, their discharge is uncontrolled, which caused destruction of ecosystem[9, 10]. Azo dyes are most widely used in the manufacture of several materials, synthetic dye products and pesticides, which polluted the environmental fresh water[11]. Such kinds of organics dyes and aromatic compounds are hard to be decomposed with conventional ways, such as active sludge method. They are used as precursor in several chemical synthesis of several azo dyes, antiseptic agents, antioxidants, pesticides and important corrosion inhibitors[12]. They are non-biodegradation compounds and their presence on wastewater shows high toxicity, carcinogenicity and mutagenicity to several organisms[13-16].
Many studies have been done on PANI and attracted largely by many scientists for the preparation of its composites with inorganic compounds such as Fe3O4, CuO, CdS, etc. to improve their stability, processability, and biocompatibility, and also to make the best linker for the hard metals in the synthesis of catalyst. The core-shell structure of PANI has also been investigated to improve their thermal stability[17, 18, 19, 20]. The catalytic activity of newly synthesized magnetic nanocatalyst of MnFe2O4@PANI@Ag has not been reported before. Many works have been done for the combination synthesis of Ag with Fe3O4[21, 22, 23] and on magnetic nanocatalyst with its application[24, 25, 26, 27], but none of them were reported with MnFe2O4 and polyaniline. In this study, a simple reflux method is applied to synthesize the MnFe2O4@PANI@Ag nanocatalyst. This method differs from the traditional sol-gel method in two aspects: one is that no expensive alkoxide reactants are required, and the other is that higher temperature calcinations are not needed to produce final product[28]. The fundamental objective of this study is to develop the value of efficient catalyst for the degradation of azo dyes such as methyl orange, methylene blue, eosin Y and rhodamine B.
FeCl3⋅ 6H2O, MnCl2⋅ 4H2O, polyaniline (PANI), AgNO3, NaBH4, NH3, methyl orange, methylene blue, eosin Y and rhodamine B were sourced from Merck and used without further purification.
X-ray diffraction (XRD) was conducted on a Rigaku Smart Lab operated at 40 kV and 35 mA using CuKα radiation (λ = 0.154059 nm). Fourier transform infrared (FTIR) spectra of the samples were recorded with a Perkin-Elmer BX FT-IR infrared spectrometer in the range of 4000-400 cm-1.
Scanning electron microscopy (SEM) was performed to investigate the microstructure of the sample, using an FEI XL40 Sirion FEG digital scanning microscope. Samples were coated with gold at 10 mA for 2 min prior to SEM analysis. Transmission electron microscopy (TEM) was performed using an FEI Tecnai G2 Sphera microscope. A drop of diluted sample in alcohol was dripped on a TEM grid and dried prior to analysis.
Thermal stability was determined by thermogravimetric analysis (TGA, Perkin-Elmer Instruments model, STA 6000). The TGA thermograms were recorded for 5 mg of powder sample at a heating rate of 10 ° C/min, over the temperature range of 30-750 ° C under a nitrogen atmosphere.
The magnetization measurements were carried out using vibrating sample magnetometer (VSM, LDJ Electronics Inc., Model 9600) in an external field up to 15 kOe at room temperature. Ultraviolet-visible (UV-vis, Shimadzu UV-Vis 2600) spectrometer was used in the range of 300-800 nm.
2.3.1. Synthesis of MnFe2O4 NPs
MnFe2O4 nanocomposite was prepared by reflux method. Analytical grade chemical initial reagents, FeCl3⋅ 6H2O, MnCl2⋅ 4H2O obtained from Merck, were used as received without further purification for the synthesis. Metal salts taken in required stoichiometric ratio (Mn/Fe = 1/2) were dissolved in 40 mL of distilled water in three-neck bottom flask, and their homogeneous solutions were prepared by magnetic stirring. After dissolving MnCl2⋅ 4H2O and FeCl3⋅ 6H2O, concentrated NH3 solution was added drop by drop under constant stirring so as the pH of the solution attains a value of 10, at which the precipitation of ferrites takes place. Then the flask was transferred to the heating mantle apparatus, where it was refluxed under Argon condition at 80 ° C for 5 h under stirring condition. Then synthesized dark brown precipitate (MnFe2O4 NPs) was separated by a permanent magnet from the reaction media and washed with distilled water-ethanol mixture solution several times to remove impurities. Finally a light brown powder product was dried at 80 ° C for 4 h in an oven.
2.3.2. Synthesis of MnFe2O4@PANI nanocomposite
MnFe2O4@PANI composite was prepared by precipitating PANI on the surface of MnFe2O4 NPs. In this typical synthesis, 50 mL of freshly prepared reaction mixture (0.1 mol/L aniline, 0.125 mol/L ammonium peroxydisulfate in 0.1 mol/L nitric acid) was added to 1.0 g of dark brown MnFe2O4 NPs at room temperature. Then the mixture was stirred during the polymerization of aniline, which was completed within 24 h under the ice bath at -4 ° C. The polymerization process was identified by the change of the starting colorless solution into deep green color. Then MnFe2O4@PANI nanocomposite was separated magnetically, washed with distilled water and ethanol for several times. Finally, the collected sample was dried in a vacuum oven at 60 ° C for 8 h.
2.3.3. Synthesis of MnFe2O4@PANI@Ag magnetically recyclable nanocatalyst (MRCs)
MnFe2O4@PANI@Ag MRCs was prepared as follows; initially 1 g of MnFe2O4@PANI nanocomposite was dispersed in 50 mL of deionized water. Then it was sonicated for 30 min, followed by the addition of 30 mL of 0.2 mol/L AgNO3 solution. After stirring the solution vigorously for 30 min, 0.6 g of NaBH4 was quickly added and the mixture was allowed to react at 60 ° C for 2 h under rapid stirring. The product was separated magnetically and washed several times with deionized water to eliminate impurities (Fig.1).
The XRD powder pattern of MnFe2O4@PANI@Ag MRCs is depicted in Fig.2. XRD analysis confirmed the presence of MnFe2O4 ((220), (311), (400), (511) and (440))[29]and Ag NPs ((111), (200), (220))[30, 31]. These observed Miller indices matched with the ICDD Card No: 73-1964 and 99-200-4306 for MnFe2O4 and Ag NPs, respectively, proved the presence of MnFe2O4 and Ag(0) NPs in the product. Notably, the diffraction peaks assigned to Ag in the patterns of the MnFe2O4@PANI@Ag MRCs are sharp and intense, indicating the better crystallization of Ag than Fe3O4 in the Ag-Fe3O4composite[32-33]. Sun et al.[34] synthesized the Ag@Fe3O4 core-shell nanospheres for reproducible SERS (surface-enhanced Raman spectroscopy) substrates, and they found out that the diffraction peaks of Ag appear to be much stronger than those for Fe3O4, which indicates that the crystallization of Fe3O4 NPs is not as good as that of the Ag NPs. The average particle diameters of Ag and MnFe2O4 measured according to TEM image are about 20.32 and 12.80 nm, respectively, using the Debye-Scherrer formula[35]. Zhang et al. had synthesized Ag/Fe3O4 composite, and the characteristic diffraction peaks of Ag were much stronger than those for Fe3O4, which revealed that the Ag nanoparticles had a larger agglomeration[32]. Li et al. synthesized the Ag/Fe3O4composites and they found that the particle size of Ag/Fe3O4 particle is about 200 nm, and the generated silver particle is only 10 nm approximately[36]. The sizes of MnFe2O4 and Ag nanoparticles calculated from the (311) and (111) peak based on Scherrer formula are 12.80 and 20.32 nm, respectively.
Polymerization of aniline and formation of MnFe2O4@PANI@Ag has been confirmed by the FT-IR spectra in the range of 4000-400 cm-1 at ambient temperature. The FT-IR spectra of MnFe2O4@PANI and MnFe2O4@PANI@Ag magnetic nanocatalyst are shown in Fig.3(a and b), respectively. In both spectra two main absorption bands at around 650 and 400 cm-1 are observed. The bands at 440, 478, 561 and 650 cm-1 are assigned to the metal-oxygen stretching bands in the material[29, 37, 38]. The tetrahedral stretching vibrations are observed at a higher wave number as compared to the octahedral stretching vibrations due to the fact that the M-O bond length in the tetrahedral site is shorter than that of octahedral site[39].
Moreover, as seen in Fig.3, the FT-IR spectra of MnFe2O4@PANI and MnFe2O4@PANI@Ag magnetic nanocatalysts exhibit all the characteristic peaks of polyaniline in addition to the Mn-O and Fe-O stretching vibrations[40, 41]. In the spectrum of MnFe2O4@PANI magnetic nanocomposite, the bands position at 1546 cm-1and 1447 cm-1 were assigned to the characteristic C-C stretching of the quinoid and benzenoid rings. The bands at position 1102 and 777 cm-1 reflected the aromatic C-H in-plane bending and out-of-plane deformation of C-H in the 1, 4-disubstituted benzene ring, respectively[18, 40, 41]. More or less the same peaks were observed in the FT-IR spectrum of MnFe2O4@PANI@Ag MRCs (Fig.3(b)). This observation clearly demonstrates that the MnFe2O4 magnetic core was coated with polyaniline to form MnFe2O4@PANI@Ag magnetic nanocatalyst.
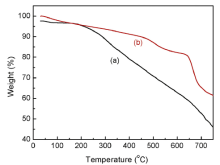
TG analysis of the MnFe2O4@PANI@Ag nanocatalyst is shown in Fig.4. PANI has a significant three-step weight loss behavior, which was studied before[19], [42, 43, 44]. InFig.4(a), PANI has a significant weight loss in the temperature interval of the measurement. However in Fig.4(b), MnFe2O4@PANI@Ag nanocomposite undergoes similar decomposition steps as that of PANI, but in this case somehow low loss weight was observed due to the higher stability and greater interaction between PANI and MnFe2O4, which restricts the thermal motion of PANI chain in the composite and enhances the thermal stability of the composite[43]. The initial weight loss up to 105 ° C is due to residual water, and the decomposition of PANI started after 300 ° C. This thermal analysis confirmed that weight percentage of organic and inorganic content in nanocomposite as 39% and 61%, respectively.
Fig.5 shows the magnetic hysteresis curve of the MnFe2O4@PANI@Ag, which exhibits a superparamagnetic behavior without the observation of coercivity and remanence. With superparamagnetic property, the catalyst can be easily recovered by applying an external magnetic field. The measured saturation magnetization value (Ms) of the MnFe2O4@PANI@Ag was found to be around 20 emu/g. The inset in Fig.5 clearly shows the color change of the system before and after the reduction of azo dye (methyl orange).
Fig.6 shows the typical SEM images of MnFe2O4@PANI@Ag MRCs. A magnified SEM image reveals the more detailed structural characteristics of the Ag-MnFe2O4nanocomposite. As shown in Fig.6, the SEM micrographs of these magnetic materials indicate globular agglomerates of irregular microcrystals. Silver nanoparticles appear as dark nearly spherical spots on the smooth bright surface of the polyaniline coating layer. Additionally, energy-dispersive X-ray spectroscopy (EDX) was used to confirm the composition of the product. EDX spectrum confirmed that the product was composed of Ag, Fe, Mn, C. N and O elementals. The presence of a certain amount of C could be expected both from the PANI and the conducting resin used for measurement.
TEM micrographs were used to evaluate the morphology of MnFe2O4@PANI@Ag MRCs. The images obtained are shown in Fig.7, which presents that MnFe2O4@PANI@Ag MRCs are spherical, and agglomerated to some extent because of magnetic dipole interactions between ferrite particles and the high surface energy of the nanoparticles. The average particle size is found to be 75 nm. TEM images reveal that the MnFe2O4nanoparticles have been already embedded in PANI matrix (the interaction between PANI shell and the oxygen atoms of the ferrite core leads to embedment of ferrite particles on PANI chains)[18]. The black core is the magnetic ferrite particles, and the light colored shell is PANI in the composite, due to the different electron penetrability.
3.7.1. Catalytic studies
The catalytic reduction experiments were done separately for different aqueous azo dyes solutions, i.e. methyl orange (MO), methylene blue (MB), eosin Y (EY) and rhodamine B (RhB) with concentration of (100 µ l, 10 mmol/L), and then aqueous freshly prepared (1 mL, 100 mmol/L) of NaBH4 were added to UV-cuvette of each dye. Then, consecutively the volume of the mixture of all dyes was adjusted up to 3 mL with distilled water. Finally, 1 mg MnFe2O4@PANI@Ag nanocatalyst was used to catalyze theses azo dyes solutions of MO, MB, EY and RhB, and the color of all four dyes solution vanished at the end of reduction process, indicating the degradation of the dyes solution. The reduction was monitored with a UV-visible absorption spectrophotometer. After the degradation was completed, MnFe2O4@PANI@Ag nanocatalysts were separated using a magnet, and the process was repeated to investigate the recyclability of the catalysts.
Each day the demand on synthetic dyes is increasing and it reaches the level where the protection of environment is much concerned by researchers. There are some toxic and stable dyes molecules being found, such as MO and MB, which are dangerous to the environment[45, 46], and their degradation is a very important subject nowadays. MO, MB, EY and RhB are organic sulfosalt azo dyes that can be reduced by reductants like NaBH4 to a non-toxic species form and can be free from any kind of pollutant[47]. However, with the help of only reductants, the process of reduction of these dyes or MO needs two consecutive days or more than days[23, 31, 48]. Thus, metal nanoparticles are the best materials to increase the rate of degradation of these dyes.
There are many research works done on catalyst base reduction process in short interval of time for these harmful and toxic dyes[49, 50]; as for example, Vidhu and Philip[51] in one research work used silver nanoparticles to catalyze the MO, MB and EY in 6, 17, and 21 min, respectively. In another work, U. Kurtan et. al. used Fe3O4@Hpipe-4@Cu nanocatalyst to reduced MB, MO, 4-NP and 4-nitroanline in 2, 18, 29 and 8 min, respectively[52], and Dang et al.[53] reduced 4-NP in 42 min with the help of Fe3O4@PS@PAMAM-Ag magnetic nanocatalyst. In another study Zhang et al.[54]synthesized Fe3O4/Ag composites and tested its catalytic activity for Rhodamine B, and they observed that 85% decomposition of Rhodamine B requires 22 min.
3.7.2. Degradation of MO
To investigate the catalytic performance of our newly synthesized MnFe2O4@PANI@Ag magnetic nanocatalyst, MO, MB, EY and RhB degradation was chosen as a model reaction in the presence of NaBH4. In Fig.8(a), it can be seen that MO took more than 24 h to reduce partially with the help of only NaBH4. But when the MO reduced by NaBH4 with addition of our synthesized nanocatalyst as shown in Fig.8(b), the spectral band of MO at 465 nm disappeared only in 9 min and a new band is formed at 250 nm, which is assigned to hydrazine derivatives, by changing the color from orange to colorless[51].
3.7.3. Degradation of MB
Similarly, the degradation of MB, which is a type of heterocyclic aromatic azo dye, was done. Initially freshly prepared aqueous MB blue solution with a relative absorbance of band at 665 nm[55, 56, 57] was reduced with the help of only NaBH4 in Fig.9(a). It consumed almost in 24 h to complete reduction process in the absence of magnetic nanocatalyst. But once the MnFe2O4@PANI@Ag magnetic nanocatalyst was added with NaBH4, the absorbance intensity has showed a decreasing trend, which indicates the reduction of MB, and it is achieved through the inclusion of Ag nanoparticles, as shown inFig.9(b). This indicates that the complete degradation of MB to leucomethylene blue (LMB) was accomplished within 7 min in the presence of silver nanoparticles by forming colorless solutions after reduction.
3.7.4. Degradation of EY and RhB
Further catalytic application of MnFe2O4@PANI@Ag magnetic nanocatalyst was done using eosin Y (EY) and rhodamine B (RhB) azo dyes. As shown in Fig.10(a, c), partial degradation of EY and RhB took place in 3 and 2 h, respectively, by using only NaBH4. However, when MnFe2O4@PANI@Ag nanocatalyst was added with NaBH4 to eosin Y aqueous solution, this time reduced to only 16 min. After the complete degradation of EY, maximum absorption peaks at 510 nm disappeared, indicating the complete and fast degradation of EY azo dye as shown in Fig.10(b). Similarly in the case of RhB, the complete degradation was done in 23 min after the addition of nanocatalyst MnFe2O4@PANI@Ag, and the characteristics peak of RhB at 650 nm disappeared after the complete degradation, which represents that this synthesized magnetic nanocatalyst plays an important role in the catalytic reduction of RhB azo dye (Fig.10(d)).
 | Fig.10 Degradation of (a) EY without catalyst, (b) with catalyst, (c) RhB without catalyst and (d) with catalyst. |
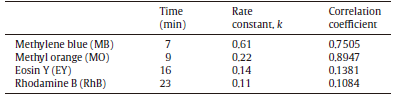
Generally, the reduction of azo dyes is considered to be a pseudo-first-order kinetic reaction due to the use of excess NaBH4[35]. Therefore, the kinetic data obtained for MB, MO, EY and RhB are fitted to first order rate equations. Upon analysis, according to the linear relationship between -ln(A/A0) and the reaction time, the rate constants (k) are in turn 0.61, 0.22, 0.14 and 0.11 min-1, as seen in Fig.11. The rate constants obtained from the kinetic data for all four samples are shown in Table 1. The above facts imply that the MnFe2O4@PANI@Ag presents the highest catalytic activity for the reduction of MB, whereas the lowest activity for the reduction of RhB and also the rate constants of MnFe2O4@PANI@Ag catalyzed reactions are found to be comparable and in some cases even higher than that of precious metal nanoparticle based catalysts [23, 47, 52, 55, 57].
 | Fig.11 Plot of In(At/A0) versus time corresponding to the reduction of azo dyes by MnFe2O4@PANI@Ag MRCs. |
| Table.1 Comparison of the completion time and catalytic activity of MnFe2O4@PANI@Ag for the reduction of various azo dyes |
The reusability of the MnFe2O4@PANI@Ag catalyst for the decolorization of dyes with NaBH4 was also investigated. After completion of one catalytic cycle, a fresh solution of dye and NaBH4 was added to the reaction mixture. Distinctly, as shown in Fig.12, the kvalue decreases after a catalytic cycle, but the catalytic efficiency of the catalyst reduced insignificantly to four cycles of operation, and the time required for 100% reduction of dyes was found to be small in change up to the 4th cycle.
 | Fig.12 Catalytic activity of MnFe2O4@PANI@Ag for the MB azo dye degradation up to four cycles. |
In the present study, Ag nanoparticle incorporated MnFe2O4@PANI has been successfully prepared by using a simple chemical method. The synthesized nanohybrids can be used as a high performance catalyst for the reduction of organic pollutants such as methylene blue (MB), methyl orange (MO), eosin Y (EY) and rhodamine B (RhB), and can be extended to catalytic reduction of other reducible contaminants. Furthermore, MnFe2O4@PANI@Ag nanocatalysts can be recycled several times by magnetic separation. Thus, as heterogeneous catalyst, MnFe2O4@PANI@Ag has shown promise to provide a cheaper and more efficient alternative than precious metal nanoparticle based catalysts for the dye reduction/decolorization reactions.
Meanwhile, the resulting catalyst illustrated a series of advantages, including high catalytic activity, easy separation and good stability, over similar catalysts. As the synthesized catalyst exhibits high catalytic efficiency toward the decolorization of the individual dyes within few minutes with high values of reaction rate constants, it is believed that promising applications of this material will be found in the field of catalysis particularly for wastewater treatment.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|