** Corresponding author. Prof., Ph.D.; Tel./Fax:þ86 24 83970826; E-mail address: zqliu@imr.ac.cn (Z.Q. Liu).
Two low-cost synthesis routes have been developed to fabricate carbon-coated Li4Ti5O12 by using H2TiO3 instead of anatase TiO2 as Ti source through solid-state reaction process. One route is a direct solid mixture of H2TiO3, Li2CO3 and pitch followed by high-temperature solid-state reaction. The other includes mixture of H2TiO3 and Li2CO3 with pitch dissolved in furanidine under vacuum and the same solid-state reaction procedure is followed after the mixture is totally dried. Microstructural investigations indicate that H2TiO3 exhibits secondary aggregates morphology with primary particle sizes of 10-20 nm. Carbon-coating layers with thickness of 2-3 nm have been observed on Li4Ti5O12 synthesized by the two routes. Cyclic performance, rate capability and electrochemical impedance spectrum of the two Li4Ti5O12/C composites have been performed, which indicate that Li4Ti5O12/C obtained by furanidine-assisted mixture exhibits better electrochemical performance than Li4Ti5O12/C synthesized by direct solid mixture. The possible reasons have been discussed. The low-cost synthesis routes of Li4Ti5O12/C using H2TiO3 as Ti source are expected to be more competitive than the traditional one for practical applications.
Currently, the anode materials for commercial lithium-ion batteries (LIBs) are carbonaceous materials with insertion/desertion potential about 0.1 V (vs Li/Li+), which may induce the formation of Li dendrites in overcharge state and further cause safety problems. Spinel Li4Ti5O12 has been considered to be a very safe anode material, since its insertion/desertion potential is 1.55 V (vs Li/Li+). Therefore no solid-electrolyte interphase films and Li dendrites can be formed even at overcharge state. Further, Li4Ti5O12has excellent cyclic lifetime since its volume variation is less than 1% during insertion/desertion process of Li+, which makes Li4Ti5O12 a very promising anode candidate of LIBs for applications in the fields of stationary energy storage systems and electric vehicle[1], [2], [3] and [4]. Although the electronic conductivity of Li4Ti5O12 is very low (∼10− 13 S cm− 1), previous reports indicate that the electrochemical performance of Li4Ti5O12 can be drastically enhanced by carbon coating or doping[5], [6], [7], [8], [9], [10], [11], [12], [13] and [14]. Up to date, commercial Li4Ti5O12 has been used in short-distance electric vehicles and energy storage field.
Generally, spinel Li4Ti5O12 is synthesized using nanosized anatase TiO2 and Li2CO3 or LiOH as starting materials through 800-900 ° C solid-state reaction process. However, the high price of nanosized TiO2 induces high cost of Li4Ti5O12, which may hinder its practical applications. According to the goals of Department of Energy of US, LIBs for electric vehicles and stationary energy storage systems should be in prices of US dollars of 125/kWh and 100/kWh by 2030, respectively[15]. However, the current price for LIBs is about US dollars of 700/kWh. Therefore, to replace TiO2 with an alternative Ti source with low cost is expected to be a very effective way to low the synthesis cost of Li4Ti5O12. H2TiO3is an intermediate product during industrial fabrication of TiO2, and therefore has lower cost. However, up to date, very few reports about synthesis of Li4Ti5O12 using H2TiO3 as Ti source have been documented in literature[16]. Herein, we report solid-state synthesis of carbon-coated Li4Ti5O12composites using H2TiO3 as Ti source. The microstructure and electrochemical performance of Li4Ti5O12/C has also been investigated and discussed.
Two methods were exploited to synthesize carbon-coated Li4Ti5O12 using H2TiO3 as Ti source. The first method was that Li2CO3 was mixed with H2TiO3 in accordance with stoichiometric Li4Ti5O12. Then pitch with 6 wt% of total mass of as-synthesized Li4Ti5O12 was added to the mixtures. After being ground for 2 h, the mixture was transferred to a tube furnace and heated at 800 ° C for 14 h under Ar gas flow, and then naturally cooled down to ambient temperature. The second method was that Li2CO3 was mixed with H2TiO3 in accordance with stoichiometric Li4Ti5O12. Then the mixture was put into a three-neck flash. Pitch with 6 wt% of total mass of as-synthesized Li4Ti5O12 was dissolved into furanidine. After the three-neck flash was mechanically pumped for 2 h, the furanidine solution was pumped into the three-neck flash and stirred for 1 h at ambient temperature. Then the solution was transferred to a water bath and heated at 70 ° C with continuous stirring until the furanidine was totally evaporated. The remainder was dried at 80 ° C overnight under vacuum and then ground for 2 h before being transferred to a tube furnace for solid-state synthesis. The following solid-state synthesis procedure was the same as the first method. Hereafter, the products synthesized with the first and the second methods are referred to as LTO-1 and LTO-2, respectively.
The microstructures of H2TiO3 and the products were characterized by thermogravimetry-differential scanning calorimetry (TG-DSC, RIGAKU/TG8120) analysis, X-ray diffraction (XRD, Bruker D8 ADVANCE, CuKα radiation), scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEM 2100).
Electrodes were prepared by drying a slurry (a mixture of 80 wt% active materials, 10 wt% acetylene black and 10 wt% polyvinylidene fluoride dissolved in N-methy1-2-pyrrolidinone) at 100 ° C for 4 h under vacuum. CR2032 coin-type cells with lithium metal as counter electrode were used. The weights of active materials in the cells are in a range of 5-6 mg. The electrolyte was composed of 1 mol/L LiPF6dissolved in a mixture of ethylene carbonate, diethyl carbonate and dimethy1 carbonate with volume ratio of 1:1:1. Electrochemical impedance spectrum (EIS) measurements were performed in an electrochemical workstation (CHI600A, CH Instruments, Inc.) with a frequency range of 105-10− 2 Hz. The discharge-charge measurements were carried out on a battery cycler in a voltage range between 1.0 and 2.5 V (CT 2001A, Land Electronic Co.).
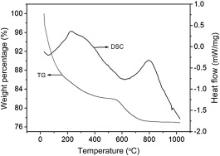
The microstructure of H2TiO3 was first characterized. Fig. 1 displays TG-DSC curves of H2TiO3. From TG curve, it can be seen that there are two weight-loss regions during heating. The weight-loss region from ambient temperature to ∼200 ° C is believed to be induced by loss of H2O physically adhered to H2TiO3. Correspondingly, an endothermal peak located at about 90 ° C is demonstrated in the DSC curve. The second weight-loss region happens between about 550 and 700 ° C, which is induced by decomposition of H2TiO3, i.e,
equation(1)
H23TiO→ 2TiO2+HO↑ .
Accordingly, an endothermal peak located at ∼600 ° C appears in the DSC curve. Besides these, a weak endothermal peak appears at ∼950 ° C. However, no weight-loss region corresponds to this endothermal reaction, which may be caused by phase transformation from anatase to rutile TiO2.
SEM images of H2TiO3 are shown in Fig. 2. From Fig. 2(a), it can be seen that H2TiO3 exhibits morphology of secondary aggregates with size in micrometer range. Fig. 2(b) is a high-magnification SEM image which indicates that the secondary aggregates are composed of primary nanoparticles with size of 10-20 nm.
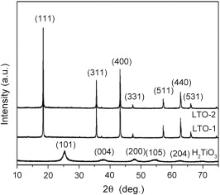
Fig. 3 displays XRD patterns of H2TiO3, LTO-1 and LTO-2. The XRD pattern of H2TiO3 can be indexed as anatase TiO2 (JCPDS No. 21-1272). The broad reflection peaks imply that the crystallinity of H2TiO3 is low and their sizes are in nanometer range. LTO-1 and LTO-2 can be indexed as spinel Li4Ti5O12(JCPDS No. 26-1198). No other phases were observed by XRD characterization, indicating that both LTO-1 and LTO-2 are pure Li4Ti5O12. The sharp reflection peaks imply that both products have high crystallinity.
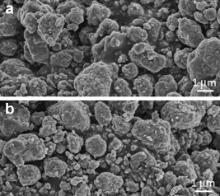
Morphologies of LTO-1 and LTO-2 are exhibited in Fig. 4(a) and (b), respectively. The two products display much similar morphology and size. The sizes of the secondary aggregates are not uniform and are in a range of several hundreds of nanometers to a few micrometers. Compared with Fig. 2(a), it can be seen that the size and morphology of the two products are roughly inherited from H2TiO3. However, the primary particle sizes of the two products increase to 100-300 nm.
Fig. 5(a) and (b) are TEM images of LTO-1 and LTO-2, respectively. Similar with SEM observation, TEM images also exhibit secondary aggregate morphology of the two products. The structure of central parts of the aggregates could not be resolved since the aggregates are too thick to allow electron beam transmission. The high-resolution transmission electron microscopy (HRTEM) images shown inFig. 5(c) and (d) are taken from LTO-1 and LTO-2, respectively, which reveal amorphous carbon coating layers with thickness of 2-3 nm on the surface of Li4Ti5O12, demonstrating the formation of the expected carbon-coated Li4Ti5O12 of both LTO-1 and LTO-2. The yield of pitch was measured to be 76.6%, therefore the carbon content in Li4Ti5O12/C was calculated to be 4.6 wt%.
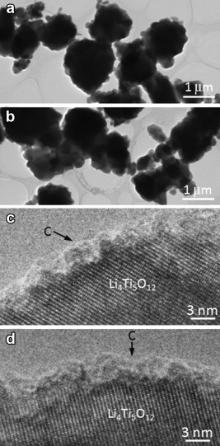 | Fig. 5 TEM images of LTO-1 (a) and LTO-2 (b). HRTEM images of LTO-1 (c) and LTO-2 (d) showing carbon-coating layers. |
The electrochemical performance of LTO-1 and LTO-2 were evaluated. Fig. 6(a) displays the first-cycle voltage profiles of the two products at 0.1 C. Both LTO-1 and LTO-2 exhibit typical long voltage plateaus at about 1.55 V. Further, it can be seen that the voltage hysteresis of the two products are very small during discharge/charge cycling. The first-cycle discharge/charge capacities of LTO-1 and LTO-2 are 162.6/161.6 mAh g− 1 and 169.8/169.0 mAh g− 1, respectively. Correspondingly, their first-cycle coulombic efficiencies are 99.4% and 99.5%, respectively.
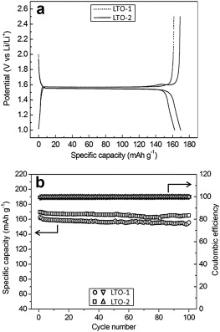 | Fig. 6 Cyclic performance of LTO-1 and LTO-2 at 0.1 C: (a) first-cycle voltage profiles of the two products, (b) discharge cyclic performance and coulombic efficiency of the two products. |
The cyclic performances of the two products at 0.1 C are shown in Fig. 6(b). It can be seen that both products display very stable cyclic performances. Overally LTO-2 exhibits higher cyclic capacities than LTO-1. After 100 cycles, the discharge capacities of LTO-1 and LTO-2 are 155.6 and 165.1 mAh g− 1, respectively. Correspondingly the capacity retentions of LTO-1 and LTO-2 are 95.7% and 97.2%, respectively. LTO-2 displays higher capacity retention than LTO-1. In addition, both products exhibit excellent coulombic efficiencies during discharge/charge cycling. After initial cycle, the coulombic efficiencies of both products are over 99.7%, implying very high structural stability of the two products.
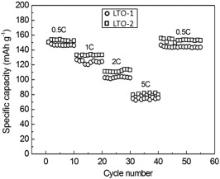
Rate capabilities of the two products were also evaluated, as shown in Fig. 7. Overally LTO-2 displays higher rate capabilities than LTO-1 at 0.5 C, 1 C, 2 C and 5 C. The 10th discharge capacities of LTO-1 and LTO-2 at 0.5 C are146.1 and 152.7 mAh g− 1, respectively. After 30 cycles at different C-rate, the initial discharge capacities of LTO-1 and LTO-2 recover to 146.5 and 155.8 mAh g− 1 at 0.5 C, indicating high capacity recovery ability of the two products. Especially the discharge capacity of LTO-2 increases by 2%.
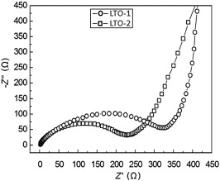
Nyquist plots of the two products at as-synthesized state are displayed in Fig. 8. It can be seen that both Nyquist plots are similar in shape, each of which is composed of a semicircle at high frequency domain and a straight line in the low frequency domain. The radius of the semicircle is an indicative of charge transfer resistance Rct happened in electrode/electrolyte interface. Apparently, Rct of LTO-2 is lower than that of LTO-1, implying that LTO-2 owns higher electronic conductivity and consequently higher rate capability than LTO-1.
The electrochemical performance of LTO-2 is better than that of LTO-1. Possible reasons might be the better carbon covering in LTO-2 than in LTO-1. The starting materials H2TiO3 exhibit secondary aggregates with size in micrometer range. Therefore the wettability of liquated pitch with nanosized primary H2TiO3 particles during synthesis process is the key point to obtain carbon-coated Li4Ti5O12with high electrochemical performance. LTO-2 was synthesized by mixing H2TiO3 aggregates with liquid furanidine dissolved with pitch under vacuum, while LTO-1 was obtained by direct solid mixture of H2TiO3 aggregates with pitch. A better covering of carbon with Li4Ti5O12 in LTO-2 can be expected, which is believed to be the main reason for the higher electrochemical performance. In addition, the rate capability of the two products can be further improved by using H2TiO3 aggregates with small size or monodispersed nanoparticles.
Carbon-coated Li4Ti5O12 composites have been synthesized using H2TiO3 as Ti source through solid-state reaction process. H2TiO3 with low cost is demonstrated to be an alternatively Ti source besides commonly used TiO2. The Li4Ti5O12/C composite obtained by mixture of H2TiO3 aggregates with liquid furanidine dissolved with pitch under vacuum exhibits better cyclic performance, rate capability and lower electron charge transfer resistance than the one synthesized by direct solid mixture of the starting materials. The low-cost synthesis route of Li4Ti5O12/C is believed to be more competitive than the traditional one using TiO2 as Ti source.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|