Silver nanowires (NWs) coated with platinum (Pt) nanoparticles were synthesized via a galvanic partial replacement of Ag NWs in an aqueous K2PtCl6 solution at room temperature. The products were characterized using a combination of electron microscopies, selected area electron diffraction, energy-dispersive X-ray mapping and X-ray diffraction. The surface morphology and Pt/Ag composition ratios are controlled by adjusting the K2PtCl6 concentration. Different concentrations result in various surface morphologies including rough nanoparticle coating, porous and relatively smooth surfaces. The formation mechanism was discussed based on the lattice constants' difference, concentration driven nucleation, consumption of Ag NWs, and stoichiometry of the replacement reaction. The effects of the bimetallic interface on the catalytic activity toward the reduction of 4-nitrophenol by sodium borohydride were studied. The activity of Ag-Pt NWs is highly enhanced over monometallic nanostructures, and optimized by a low Pt loading of 1.34 at.%, which indicates a catalytic role of the inter-metallic interface for the electron transfer.
One dimensional (1D) metal nanostructures have attracted great interests owing to their excellent electrical, thermal, optical, and mechanical properties[1-4]. Metallic nanowires (NWs) are considered key building blocks future microelectronic and microelectromechanical systems as both interconnect and active components[5-8]. Also, they provide an ideal model system to experimentally investigate many physical phenomena such as quantized conductance, size[9] and geometric[10] effects, and localization effects[11, 12]. The small diameter, high flexibility, and large aspect ratio make it possible to form flexible transparent conductive films[13] and to provide structures with easily formed percolation pathways[1], such as array[2], network[3] and other 3D structures[4].
As for the usefulness as an active component in functional devices, an improvement of activity of metal NWs is highly desirable. For instance, a nanowire network is expected for potential applications in tail-gas and wastewater treatment based on both the network structure and catalytic properties of NWs[2]. Monometallic NWs have a quite high electrical conductivity, a high thermal conductivity, but their functions are not enough. Integration of different metals can improve activity and reaction selectivity of monometallic counterparts[14, 15, 16]. For example, Pt-Pd[14], Ag-Au[15], and Co-Pt[16] bimetallic nanodendrites showed much enhanced catalytic activities over monometallic structures. The property enhancement of bimetallic structures is partially due to a combination of their activities, but more importantly is from the resulting microscopic fine structures, such as novel surface morphologies[14] and metal-metal interfaces[16, 17]. Recently, the catalytic activity of dispersed metal NPs supported on oxides[18] or sulfides[19] has been studied. The improved activity is due to the interface sites. Interfaces such as metal/oxide, metal/sulfide, and bimetallic ones play an important role in improving the properties of nanostructures. Additionally, bimetallic systems allow tuning reaction activities via the composition ratio and structure tailoring[20]. In recent years, various bimetallic nanoparticles (NPs) have been investigated[21, 22, 23, 24]. They are mostly applied dispersed in mediums, and if nonmagnetic, recovery is difficult[25]. While long 1D metallic NWs are easier to retrieve after applications as catalyst, and they can be easily assembled into networks, for example honeycomb-like arrays[26]. The controllable synthesis of bimetallic NWs combining the advantages of 1D structures and bimetallic composition is of great significance.
Among the available methods for the preparation of bimetallic compounds, the reduction of metal ions on the surface of sacrificial particles, better known as galvanic replacement reaction (GRR), is a simple yet versatile method that has been extensively employed to synthesize bimetallic nano/microstructures[14, 15, 16, 27]. Scalable preparation of monometallic (e.g. silver[28, 29, 30]) NWs has been achieved, which allows controllable synthesis of bimetallic ones based on GRR. Due to the consumption of substrate material, GRR can result in the formation of very rough surfaces with large surface areas and many active corners and edges[15, 16]. This kind of structure is more stable than those synthesized by loading small noble-metal nanoparticles onto surfaces[31]. The rough surface is especially promising for enhancing catalytic activities[14, 16].
Herein, we report the fabrication of bimetallic Ag-Pt NWs via a GRR by dispersing hydrothermally synthesized Ag NWs in an aqueous K2PtCl6 solution at room temperature. Ag NWs with uniform diameters of about 250 nm were synthesized. The products were characterized using a combination of scanning- and transmission-electron microscopy, selected area electron diffraction, energy-dispersive X-ray mapping and X-ray diffraction analysis. The effect of the K2PtCl6 concentration was investigated systematically. The growth mechanism is discussed based on the lattice constants' difference and the influence of the reaction parameters. Catalytic activity is investigated via the activity toward the reduction of 4-NP by NaBH4. The results indicate a dominant role of the Ag/Pt bimetallic interfaces.
Silver nitrate (AgNO3), polyvinylpyrrolidone (PVP, ≥ 95 wt%), and sodium sulfide (Na2S⋅ 9H2O, ≥ 98 wt%) were purchased from Shanghai Shenbo Chemical Co. Ltd. Potassium hexa-chloroplatinate (K2PtCl6) and ethylene glycol (EG) were from Shanghai Shiyi Chemical Reagent Co. Ltd. 4-nonylphenol (4-NP) and NaBH4 (≥ 96 wt%) were bought from Sinopharm Chemical Reagent Co., Ltd. All chemical reagents were of analytical grade and used without further purification. Deionized water with a resistivity exceeding 18 MΩ cm was used throughout the experiments.
Ag NWs were synthesized via a modified solvothermal method[30]. In a typical procedure, a 20 ml EG solution of Na2S was magnetically stirred after an addition of 6 mmol PVP. This solution was injected drop by drop into 20 ml of stirred EG solution of AgNO3. The solution became gradually wine-red colored. The mixture was then transferred into a 50 ml Teflon-lined stainless steel autoclave and sealed. After being maintained in an oven at 160 ° C for 1.5 h, the autoclave was allowed to cool to room temperature (r.t.). The products were washed with ethanol and deionized water for several times to remove remnant EG, PVP and ions. Finally, NWs were collected by centrifugation at a rotation rate of 6000 r/min and dried in a vacuum oven at 40 ° C.
Typically, 1 ml aqueous K2PtCl6 solution was added drop by drop into a 20 ml aqueous suspension containing 2 mg Ag NWs, which was stirred in water bath at 90 ° C. The initial concentration of the aqueous K2PtCl6 solutions (CPt-ion) was varied in the range of 0.06-7.7 mmol/l. After a reaction for 5 min, the color of the solution turned from pale white, which is the characteristic color of Ag NW suspensions, to gray. The higher the CPt-ion, the darker the solution color. The products were washed and collected as described above.
To compare the catalytic activity between Ag-Pt structures and monometallic Pt ones, pure Pt nanostructures were prepared by ultrasound assisted chemical reduction using EG as reducer. Five milliliters of K2PtCl6 (8 mmol/l) aqueous solution were placed in a beaker and ultrasonicated at r.t. for 1 h. The products were collected by centrifugation (12, 000 r/min), washed, and vacuum-dried.
Scanning electron microscopy (SEM) was performed on a field-emission SEM microscope (Hitachi S-4800) at an acceleration voltage of 5 kV. The crystal structure of the products were analyzed by X-ray diffraction (XRD) (Shimadzu 6000, Cu Kα radiation, λ = 0.15418 nm). Transmission electron microscopy (TEM), high resolution TEM (HRTEM) and selected area electron diffraction (SAED) were carried out on a JEM-200CX electron microscope at an acceleration voltage of 200 kV. The contents of the individual elements contained in the bimetallic structures were measured by energy dispersive X-ray (EDX) spectroscopy, which was carried out on the same SEM microscope. Repeated measurements of compositional ratios in the SEM-EDX analyses were subsequently averaged.
Catalytic activity toward the reduction of 4-NP by NaBH4 was measured on a Shimadzu UV-3600 UV-vis-NIR spectrophotometer at RT. In a measurement, 0.6 mg of dried solid product was added into 2.8 ml of 4-NP aqueous solution (7.5 × 10-5mol/l) in a quartz cuvette with an optical path length of 1 cm. Then, 0.2 ml freshly prepared NaBH4 aqueous solution (0.15 mol/l) was added. UV-visible absorption started to monitor changes in the reaction mixture simultaneously. The strongly alkaline NaBH4 raises the solution's pH above 12 and so the 4-NP converts to the 4-nitrophenolate ions, which makes the absorption peak shift from 317 nm to 400 nm[32]. This peak, i.e. the absorbance A400nm, is monitored to infer the conversion of the 4-NP into its reduction product 4-aminophenol (4-AP), whose absorption can also be monitored, namely at 290 nm. At such low concentrations of catalyst as employed here (ca. 0.33 g/l), the absorption by the Ag NWs' surface plasmon, which is centered around 400 nm, can be disregarded. The high concentration of BH4- protects the 4-AP from aerial oxidation and it is so far above the concentrations of 4-NP and the catalysts, that it can be assumed constant during the whole reaction. The catalytic rates are thus calculated via pseudo-first-order kinetics[32].
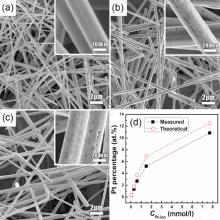
Fig. 1(a) shows a low-magnification SEM image of the initial Ag NWs synthesized. The NWs have lengths over 15 µ m. They are uniform, narrowly distributed in diameter, and their average diameter is ca. d = 250 nm. A higher magnification (the inset) reveals a smooth surface of an individual wire. These NWs served as precursors for the subsequent synthesis of Ag-Pt NWs. The reduction of PtCl62- ions happens because the standard reduction potential of the PtCl62-/Pt pair (1.435 V vs. standard hydrogen electrode (SHE))[33] is higher than that of the Ag/Ag+ pair (0.80 V vs. SHE)[34]. The reaction occurs according to the following equation:
After reacting with 1 ml of 0.06 mmol/l K2PtCl6 solution, no change of the surface can be seen in the SEM images (see inset). At CPt-ion = 0.06 mmol/l, the NWs' surfaces become relatively rough since they are encrusted with small Pt NPs (see inset). Holes with diameters up to 10 nm are visible in the Pt surface layer (Fig. 1(b)). Increasing the CPt-ionto 0.30 mmol/l results in larger Pt particles, as shown in the inset of Fig. 1(c). The reduction of K2PtCl6 ions via a GRR and Pt nucleation on the NWs happens rapidly at 90 ° C, which leads to the rough surfaces. It is observed that a rougher surface with more NPs is obtained at higher CPt-ion. Fig. 1(d) shows a comparison between the Pt atomic percentages calculated from the equation (1) and those measured from EDX analysis, which will be discussed in detail later. When the CPt-ion is lower than 0.3 mmol/l, the atomic percentage of Pt obtained from EDX patterns roughly matches the calculated values. When CPt-ion reaches 0.60 mmol/l and larger, the measured values fail to reach the theoretical ones, which indicates an incomplete GRR. In particular, when CPt-ion reaches 7.7 mmol/l, PtCl62- are enough for complete replacement of Ag NWs theoretically, but a little Ag remains. Since the GRR happens only on the surface of Ag NWs, no Pt NPs are formed in solution, which is confirmed by the fact that no Pt NPs are collected by repeated washings. The difference between EDX analysis and calculated values is because pre-grown Pt covers prevent GRR between the Ag below the surface and the residual PtCl62-ions.
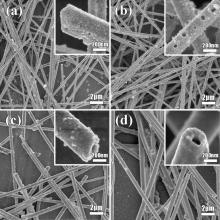
The products from high CPt-ion are shown in Fig. 2. At a higher CPt-ion of 0.6 mmol/l, a dense Pt cover consisting of more NPs is formed on the surface (Fig. 2(a)). It is supposed that they are tiny holes (ca. 30 nm) and particles (ca. 10 nm). When CPt-ion increases to 1.0 mmol/l, the surface of the NWs becomes rougher with larger holes and particles along the wires. Holes and particles can be clearly seen on the walls of NWs (Fig. 2(b)). The formation of Ag-Pt bimetallic NWs confirms the GRR reaction. After reacting with 1.5 mM K2PtCl6 solution, the Pt layer covers the Ag NWs smoothly without holes, but some hollow tubes are obtained (Fig. 2(c)). Especially, when CPt-ion is increased to a high value of 7.7 mmol/l, the products turn out to be nanotube-like with holes on the ends (inset of Fig. 2(d)).
As can be seen in the inset of Fig. 2(a), regardless of the structural anisotropy of Ag NWs, the reduction exhibits no chemically distinguishable activities on their tips and sides. The morphology of the bimetal NWs can be tuned by the relative amount of Pt and Ag from a smooth coating to a rough surface with nanoholes and NPs. In a typical GRR, the reaction process can be described as follows: the reduction of PtCl62- firstly takes place at the sites with relatively high surface energies, thin films of Pt NPs are formed and Ag starts to dissolve into the solution. For every Pt atom added, four electrons are necessary [
Representative XRD patterns of the initial Ag NWs and the Ag-Pt bimetallic NWs obtained with CPt-ion = 0.3 mmol/l (Fig. 1(c)) are shown in Fig. 3. The strong diffraction peaks indicate that the Ag NWs are well crystallized. Characteristic peaks appear at 2θ = 38.12° , 44.30° , 64.43° , 77.39° , and 81.53° , which match the reflection planes of (111), (200), (220), (311), and (222) of face centered cubic (fcc) silver (JCPDF 04-0783). The XRD pattern indicates that the Ag-Pt product is in the fcc phase with a lattice constant (0.4078 nm) very close to that of pure Ag (0.4086 nm, JCPDF 04-0783). Compared with the referenced XRD pattern of Pt (JCPDF 18-0972) and the original Ag NWs (black curve), the pattern of Ag-Pt NWs (red curve) shows no diffraction peaks assigned to Pt. The absence of Pt diffraction peaks after the GRR may result from the very small amount of Pt (as demonstrated by EDX analysis below); hence, its signal may be covered by the strong signals of Ag NWs [35]. This also implies that much of the crystalline lattice of the Ag-Pt structure is dominated by the framework of Ag[14]. This result is similar to that for bimetallic Pd-coated-Ag dendrites[15] and AgPd alloy-coated-Ag NWs[36].
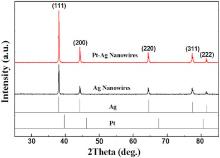 | Fig. 3. XRD patterns of the initial Ag NWs (shown in Fig. 1(a)) and the resulting bimetallic Ag-Pt NWs as shown in Fig. 1(c). |
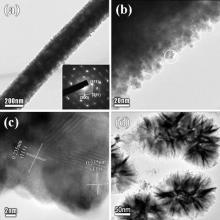
A typical TEM image of a single Ag-Pt nanowire with a diameter about 250 nm (Fig. 4(a)) shows that it has a core-shell structure due to an obvious contrast, showing D— d ~ 80 nm. The SAED pattern (inset of Fig. 4(a)) shows darker discontinuous concentric rings, which implies a polycrystalline structure. The (111), (200), and (311) planes of face-center-cubic silver can be identified. The discontinuous concentric rings should be from the diffraction of many Pt NPs. There are many tiny Pt particles (average diameter is ca. 10 nm) on the Ag-core (Fig. 4(b)). The nanoparticles are distributed continuously on the outside of the wire, forming a dense coating. The crystal grains within the surface region (recorded from the circled area in Fig. 4(a)) are shown in the HRTEM image (Fig. 4(c)), from which we can also detect a lattice spacing of 0.235 nm, which is the inter-planar spacing of Ag (111). The polycrystalline feature is seen in the HRTEM image as well, several domains are assigned to Pt (111) planes with different lattice orientations overlapping each other. A bright-field TEM image (Fig. 4(d)) shows that the Pt nanostructures obtained from the ultrasound assisted reduction consist of many nanoflakes, and these loosely inter-connected flakes result in many internal pores and rough surfaces. An EDX pattern (Fig. 5(a)) of a typical product obtained with CPt-ion = 0.3 mmol/l verifies the existence of both Pt and Ag with a Pt atomic percentage of 1.34 at.% and Pt mass percentage of 2.46 wt%. The X-ray elemental mapping (Fig. 5(b)-(c)) examines the distribution of Pt and Ag in Ag-Pt bimetallic NWs, which implies Agcore-Ptshell structure. The Ag element is intensively distributed along the wire and the Pt element is unevenly coated on the Ag wire. Thin coating of Pt element is presented as many spots with a low number density.
 | Fig. 5. EDX pattern (a) and X-ray elemental mapping of an isolated Ag-Pt nanowire (b-c) recorded from the sample as shown in Fig. 1(c). |
Thin Pt layers promise better catalytic activity because of the accessible bimetallic interfaces, which may be covered in Pt in case of thicker coverings. The reduction of 4-NP by NaBH4 in aqueous solution is easily monitored by UV-visible absorption, and it is thus selected as a model reaction for an evaluation of catalytic properties. Without addition of a catalyst, the reduction will not proceed, the absorption spectrum remains unaltered, and the mixture maintains a yellow color. However, when even only 0.6 mg of the Ag-Pt bimetallic NWs with 1.34 at.% Pt was added, the reduction of 4-NP proceeded rapidly as can be immediately seen from the bleaching of the yellow color. Time-dependent UV-Visible absorption spectra monitor the consumption of the 4-NP (Fig. 6). While the 400 nm peak decreases, a new peak corresponding to 4-AP appears at 300 nm. A linear decline of log (A400nm) vs. the reduction time t is obtained as expected from pseudo-first-order kinetics (the inset of Fig. 6). Using only Ag-NW or only Pt-NPs as catalysts, the curve stays almost parallel to the t-axis, indicating a very weak catalytic activity for the reaction. The apparent rate constants (kapp) is the slope of log (A400nm) and here calculated to be 6.93 × 10-3s-1 for the Ag-Pt bimetallic NWs.
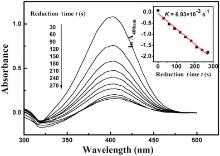 | Fig. 6. UV-Vis absorption spectra of the reduction of 4-NP by NaBH4 in the presence of 1.0 mg Ag-Pt bimetallic NWs (as shown in Fig. 1(c)); the inset shows the logarithm of the absorbance at 400 nm vs. reduction time t. |
The apparent rate constants kapp of pure Ag NWs, pure porous Pt nanostructures, and Ag-Pt bimetallic NWs are shown in Table 1 and are summarized in Fig. 7. The reductions catalyzed by the Ag-Pt bimetals are much faster than those in the presence of the monometallic structures. The importance of bimetallic interfaces is supported by the observation that increasing Pt content results in the rapid enhancement of the catalytic activity, as can be inferred from the rapid rise of the kapp vs. Pt atomic percentage (Fig. 7). Catalytic rate constants increase initially and then decrease with the Pt atomic percentage. When adding little Pt, the catalytic rate increases strongly because all the added Pt particles create bimetallic interfaces to the accessible surface. However, when more Pt is added, the newly added Pt just locates on already present Pt, thus few new bimetallic interfaces are created. A maximum catalytic rate activity should be expected when the NWs are completely covered by Pt. At some point, the already present bimetallic interfaces become even covered with additional Pt, and the activity is expected to decrease. The catalytic rate activity decreases after about 1.5 at.% Pt. When the atomic Pt percentage is increased to ~11 at.%, the resulting tubes with high Pt content bimetals behave similar to the pure Pt nanostructures as shown in Fig. 4(d) (kapp = 0.07 × 10-3s-1). This is consistent with the surface of the bimetallic structure being almost completely covered by Pt and the structures also not being rough anymore as was seen from the SEM image (see Fig. 2(d)). This is consistent with our previous investigation on Co-Pt bimetallic structures with nanoflake-built Pt covers[15] and due to the accessibility of bimetallic interfaces. When more and more Pt is added, it joins the already present Pt, so few new bimetallic interfaces are formed. Moreover, the already-formed interfaces are covered by additional Pt. To compare our result with previously reported catalytic activities, the activity factor (K) was defined as the values of kapp over the total weight of the catalyst[37]. The Kvalue for our optimal Pt-coated-Ag NWs is 11.55 × 10-3s-1mg-1. This value is much higher than those for many previously reported catalysts (as listed in Table 2), such as pure metal (Ag[38], Ni[39], Pt[42] and Pd[43]), nanowires, bimetallic (Ag-Au[38], Au-Ni[39]) NWs, and some other bimetallic catalysts (e.g. Au-Ag nanocubes[40] and Pt3Te4nanoparticles[41]).
| Table 1 4-NP reduction reaction apparent rate constants (kapp) in the presence of monometallic Ag and Pt nanostructures and the Pt-Ag bimetallic NWs obtained with different CPt-ion |
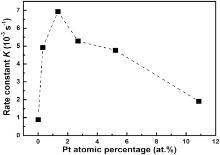 | Fig. 7. Apparent rate constants kapp of pure NWs and the Pt-Ag bimetallic wires vs. Pt atomic percentages. |
| Table 2 Comparison of apparent rate constants (kapp) and activity factor (K) of different catalysts for the reduction of 4-NP |
Bimetallic Ag-Pt NWs were synthesized via GRR. Different CPt-ion resulted in different morphologies and compositions, which were characterized by a series of techniques. Especially when NWs with thinner Pt coating, the bimetallic interfaces formed between Pt and Ag perform highly catalytic activity in hydrogen reduction reactions as has been benchmarked via the model 4-NP/NaBH4 system. By tuning the morphologies and compositions, it is very promising to provide a reaction selective catalyst. By synthesizing the Ag-Pt bimetallic NWs with Pt coated on a much less expensive Ag substrate, we obtain a catalyst with very strong catalytic activity with a rather small Pt content, i.e. to form the interfaces accessible. By dissolving the non-form-interface Ag substrate, we can obtain bimetallic nanotubes and recycle the Ag ions to get a high yield of catalyst. It is easy to collect and it can be used repeatedly. This method can be easily extended to other bimetallic or multimetallic NWs, since GRR is a very pervasive route. Our follow-up work is to investigate the electronic and mechanic properties of the Ag-Pt bimetallic NWs.
The authors gratefully acknowledge the financial support of the project from the PAPD(No. 50831004), the Fundamental Research Funds for the Central Universities (Nos.021314380019 and 1106021343), the Innovation Fund of Jiangsu Province (No.BY2013072-06), the Natural Science Foundation of Jiangsu Province (No. 2012729), the National Natural Science Foundation of China (No. 11374136), and the State Key Program for Basic Research of China (No. 2010CB631004).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|