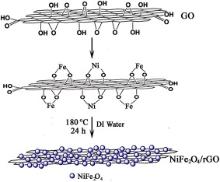
In this work, a facile, one-pot route has been applied to synthesize nanohybrids based on mixed oxide NiFe2O4 and reduced graphene oxide (rGO). The hybrid is constructed by nanosized NiFe2O4 crystals confined by few-layered rGO sheets. The formation mechanism and microstructure of the hybrids have been clarified by X-ray diffraction, Raman spectroscopy, scanning electron microscopy, and transmission electron microscopy. Electrochemical tests show that the performance of NiFe2O4 can be considerably improved by rGO incorporation. The performance improvement can be attributed to the two-dimensional conductive channels and the unique hybrid structure rGO constructed. The easy synthesis and good electrochemical performance of NiFe2O4/rGO hybrid make it a promising anode material for Li-ion batteries.
Graphene, a two-dimensional (2D) flat monolayer sheet of sp2-bonded carbon atoms, has attracted a considerable interest in various fields such as materials science, chemistry, and condensed matter physics, since it was first discovered by Novoselov group[1], [2] and [3]. The unique structure endows graphene with some attractive properties, including excellent chemical/thermal stability[4], high electrical conductivity[5], superior mechanical strength[6], and large specific surface area[7]. Despite these unique properties, bare graphene is not an ideal Li-storage host because of its poorly developed potential plateaus, large potential hysteresis, and high irreversible capacity on the first cycle, even though it could exhibit moderate capacity, satisfactory rate capability, and good cycling stability[8], [9] and [10]. Instead, graphene is more suitable to act as support for nanostructured Li-storage materials[11], [12] and [13], for example transition metal oxides (TMOs) which generally show high capacity but poor cycling performance[14].
Previous work has shown that the electrochemical performance of some TMOs, Co3O4[15] and [16], CoO[17] and [18], NiO[19], Fe2O3[20] and [21], Fe3O4[22], Mn3O4[23] and [24], MnO[25] etc., can be remarkably improved by forming hybrids with graphene. Besides simple oxides, some mixed oxides, such as CoFe2O4[26] and [27], MnFe2O4[28], NiFe2O4[29], and ZnFe2O4[30] and [31] also show improved electrochemical properties by introducing graphene. NiFe2O4 is a typical mixed oxide and has been investigated as a Li-storage host[32], [33], [34], [35], [36], [37] and [38]. The theoretical capacity of NiFe2O4 is calculated to be 914 mA h g− 1 according to the reactions[36]:
equation(1)
NiFe2O4 + 8Li → Ni + 2Fe + 4Li2O
equation(2)
Ni + 2Fe + 4Li2O ↔ NiO + Fe2O3 + 8Li
After the first cycle, reversible reaction between Ni/Fe/Li2O and NiO/Fe2O3/Li occurs while NiFe2O4cannot be recovered. Compared with Fe2O3, NiO exhibits a low conversion potential[14], leading to a low Li-storage potential of NiFe2O4 compared with Fe2O3. In addition, the mixed oxide may show improved cycling performance than the corresponding simple oxide since one component may act as the buffer for the other because of the different conversion potentials. As previously reported, NiFe2O4/carbon-nanotubes (CNTs) exhibited better electrochemical performance than Fe2O3/CNTs[39], indicating the advantage of mixed oxides.
It was found that the electrochemical performance of NiFe2O4 can be improved using nanostructured materials[38] and [40], or forming hybrids with carbon materials[37] and [39]. A low-temperature solution route is favored to synthesize small sized NiFe2O4. Besides, a carbon matrix is usually needed to further improve the electrochemical performance. However, it is difficult to achieve a uniform dispersion of nanoparticles on carbon matrix, for example graphene, because of its hydrophobic nature. In contrast, graphite oxide (GO), the precursor for graphene, can be sufficiently exfoliated to single-layered or few-layered graphene oxide sheets in water or some polar solvents[41] and [42]. In addition, the negatively charged graphene oxide can absorb positively charged metallic ions by electrostatic attraction[43]. Also, the oxygen-containing groups can be removed after the hydrothermal or solvothermal reaction, leading to the formation of reduced graphene oxide (rGO). Unlike perfectly crystallized graphene, rGO processed by solution route still contains residual oxygen-containing groups. Thus, it is possible to synthesize graphene-based hybrids with a facile in situ route by using GO as the precursor.
In our previous work[26], a controllable route was proposed to prepare CoFe2O4/rGO and MnFe2O4/rGO hybrids. The hybrid could exhibit better electrochemical performance than bare CoFe2O4. To synthesize uniform CoFe2O4/rGO and MnFe2O4/rGO, a low-temperature pre-heating step is necessary and organic solvent such as ethylene glycol (EG) is needed. Without the pre-heating step, large particles will form. It suggests that the oxygen-containing groups can be rapidly removed in the reductive solvent, for instance EG. Without the pinning effect of these groups, small crystals tend to aggregate into large particles. In this work, we found that NiFe2O4/rGO can be synthesized by a facile in situ route in deionized (DI) water which is less reductive compared with some organic solvents. In the hybrid, NiFe2O4nanocrystals of 5-10 nm can be uniformly dispersed on rGO without the pre-heating step. The hybrid shows obviously improved electrochemical performance than bare NiFe2O4 due to rGO incorporation and formation of the unique hybrid structure.
To synthesize NiFe2O4/rGO, first, 50 mg of GO prepared by a modified Hummers method[44], was uniformly dispersed in 50 mL of DI water by vigorous sonication to form solution A. 0.5 mmol of Ni(NO3)2· 6H2O (Alfa Aesar) and 1 mmol of FeCl3· 6H2O (Alfa Aesar) were dissolved in 10 mL of DI water with stirring to form solution B. Then, solution A was slowly added into solution B with stirring. Afterwards, the pH of the mixed solution was adjusted to ∼10 using 25 wt% ammonia water. Finally, the mixed solution was transferred to a Teflon-lined stainless steel autoclave (100 mL) and heated in an electric oven at 180 ° C for 24 h. The resulting product was collected by centrifugation, washed with DI water and absolute ethanol repeatedly, and dried at 40 ° C under vacuum overnight. A control experiment was performed to prepare bare NiFe2O4 (NFO) using the same procedure without the addition of GO. Bare rGO was also prepared by hydrothermal reaction at 180 ° C for 24 h using GO as the precursor.
The phases of the obtained products were analyzed by X-ray diffraction (XRD) on a Rigaku D/Max-2550pc powder diffractometer equipped with Cu Kα radiation (λ = 0.1542 nm). The morphologies of the products were observed by field-emission scanning electron microscopy (SEM) on an FEI-sirion microscope and transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) on a JEM 2100F microscope. Raman spectra were collected on a Jobin-Yvon Labor Raman HR-800 Raman system using a 514.5 nm Ar-ion laser at 10 mW. N2 absorption/desorption isotherms were measured on an AUTOSORB-1-C apparatus. Thermogravimetric (TG) analysis was conducted on a DSCQ1000 instrument from 35 to 800 ° C at a ramp rate of 10 ° C min− 1 in air.
The electrode slurry was made by mixing 75 wt% active material (NiFe2O4/rGO, NiFe2O4), 15 wt% acetylene black and 10 wt% polyvinylidene fluoride (PVDF) in N-methyl pyrrolidone (NMP) with magnetic stirring for 2 h. The slurry was pasted onto Ni foam to make the working electrodes. The loading of electrode is around 2 mg. The electrodes were dried at 100 ° C under vacuum for 10 h before the batteries assembly. CR2025-type coin cells were assembled in an argon-filled glove box using metallic Li foil as the counter electrode, 1 mol L− 1 LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1:1 in volume) as the electrolyte, and polypropylene microporous film (Celgard 2300) as the separator. The cells were charged and discharged on a NEWARE battery tester (Shenzhen, China) in a voltage range of 0.005-3.0 V (vs. Li/Li+) at various current densities. All of the electrochemical measurements were conducted at 25 ° C.
NiFe2O4/rGO was synthesized by a facile one-pot hydrothermal route at 180 ° C for 24 h. As shown inFig. 1(a), all the diffraction peaks of NFO/rGO and NFO can be indexed to cubic NiFe2O4 phase (space group Fd-3m, JCPDS cards No.74-2081). The broad diffraction peaks of the two samples suggest that their particle sizes are rather small. Fig. 1(b) gives the TG curve of NFO/rGO, from which the rGO content is estimated to be 30 wt%. At such a high rGO content, however, the diffraction peak of rGO was not detected, while bare rGO exhibits its (002) characteristic peak at around 2θ = 25° . This indicates that the restacking of rGO sheets after reduction has been effectively refrained by anchoring NiFe2O4 particles as spacers. As seen in Fig. 1(c), the Brunauer-Emmett-Teller (BET) specific surface area (SBET) of NFO increases from 85 to 107 m2 g− 1 by rGO incorporation. Because of severe restacking, bare rGO exhibits a low SBET of 29 m2 g− 1 [25]. This indicates that the increase in SBET is mainly due to the formation of the hybrid structure. Fig. 1(d) presents Raman spectra of NFO/rGO and GO. Both samples exhibit two bands at 1350 and 1580 cm− 1, corresponding to the D line and G Line of carbon materials. NFO/rGO shows an increased D/G intensity ratio compared with GO, due to a decreased average size of the sp2domains and an increased number of these domains during the reduction of GO [45].
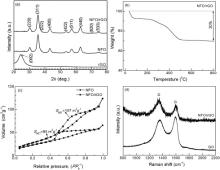 | Fig. 1 (a) XRD patterns of NFO/rGO, NFO, and rGO, (b) TG curve of NFO/rGO, (c) N2 absorption/desorption isotherms of NFO/rGO and NFO, and (d) Raman spectra of NFO/rGO and GO. |
The morphology of NFO/rGO is characterized by SEM, TEM and HRTEM. Unlike CoFe2O4 and MnFe2O4[26], NiFe2O4 cannot form in EG because Ni2+ will be reduced to metallic Ni by EG. Instead, it was synthesized in DI water. The hydrothermal product exhibits a thin sheet-like structure with a lateral size of several microns as seen in Fig. 2(a). TEM image in Fig. 2(b) demonstrates that the thin rGO sheet is densely decorated with nanosized NFO without the formation of large aggregations. HRTEM image inFig. 2(c) shows that each NFO particle is a single crystal with a size of 5-10 nm. This means that the attachment of primary nanocrystals to large secondary particles did not occur for NFO, unlike the case in CoFe2O4 or MnFe2O4. The inhibition of crystals attachment can be attributed to the pinning effect of the residual oxygen-containing groups on NFO due to the use of less reductive water instead of EG. HRTEM image also displays that NFO nanocrystals are firmly confined by few-layered rGO sheets. Fig. 2(d) presents energy dispersive X-ray spectroscopy (EDS) of NFO/rGO from which the Ni/Fe atomic ratio is roughly determined to be 1:2. The SEM and TEM images of bare NFO are presented in Fig. 2(e, f). Note that without the fixing effect of rGO, NFO crystals tend to aggregate.
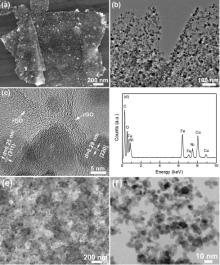 | Fig. 2 (a) SEM, (b) TEM, and (c) HRTEM images of NFO/rGO, (d) EDS of NFO/rGO, and (e) SEM and (f) TEM images of bare NiFe2O4. |
The formation process of NFO/rGO hybrid is schematically illustrated in Fig. 3. Positively charged metallic ions (Ni2+, Fe3+) are first attracted by negatively charged graphene oxide sheets by electrostatic attraction, followed by hydrolysis and the nucleation. The oxygen-containing groups on graphene oxide are not completely removed at the early reaction stage which can act as the nucleation sites for NiFe2O4crystallization and as the barriers for the slide and aggregation of NiFe2O4 crystals, refraining the crystal growth of NiFe2O4. Interaction between rGO and Ni or Fe is possible by forming C-O-Ni[46] or C-O-Fe[47] linkages, further preventing the crystal growth of NiFe2O4. When the reaction proceeds, reduction of graphene oxide to hydrophobic rGO sheets occurred, leading to the confinement of NFO crystals by restacked rGO sheets. The confining effect of rGO sheets also inhibits the aggregation of NFO nanocrystals.
Charge and discharge cycling was performed to investigate the effect of rGO introduction on electrochemical performance of NiFe2O4. Fig. 4(a) shows the first three voltage profiles of NFO/rGO charged (de-lithiation) and discharged (lithiation) at 50 mA g− 1. The specific capacity and specific current of NFO/rGO were calculated based on the total mass of NFO and rGO. The first charge and discharge capacities of NFO/rGO are 837 and 1546 mA h g− 1, respectively. After the first cycle, reversible reactions occur for NFO/rGO evidenced by its almost overlapped charge or discharge curves. In contrast, the charge capacity of bare NFO decreases from 361 to 260 mA h g− 1 within only three cycles as shown in Fig. 4(b). The first charge capacity of NFO/rGO is calculated to be 1195 mA h g− 1 if the mass of rGO (30 wt%) is excluded, higher than the theoretical capacity (914 mA h g− 1) of NiFe2O4. The extra capacity can be attributed to the enhanced exfoliation of rGO sheets by NFO loading which renders more exposed vacancy defects for Li-storage[9]. The reversible Li-storage capacity of rGO itself is low as observed in our previous work[25].
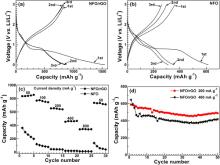 | Fig. 4 The first three voltage profiles of (a) NFO/rGO and (b) NFO, (c) comparison of rate capability between NFO/rGO and NFO, and (d) cycling stability of NFO/rGO charged at 200 and 400 mA g− 1. |
Fig. 4(c) compares the rate capability between NFO/rGO and NFO. The cells are charged at various current densities and discharged at 50 mA g− 1. Clearly, the rate capability of NFO is significantly improved by forming a hybrid with rGO. At 800 mA g− 1, NFO/rGO can still deliver a charge capacity of around 340 mA h g− 1. By contrast, the value of bare NFO at this current density is only 11 mA h g− 1. The improved rate capability of NFO/rGO is related closely to the unique microstructure of the hybrid. First, rGO offers 2D electronically conductive channels for the active particles since they are firmly and uniformly anchored on rGO sheets because of their small size; second, although NiFe2O4 crystals are densely anchored on rGO, there is still sufficient free space in hybrid that facilitates the wetting of the active materials by electrolyte and rapid Li-ion diffusion at the particle/electrolyte interface; third, the small size (5-10 nm) of NFO nanocrystals reduces the Li-ion diffusion length, rendering rapid Li-ion transport in bulk particles; Fourth, few-layered rGO also supplies rapid Li-ion diffusion channels. Clearly, the improvement in rate capability is closely related to the unique structure of the hybrid composed of small sized NiFe2O4 and few-layered rGO.
Fig. 4(d) shows the cycling performance of NFO/rGO at charge current of 200 and 400 mA g− 1. Stable cycling of NFO becomes possible by fabricating a hybrid with rGO. At 200 mA g− 1, NFO/rGO delivers an initial charge capacity of 590 mA h g− 1. After 50 cycles, the capacity can be maintained at 489 mA h g− 1. Note that the capacity is on the increase after 35 cycles. It suggests that the increase in capacity is attributed to the gradual activation of NiFe2O4 particles after initial cycles. The pulverization of NiFe2O4into smaller particles during cycling may also contribute to the capacity rise since size reduction generally increases the utilization of the active material, provided that the smaller particles still anchor on rGO sheets. The good cycling stability of NFO/rGO can be ascribed to the following factors: (1) rGO can tightly immobilize the active particles even though pulverization occurs; (2) the high Li-ion/electronic conductivity of rGO makes it possible for rapid electrochemical reactions at high current densities; (3) both the free space and rGO in the hybrid provide buffering effect for the volume changes. The electrochemical performance of our NiFe2O4/rGO is better than those of NiFe2O4 samples which usually exhibited rapid capacity fade without carbon support[34] and [35], and is comparable with or superior to those using carbon matrix considering the applied current density[29] and [37]. Note that although CNTs addition could significantly improve the electrochemical performance of NiFe2O4, the synthesis of NiFe2O4/CNTs involved the use of costly precursors and a relatively complicated procedure[39]. The facile synthesis method and good electrochemical performance make our NiFe2O4/rGO hybrid a promising anode material for Li-ion batteries.
NiFe2O4/rGO nanohybrid has been successfully synthesized by a facile in situ hydrothermal route. The formation of NiFe2O4 and reduction GO can occur simultaneously through a one-pot route. In the hybrid, NiFe2O4 nanocrystals (5-10 nm) are uniformly distributed on rGO. NiFe2O4/rGO hybrid shows better rate capability than bare NiFe2O4 due to the formation of 2D Li-ion/electronic conductive channels and easy electrode wetting of the hybrid structure. The hybrid shows good cycling stability because of the confining effect of rGO for NiFe2O4 crystals as well as the buffering effect of free space and rGO for the volume changes. Our NiFe2O4/rGO sample is among the best ones for NiFe2O4-based anode materials. This work provides a facile, cost-effective, and controllable route to synthesize high-quality anode material based on mixed oxides.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|