The purpose of this paper is to investigate heat dissipation performance of porous copper with long cylindrical pores fabricated by a unidirectional solidification method. Three samples with porosity of 29.87%, 34.47% and 50.98% were chosen and cut into size of 60 mm (length) × 26 mm (width) × 2 mm (thickness) along the vertical direction of pore axis. Their heat dissipation performance was evaluated by a nonsteady method in air and compared to those of not only bulk copper but also bored coppers with porosity of 30.61% and 32.20%. It is found that the porous copper dissipated heat faster by a forced air convection than that by natural convection from 80 °C to room temperature and both porosity and pore size play an important role in the performance for the porous copper. Furthermore, the heat dissipation rate is higher when the forced air was circulated along the specimens than that perpendicular to the specimens for the porous copper. It is revealed that porous copper with bigger porosity and a proper pore size possesses a higher heat dissipation rate. It is concluded that the porous copper with elongated cylindrical pores has larger heat dissipation performance than both the bulk copper and the bored copper, which is attributed to its higher specific surface area. Application of the porous copper for heat dissipation is promising.
Heat dissipation in high power electronics and laser diodes faces serious challenges under the trend of high frequency, miniaturization and growing capacity. Generally, a novel heat sink or a heat dissipation-assisted equipment with large heat transfer performance has to be attached to a power device or a laser diode for a normal temperature level[ 1].
Among various types of heat sinks, those utilizing micro-channels with channel diameters of several tens of microns are expected to have excellent cooling performance because a higher heat transfer capacity was obtained with smaller channel diameters[ 2]. Recently, porous metals have been considered as preferable for three-dimensional micro-channels, and a promising alternative for compact heat exchangers due to the high surface area density, superior thermodynamic characteristics and good mechanical properties[ 3], [ 4], [ 5], [ 6],[ 7]. Among porous materials such as sintered porous metal, cellular metal and fibrous composite, the porous metal with elongated cylindrical pores, which is also called gasars[ 8] and lotus-type porous metal[ 9],[ 10], is preferable for heat sinks due to small pressure drop of cooling water flowing through the pores[ 11]. It has been reported that the lotus-type porous copper heat sink showed a very large heat transfer coefficient of 8 W/(cm2 K) under the velocity of 0.2 m/s of cooling water[ 2], which is 1.7 times higher than that for the micro-channels and 6.5 times higher than that for the conventional groove fins. Chen et al.[ 12] reported that the porous copper heat sink with pore length about 20 mm has a heat transfer coefficient of 5 W/(cm2 K) when its porosity is 29% and mean pore diameter is 400 μm, which can be increased to 6.5 W/(cm2 K) after cutting the porous copper along the vertical direction of pore axis into two section. Their observation implies that the length of pores may play an important role in the heat transfer coefficient of the porous metal. By the theoretical analysis, Chen et al.[ 13] predicted that the porous copper used for heat sink with excellent heat transfer performance should have the following porous structure: the pore diameter is 0.1-0.6 mm; the porosity is 30%-70%.
The porous metal with elongated cylindrical pores has shown an excellent heat transfer performance with a liquid as heat transfer medium[ 14]. However, knowledge with air as heat transfer medium is absent on the porous metal. Actually, most of the investigations and applications on heat transfer for porous metals were performed with air, due to the high pressure drop associated with liquids[ 15]. In the present work, heat dissipation performance of the porous copper with elongated cylindrical pores with air as heat transfer medium was firstly investigated with consideration of the effects of porosity and pore size on heat flow. Secondly, the effect of forced air convection on heat dissipation performance of the porous copper was examined. Finally, the results mentioned above were discussed.
The porous copper was fabricated by a vacuum-assisted and pressurized casting apparatus, comprised a high-purity graphite crucible (172 mm in inside diameter, and 320 mm in length), a middle-frequency induction heating coil and a mold (135 mm in inside diameter, and 380 mm in length) with a water-circulated chiller under bottom and a thin ceramic wall adjacent to the lateral side of the mold for the solidification from the bottom to top only. The crucible in the apparatus was placed perpendicularly with respect to the axis of a funnel and the mold. After the chamber was evacuated to 0.5 Pa, high-purity copper (99.99 wt%) was melted in the crucible by middle-frequency heating under 0.1 MPa of hydrogen to a temperature of 1603 K, additional hydrogen and argon were introduced into the chamber and a pressurized condition was maintained at 1603 K for 2400 s. The purity of each gas used was 99.999%. The pressure values during melting and solidification in this work were 0.2 and 0.5 MPa for hydrogen ( pH2), while 0.1, 0.3, 0.5 MPa for argon ( pAr), as listed in Table 1. Then the apparatus was rotated by 90 ° to pour the melt into the mold through the funnel, in which the metal liquid was solidified unidirectionally. The details about this apparatus and the fabrication technique were given in our previous paper[ 16].
| Table 1. Fabrication conditions for the porous copper samples |
The porous copper ingots were cut in transverse section using an electric discharge machine (DK7763, Longhao digital-controlled machine Corp., China) between 30 mm and 130 mm from the bottom and further cut into some plates with size of 60 mm (length) × 26 mm (width) × 2 mm (thickness) along the vertical direction of pore axis.
The porous copper specimens were examined using optical technique on the cross section. Three images on each sample were analyzed by using an SISC Image Analyzing software (KYKY Technology Development Ltd., China) for determining the pore size and the pore density. The porosity of the porous copper specimens was evaluated from their weights and volumes. The average values of porosity, pore size and pore density of the three porous copper samples are also listed in Table 1.
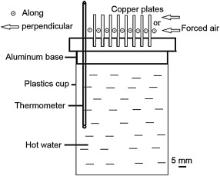
A nonstandard and nonsteady method was employed to evaluate heat dissipation performance for the porous copper plates. Fig. 1 shows the schematic view of the experimental apparatus for the measurement. Hot water with temperature of 80 °C was poured into a cylindrical plastics cup with both bottom and side thermally insulated. A pure aluminum base was employed as the top cover with one side connected to the hot water. Eight sheets of the porous copper specimens were stacked and soldered to the aluminum base with tin on the other side. In addition, aluminum foil was inserted between the copper plates and the ditch of the aluminum base for reducing contact thermal resistance between them. A thermometer was inserted into the hot water through the aluminum base to measure its temperature. A fan (Lileng-815, Rongzhifa Electronic Co., Ltd., China) was located with a distance of 20 cm for a forced air circulation along or perpendicular to the porous copper plates with air speed of 2 m/s.
In this system, a certain amount of heat is considered to be transferred and dissipated through the aluminum base, the porous copper specimen to air, which can be calculated by mass, temperature drop of the hot water. The dissipated heat through the porous copper plates is available if the part through the aluminum base can be deducted. It is the advantage of this method that the heat dissipation at different temperature from 80 °C to room temperature can be evaluated.
The temperature variation of the water with aluminum base and copper plates is compared to that with aluminum base only under a forced air convection perpendicular to the copper plates as shown in Fig. 2. It is indicated that the temperature drops faster when the porous copper plates (L) or nonporous copper plates (N) are attached to the aluminum base although the difference is not distinctive for the huge volume of the hot water. By Q = cmΔ T, where c is the specific heat, m is the total mass, Δ T is the difference between the beginning and the measured temperature of the water, the figure is changed to the dependence of the heat dissipation on time, as shown in Fig. 3.
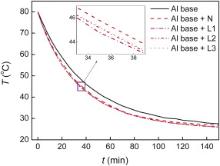 | Fig. 2. Temperature variation of the water with aluminum base and copper plates under a forced air convection perpendicular to the copper plates. |
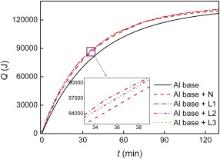 | Fig. 3. Heat dissipation variation of aluminum base and copper plates under a forced air convection perpendicular to the copper plates. |
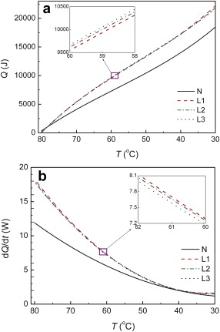
As mentioned above, the heat dissipation of the whole system is composed of two parts: one is the part dissipated through the aluminum base, the other through the copper plates. It is assumed that the heat dissipation rate is stable for the aluminum base under a given temperature, as the temperature difference between the hot water and the room air is the main force for the dissipation. So, the dependence of the heat dissipation on temperature of the copper plates is available by deducting the part of the aluminum base, as shown in Fig. 4(a), by which the heat dissipation rate is calculated and shown in Fig. 4(b). It is proven that the temperature gradient is the main force for the heat dissipation as the heat dissipation rates of both porous copper plates and nonporous copper plates decrease with decreasing temperature. In this case, the heat dissipation rate of the porous copper plate reaches 0.1 W/cm2 at temperature of 80 °C, which is 1.5 times of that of nonporous copper plate.
To elucidate how heat is dissipated through the copper plates in this work, the variation of the heat dissipation rate with temperature under natural convection and a forced air convection parallel or perpendicular to the copper plates is compared as shown in Fig. 5. In this case, the porous copper with porosity 50.98% and 29.87% are chosen as examples. It indicates that, in each case for the porous copper, the heat dissipation rate is higher when a forced air convection to the porous copper plates is employed, which is about 5-6 times of that by natural convection. Between the two forced air flowing directions, the heat dissipation rate is higher in the case of the forced air convection along the copper plates.
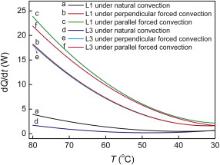 | Fig. 5. Dependence of heat dissipation rate on temperature of the porous copper plates under natural convection and a forced air convection parallel or perpendicular to the plates. |
Since the thermal conductivity of the air in the pores is negligible in comparison to copper, heat from the hot water in the plastics cup is transported through these copper plates via two competing mechanisms: thermal radiation and air convection. The thermal radiation is almost the same when the temperature gradient and the exposed area are kept relatively stable. Therefore, the radiation is insignificant as the effective exposed area is sheltered by neighbor plates. In the case of natural convection, heat dissipation is carried out by warmer air flowing upward. In the case of forced air convection, heat dissipation is enlarged for the flowing air carrying the heat away. It should be noted that there is a resistance when the flowing air reaches the porous copper plates, especially when the air is circulated perpendicular to the copper plates with pores in small size. As the heat dissipation through the second, third until the eighth copper plate becomes poorer and poorer as compared to the first plate by the flowing resistance in the case of the force air convection perpendicular to the copper plates, the heat dissipation is larger in the case of the forced air convection along the copper plates.
The results indicate that the forced air convection plays an important role in the heat dissipation for the porous copper. It is also indicated that the forced air convection along the porous plates is preferable for heat dissipation considering the flowing resistance.
The dependence of heat dissipation rate of the porous copper plates on temperature under the forced air convection parallel the plates is shown in Fig. 6. The porous copper with porosity of 50.98% shows the highest heat dissipation rate, which reaches 0.125 W/cm2 at temperature of 80 °C. It seems that the heat dissipation rate increases with increasing porosity for the porous copper, especially at temperature higher than 50 °C.
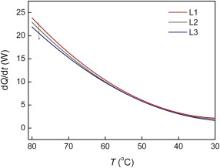 | Fig. 6. Dependence of heat dissipation rate on temperature for the porous copper under the forced air convection along the copper plates. |
When the porous copper is used to cool the hot water under a forced convection, almost all heat is transferred to the flowing air through the pore surface in the porous copper. So, the pore surface area plays an important role in the heat dissipation. As the specific surface area of pores Apore of the porous copper can be expressed as Apore = 4 PL/ d[ 14], where P is porosity, L is pore length, and d is pore diameter, the porous copper with the biggest porosity possesses the biggest surface area as shown in Table 2, so its heat dissipation rate is also the highest. However, sample L3 with lower porosity holds a lower heat dissipation rate although its surface area is bigger, which may be attributed to another important role from pore size and will be discussed in Section 3.4.
| Table 2. Surface area in pore and on surface of the porous copper plates with thickness of 2 mm |
It should be noted that the relationship between heat dissipation rate and porosity is still not clear. It is accepted that there is a decrease in flowing resistance with an increase in porosity[ 13], resulting in an increase in the air volume passing the pores. At the same time, the effective area for heat dissipation increases with an increase in porosity, resulting in a decrease in convection heat resistance. On the other hand, with an increase in porosity for the porous copper, the efficiency to conduct heat from aluminum base through pore wall (copper frame) decreases. So, the relationship between heat dissipation performance and porosity for the porous copper will rely on a compromise or a balance of the three effects mentioned above.
The result that sample L3 with lower porosity holds a lower heat dissipation rate although its surface area is bigger than sample L2 indicates that porosity is not the only parameter to determine the heat dissipation for the porous copper. So, we turned to the effect of pore size on the performance. As there are only two porous copper samples with almost the same porosity, nonporous copper plates obtained by the same cutting process were bored using a φ2 mm and a φ4 mm drill to obtain some axial penetrable holes on the surface uniformly to the porosity in range of 30%-34%, as shown in Fig. 7. The same measurement was carried out on the bored copper plates for heat dissipation performance.
The heat dissipation rate of the porous copper and the bored copper are shown in Fig. 8. In this case, the porosity of samples L2, L3, B1 and B2 is almost the same and in a range of 30%-34%. However, the heat dissipation rate of the porous copper is higher than that of the bored copper, which is attributed to their smaller average pore size and bigger surface area, as shown in Table 3. On the other hand, in the case of the porous copper, sample L2 possesses a higher heat dissipation rate although its pore size is bigger than sample L3, indicating that there is no simple linear relationship between the heat dissipation performance and the pore size.
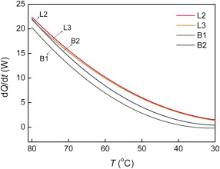 | Fig. 8. Dependence of heat dissipation rate on temperature for the porous copper and the bored copper with porosity around 30% under the forced air convection along the copper plates. |
| Table 3. Comparison on surface area in pore and on surface of the copper plates between the porous copper and the bored copper |
It is accepted that the heat dissipation performance is dominated by the surface area the air passing through. It was reported in reference[ 14] that different size pores of lotus-type porous copper will have different micro-channel effects, and smaller size pore will have bigger heat transfer capacity. However, it should be noted that the result mentioned above appeared in the case of cooling water passing through the pores in the porous copper. In this work, air flows along the gap between porous copper plates (Fig. 1), the air current brings the heat away through pore wall. Under the same condition, the smaller pore size results in a decrease in flowing volume and flowing velocity of passing air. Furthermore, the pore size of porous metal is coupled with the thickness of the ligament. By decreasing the pore size, the thickness of the copper frame decreases, resulting in a reduction in heat conduction through thin struts, as reported by Zhao et al.[ 17]. So, less heat is transferred, which may result in poorer heat dissipation performance. On the other hand, the specific surface area of a porous metal increases with decreasing pore size[ 14]; the heat transfer between air and the pore wall is more sufficient, so the porous copper with small pores should possess a better heat dissipation performance. Considering both effects, it is predicted that there is an optimal pore size corresponding to the highest heat dissipation performance.
The porous copper with elongated cylindrical pores fabricated by a unidirectional solidification method in a mixture of hydrogen and argon shows a good heat dissipation performance evaluated in air by a forced air convection. It shows that the porous copper can remove heat approximately 1.5 times more efficiently in comparison with nonporous copper. It indicates that the heat dissipation is increased by a forced air convection both along and perpendicular to the porous copper plates, in which the forced convection along the porous plates is preferable as the flowing resistance is smaller. It is found that the porous copper with bigger porosity possesses a higher heat dissipation rate. On the other hand, pore size also plays an important role in the performance for the porous copper at a stable porosity.
Acknowledgments
The financial supports of Cooperative Foundation between Industry, Colleges or Scientific Institutes and Relevant Issues from Guangdong Province (00124720225267058), Natural Science of Foundation from Liaoning Province (No. 201102222), and Science and Technology Project (No. 2010AZ2010) from Jiaxing City are acknowledged.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|