Black nanostructured BiOCl microspheres were directly synthesized by a hydrothermal method. The black ball-like BiOCl microspheres and black flower-like BiOCl microspheres were obtained using different surfactant. The color of the BiOCl microspheres turned from black to white when being annealed at 400 °C in air for 3 h and could be recovered to black by exposure to ultraviolet light for a few hours. The photocatalytic activity of both the black and the white BiOCl microspheres was characterized by the photo-degradation of methyl orange dye under visible light irradiation. The black ball-like nanostructure BiOCl displayed the best photocatalytic activity, compared with the white BiOCl and the black flower-like BiOCl. It can degrade the methyl orange dye to 20% within 70 min under visible light irradiation. The high activity of the BiOCl ball-like sphere may own to its special morphology, strong absorption in visible light range and the existence of oxygen vacancies.
Semiconductor photocatalysts are widely used in hydrogen generation, solar energy utilization and environmental remediation[1] and [2]. Recently, the semiconductor material BiOCl with a layer structure is more active than TiO2 for promising practical industrial application due to its efficient photocatalytic activities, high physical and chemical stability, low cost and nontoxicity[3] and [4]. Until now, two dimensional BiOCl lamellae[5], flower-like BiOCl[6], BiOCl nanofibers[7], and other morphologies of BiOCl including BiOCl micro/nanostructures have been synthesized by low-temperature sonochemical route ultrasonic treatment, low-temperature chemical vapor transport, electrospinning method, respectively. And our group[8] and [9] has synthesized the BiOCl nanoflakes and nanowires array by anodic aluminum oxide (AAO) template via sol-gel combined with the vacuum air extraction method. Nevertheless, BiOCl is a type of wide band gap (ranging from 3.02 to 3.5 eV[10] and [11]) semiconductor material which can only be excited by UV light (5% of the solar light) or solar light irradiation. Hence, many efforts have been made to develop functional BiOCl based visible light photocatalysts by incorporating metal ions or compositing with other semiconductors[12], [13], [14], [15] and [16].
However, to further improve the photocatalytic activity of BiOCl and obtain the efficient photocatalysis applied to environmental protection, some researchers have proved that intrinsic semiconductor photocatalysts with oxygen vacancies can absorb visible light and exhibit excellent photocatalytic activity under visible light[17], [18], [19] and [20]. Chen et al.[17] observed that disorder-engineered black TiO2can more efficiently harvest the infrared photons for photocatalysis than what bulk anatase can do. And the presumable reason was that the localization of both photoexcited electrons and holes prevented fast recombination. Both our group[11] and Ye et al.[19] ever reported black BiOCl had the ability to absorb visible light irradiation which was obtained from white BiOCl by UV light irradiation. And the reason for color of change was the creation of oxygen vacancies. The oxygen vacancies only trapped electrons for its positive charge and reduced the recombination of electrons and holes, which increased the mobility of holes.
In the present work, two kinds of black BiOCl nanostructured microspheres were synthesized directly with acetylacetone (AAT) and diethylene glycol (DEG), respectively, by a hydrothermal method. We adopted different solvents to control the formation of the black BiOCl crystals in order to obtain effective photocatalytic activities. The photocatalytic activities of the BiOCl nanostructure were investigated. And photoluminescence (PL) analysis was applied to understand the oxygen vacancy function.
2 mmol Bi(NO3)3 5H2O was dissolved in 40 ml AAT or DEG, followed by adding 1 mmol BiCl3. Subsequently, the solution was transferred into a teflon-lined stainless-steel autoclave, which was sealed and heated at 180 ° C for 12 h, and then cooled down to room temperature. The precipitates were collected and washed by ethanol and deionized water repeatedly. Finally, the products were dried in vacuum at 80 ° C for 10 h and denoted as S1 (the solvent is AAT) and S2 (the solvent is DEG), respectively. In addition, the samples S3 and S4 were obtained from the samples S1 and S2 which were annealed at 400 ° C for 3 h in air, respectively.
The morphology of the as-synthesized BiOCl was characterized by scanning electron microscopy (SEM, Hitachi S-4800). The phase structure of BiOCl was carried out by X-ray diffraction (XRD) on a Bruker D8 diffractometer with CuKα radiation. UV-Vis diffuse reflectance spectroscopy (DRS) was recorded on a UV-Vis spectrophotometer (HITACHI U-3010, Japan). The UV-Vis absorption spectra were also examined using the spectrophotometer U-4100. Photoluminescence (PL) spectra of the samples were obtained on laser confocal micro-Raman spectroscope (LabRAM Aramis) with 325 nm semiconductor laser as exciting sources at room temperature.
The photocatalytic activity of the as-prepared samples was investigated by the photo-degradation of methyl orange (MO) dye. The photo-degradation experiments were carried out under visible light irradiation whose source is a 300 W Xe lamp equipped with UV cut off filter to provide only visible light (λ ≥ 420 nm). The distance between the Xenon lamp and the sample was about 30 cm. 20 mg of the as-prepared photocatalyst was suspended in 20 ml MO aqueous solution (C0 = 10 mg/l) with constant stirring. Prior to irradiation, the suspensions were stirred in the dark for 2 h to ensure the adsorption-desorption equilibrium. The HITACHI U-4100 UV-Vis spectrometer was used to determine the concentration of MO solution during the photocatalytic degradation process. Ci is the photodegrade concentration. The percentage of degradation is reported as Ci/C0.
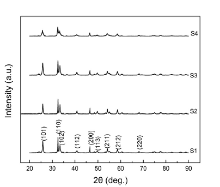
The phase structure and morphology of the as-synthesized products were characterized by XRD and SEM. Fig. 1 presents the XRD patterns of the BiOCl samples. Their diffraction peaks correspond to the (101), (110), (102), (112), (200), (113), (211), (212), and (220) planes, respectively, in which all the diffraction peaks can be perfectly indexed to the pure products of BiOCl with the tetragonal phase BiOCl (JCPDS card No. 06-0249). No other peaks arising from impurities can be found, indicating that pure BiOCl crystals have been successfully synthesized.
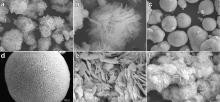
By visual observation, the BiOCl samples S1 and S2 display black. In order to shed more light on the nanostructure of these products, the SEM images of the samples are shown in Fig. 2. Fig. 2(a) is a large-scale overview with flower-like morphology of the sample S1. A magnified image of the sample S1 shown in Fig. 2(b) provides the detailed view of the structure. It can be found that the flower shape of the sample S1 consists of lots of thin flakes with thickness of 60 nm. As to the sample S2, the highly monodispersed rough ball-like spheres were obtained as shown in Fig. 2(c). The magnified SEM image for the sample S2 (Fig. 2(d)) reveals that the sphere is composed of thin plates. The nanoplates align radically and tightly to assemble into the uniform spheres, which is very different from the sample S1. Sample S1 and sample S2 after annealing in air at 400 ° C for 3 h are noted as sample S3 and sample S4, respectively. The color of the samples S3 and S4 was found to be white. The SEM images of the samples S3 and S4 are shown in Fig. 2(e, f). From Fig. 2(e), it can be found that flower-like morphology of the sample S3 grows and the sizes of nanoplates are enlarged. Fig. 2(f) shows that the ball-like spheres of the sample S2 turn to the flower-like morphology of the sample S4. The formation of flower-like morphology was observed by SEM and many nanoplates were found.
Unlike conventional white BiOCl, in this study the black nanostructured BiOCl microspheres were successfully synthesized by a hydrothermal method. In early 2006, our group[11] found that white BiOCl nanopowder can become black under UV illumination. And it was due to the oxygen vacancy. In this paper, the black BiOCl microspheres turns to white when being annealed at 400 ° C in air (Fig. 3) and can be recovered by exposure to ultraviolet light for a few hours. Under UV irradiation using Xeon lamp with 300 W power, it was found that the color of the BiOCl (S3, S4) was reverted to the original black. In addition, the thermochromic phenomenon further validates the existence of oxygen vacancy in black BiOCl. However, the color of as-prepared black BiOCl (S1, S2) which were annealed at 400 ° C for 3 h in Ar gas remained unchanged.
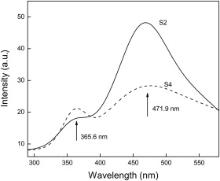
PL emission was a common and useful technique to survey the oxygen vacancy function[21], [22], [23] and [24]. Fig. 4 shows the room temperature PL spectra of the BiOCl samples S2, S4, with a 325 nm laser used as the exciting source. The emission spectra could be divided into two broad zones: the ultraviolet-blue and the visible emissions. To as-obtained sample S2, two obvious PL peaks were observed: weak ultraviolet (UV) emission, and strong green emission peaks centered at 365.6 nm, and 471.9 nm, respectively. After being annealed for 3 h in air, the two PL peaks of sample S4 were observed. To the sample S4, two PL peaks were also observed: UV emission and visible emission peak centered at around 365.6 nm and 471.9 nm, respectively. The strong visible emission was believed to be caused by the trapping of free electrons in the conduction band by the recombination centers originating from oxygen vacancy inside of the BiOCl microsphere[17], [18], [19] and [20].
As observed with experiment of heat treatment, this BiOCl exhibits a thermochromic behavior. The electron paramagnetic resonance (EPR) spectra shown in Fig. 5 prove the emergence of oxygen vacancy. The signal at g = 2.005 observed is assigned to the electron trapped at the oxygen vacancy position [11].
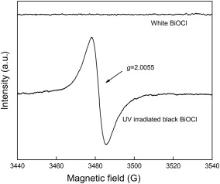 | Fig. 5 EPR spectra of the white and black BiOCl[11]. |
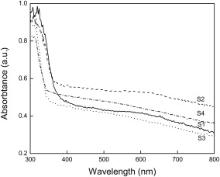
The optical properties of the BiOCl samples were characterized by UV-Vis. Diffuse reflectance spectroscopy and the results are shown in Fig. 6. It indicates that the absorption of the black BiOCl in the visible light range is greater than that of the white BiOCl. The black BiOCl has strong absorption in the whole spectra of visible light range, which can explain why the photocatalytic property of the black BiOCl is better than that of the white BiOCl. However, the absorption edge of the black BiOCl does not show any obvious red shift in the spectrum, which was mentioned in Ref.[17].
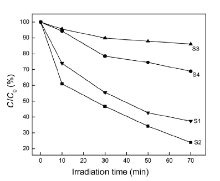
The MO photo-degradation curves of the BiOCl samples from S1 to S4 are shown in Fig. 7. It can be seen that the sample S2 degrades about 80% of MO dye within 70 min under visible light irradiation. In contrast, the sample S1 only degrades about 60% of MO dye at the same condition. The results reveal that the BiOCl ball-like microspheres (S2) show a better photocatalytic activity on the degradation of pollutants under the visible light irradiation, compared with the S1 products. During the same time of visible light irradiation, it can be seen that the white BiOCl samples S3, S4 show weak photocatalytic activity with MO dye removal efficiency of 15% and 28%, respectively. Comparing degradation curves of the samples S2 with S4, the photocatalytic activity of the samples S2 is better. On the other hand, it was found that after the irradiation for 70 min the concentration of MO dye was degraded about 60%, 15% by the samples S1, S3, respectively.
From the comparison of the photocatalytic activity of the samples from S1 to S4 with each other, the black BiOCl has the ability to absorb visible light irradiation. In comparison with the white BiOCl (S3, S4), although the black BiOCl (S1, S2) samples have very similar XRD patterns and the absorption edge, they exhibit higher photo-reactivity under visible light. However, the generated oxygen vacancies, as color center, extend the light reposing range of BiOCl from UV light to visible light[17], [18], [19] and [20]. Between the black BiOCl samples S1 and S2, the hierarchical sample S2 supplies the highest photocatalytic activity, owing to the assembled nanoplates into ball-like spheres, which has an ability to increase their specific surface area. It is well known that the specific surface area of the photocatalyst is an important factor influencing the photocatalytic activity. Work of Zhang et al.[25] explained that the ball-like Bi2WO6 nanospheres had good photocatalytic activity because of its large specific surface area. The black BiOCl sample S2 has good photocatalytic activity which should be attributed to the special surface structure.
Black BiOCl nanomaterial was directly synthesized by a hydrothermal method with AAT or DEG as the solvent. Two types of morphologies were obtained. The color of the BiOCl turned from black to white when it was annealed in air at 400 ° C for 3 h. Under UV irradiation, it was found that the white color of the BiOCl reverted to the original black. The photocatalytic activity of the black BiOCl was better than that of the white one which degraded to 20% of MO dye at the same condition. And the photocatalytic activity of the black BiOCl (DEG) was better than that of the black BiOCl (AAT). The higher activity of the BiOCl (DEG) ball-like sphere assembly may benefit from its unique morphology and strong absorption in the visible light range. In addition, PL spectra showed that the samples of the black BiOCl had a large number of oxygen vacancies, which were in favor to absorb much visible light, as a result, to improve the photocatalytic property. Therefore, the black ball-like BiOCl nanomaterial would be a potential candidate as an efficient visible light photocatalyst.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|