The isothermal and cyclic oxidation behaviors in air and hot corrosion behaviors in Na2SO4 + 25 wt% K2SO4 salt of M951 cast superalloy and a sputtered nanocrystalline coating of the same material were studied. Scanning electron microscopy, energy dispersive X-ray spectroscope, X-ray diffraction, and transmission electron microscopy were employed to examine the morphologies and phase composition of the M951 alloy and nanocrystalline coating before and after oxidation and hot corrosion. The as-sputtered nanocrystalline layer has a homogeneous γ phase structure of very fine grain size (30-200 nm) with the preferential growth texture of (111) parallel to the interface. Adherent Al2O3 rich oxide scale formed on the cast M951 alloy and its sputtered coating after isothermal oxidation at 900 and 1000 °C. However, when being isothermal oxidized at 1100 °C and cyclic oxidized at 1000 °C, the oxide scale formed on the cast alloy was a mixture of NiO, NiAl2O4, Al2O3 and Nb2O5 and spalled seriously, while that formed on the sputtered coating mainly consisted of Al2O3 and was very adherent. Nanocrystallization promoted rapid formation of Al2O3 scale during the early stage of oxidation and enhanced the adhesion of the oxide scale, thus improved the oxidation resistance of the substrate alloy. Serious corrosion occurred for the cast alloy. The sputtered nanocrystalline coating apparently improved the hot corrosion resistance of the cast alloy in the mixed sulfate by the formation of a continuous Al2O3 and Cr2O3 mixed oxide layer on the surface of the coating, and the pre-oxidation treatment of the coating led to an even better effect.
Nickel-based superalloys have been widely used in industrial gas turbines, aircrafts, jet engines owing to their excellent mechanical strength at high temperature[ 1], [ 2], [ 3], [ 4], [ 5],[ 6]. M951 alloy[ 7], [ 8], [ 9], [ 10] is one of the cast nickel-based superalloys, which is newly developed by Institute of Metal Research, Chinese Academy of Sciences. It is a combination of many attractive advantages, such as low density, high thermal fatigue resistance, good tensile and stress-rupture properties, high incipient melting temperature, low cost, and in particular excellent oxidation resistance, which make it a material of great interest in modern advanced aircrafts and industrial gas turbines.
Conventional high temperature protective coatings[ 11], [ 12], [ 13], [ 14], [ 15], [ 16], [ 17],[ 18], such as aluminized coating, MCrAlY overlay coating, play a vital role in protecting substrate alloy from oxidation and corrosion, but they also have some inevitable shortcomings. For instance, the inter-diffusion between substrate and coating may cause the formation of harmful brittle phases in the substrate after high temperature exposure.
Wang's group[ 19], [ 20], [ 21], [ 22],[ 23] invented a kind of high temperature “nanocrystalline coating”. The main difference between the nanocrystalline coating and the traditional protective coatings is that the nanocrystalline coating exhibits excellent compatibility with the substrate, since it has the same chemical composition as the substrate alloy. Therefore, any possible harmful effects induced by the inter-diffusion between the coating and the substrate can be avoided.
Extensive studies have been made to investigate the effects of nanocrystallization on the oxidation resistance of superalloy K38G, KI7F, IN738 and intermetallic TiAl, Ni3A1, NiAl[ 24], [ 25], [ 26], [ 27], [ 28],[ 29] in air. However, the studies on the effect of nanocrystallization on hot corrosion resistance of alloys are quite few[ 30]. In addition, the Cr content in M951 is lower while Mo and W contents are higher than those in the traditional superalloy, which is not beneficial for the corrosion resistance. It is necessary to study the effect of nanocrystallization on the oxidation and corrosion resistance of M951 alloy. In this paper, M951 sputtered nanocrystalline coating was deposited on the M951 substrate. Isothermal oxidation at 900 °C, 1000 °C and 1100 °C, cyclic oxidation at 1000 °C and hot corrosion behaviors in molten Na2SO4 + 25 wt% K2SO4 salt at 850 °C of the M951 cast alloy and its sputtered nanocrystalline samples were investigated.
The nominal chemical composition of the cast M951 superalloy used in this paper was listed in literature[ 31]. The samples were cut into 15 mm × 10 mm × 2 mm from an ingot and ground down to 600 grit using SiC papers. They were sandblasted and then degreased by an ultrasonic cleaner in acetone and ethanol before sputtering. The target used for sputtering was a 382 mm × 128 mm sheet whose composition was the same as that of the M951 superalloy. Sputtering parameters were depicted as follows: argon pressure: 0.24 Pa; sputtering current: 4.95 A; substrate temperature: 200 °C. The samples were rotated in front of the target during sputtering to ensure better uniformity. The coating was approximately 26 μm thick.
The oxidation kinetic measurements were carried out in a thermal balance with ambient atmosphere at 900, 1000 and 1100 °C respectively for 50 h. Thermal cyclic oxidation tests were conducted in an automated rig in air at 1000 °C for 100 cycles. In each cycle the specimens were exposed to the elevated temperature for 1 h and then taken out from the furnace and cooled in air for 10 min. The mass changes of the specimens were measured using an electronic balance with sensitivity of 0.01 mg every 20 cycles.
Hot corrosion tests were conducted in molten Na2SO4 + 25 wt% K2SO4 salts at 850 °C. The specimens were immersed into the blended salts completely before the test. In each test, one sample and mixed salts were put into an alumina crucible, and then inserted in a muffle furnace. The crucibles were taken out from the furnace at every certain interval and cooled down to room temperature. After that the samples were washed in boiling distilled water for at least 30 min to dissolve the residual salts and then dried in a dryer. Each sample was weighted on an electronic balance after the test. Then, they were re-immersed in fresh mixed salts to repeat the above procedures. Three parallel samples were employed at the same time for all the tests.
Scanning electron microscopy (SEM) equipped with an energy dispersive X-ray spectroscope (EDX), transmission electron microscopy (TEM) and X-ray diffraction (XRD) were employed to examine the morphology and the phase structure of the as-sputtered M951 nanocrystalline coating and the oxide scale formed on the surface of the M951 cast alloy and its nanocrystalline coating after oxidation and hot corrosion.
It is well known that cast nickel-based superalloy is mainly composed of phases γ, γ′ and carbides. The average grain size is in the order of millimeter. The cast microstructure of M951 alloy comprises a uniform dispersion of small ordered γ′ precipitates together with carbide particles, both of which are distributed within a face-centered cubic γ matrix[ 9]. It is a γ′-strengthened alloy containing about 58 vol.% γ′ phase in the as-cast condition, and Al is the main γ′ stabilizer in this alloy.
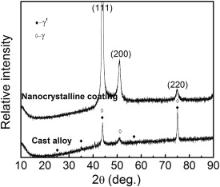
The surface and cross-sectional morphologies of the as-sputtered sputtered coating are shown in Fig. 1. The XRD patterns of the coating and the cast alloy are shown in Fig. 2. The as-sputtered coating had a rough surface (Fig. 1(a)) with the thickness of about 26 μm. The cross-sectional image (Fig. 1(b)) indicates that the sputtered coating consisted of typical column-like grains along the deposition direction. Only γ phase was detected in M951 sputtered coating. Compared with the cast alloy, sputtered coating possesses a preferential growth texture of (111). The diffraction peaks broaden, which is associated with the decrease of the grain size.
The plan-view and cross-sectional TEM bright field images and the corresponding electron diffraction patterns of the as-sputtered M951 nanocrystalline coating are presented in Fig. 3. It can be seen from the plan-view bright field images (Fig. 3(a)) that a “texture” structure exists in the coating and the grains grow along a preferred direction. According to the XRD patterns (Fig. 2), the preferred growth direction is (111). The width of the grain is less than 30 nm and the length is usually less than 200 nm. The cross-sectional bright field image displayed in Fig. 3(c) reveals that the column-like grains in the deposition direction (Fig. 1(b)) are composed of nanocrystalline grains, whose sizes are less than 30 nm. So the sputtered coating was typical nanocrystalline. The selected area electron diffraction (SAED) patterns display rings corresponding to γ-Ni. The analysis of the SAED rings is listed in Table 1.
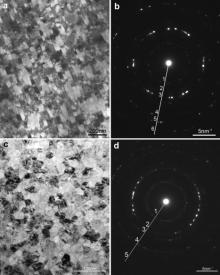 | Fig. 3. The plan-view (a) and cross-sectional (c) TEM bright field images and corresponding electron diffraction patterns (b) and (d) of the as-sputtered M951 nanocrystalline coating. |
| Table 1. Theoretical and experimental values for dhkl of phases of the M951 nanocrystalline coating |
3.2.1. Isothermal oxidation
The isothermal oxidation kinetics curves of M951 cast alloy and its sputtered coating at 900, 1000 and 1100 °C are shown in Fig. 4. It can be seen that the mass gain of sputtered coating at 900 °C is greater than that of the M951 alloy in the former 45 h, after which they are quite close. While at 1000 °C the mass gain of the coating and the cast alloy are almost same. The mass gain of the cast alloy was nearly 1.4 times that of the sputtered coating at 1100 °C when the oxidation time reached 50 h.
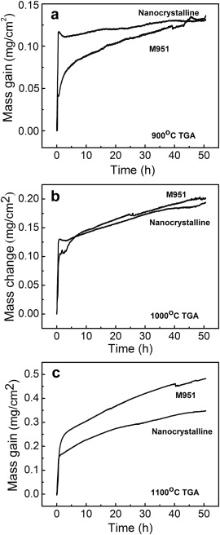 | Fig. 4. Isothermal oxidation kinetics curves of M951 cast alloy and its nanocrystalline coating at: (a) 900 °C, (b) 1000 °C, (c) 1100 °C. |
Surface and cross-sectional morphologies of M951 cast alloy and its sputtered coating after oxidation at 900-1100 °C for 50 h are shown in Fig. 5, Fig. 6 and Fig. 7. The oxide scales formed on the M951 alloy at 900 °C and 1000 °C are thin and flat (Figs. 5(a and c) and 6(a and c)). Some grid-like oxides show up on the surface of the alloy (Figs. 5(a) and 6(a)), which are Nb-rich oxide analyzed by EDX. XRD results (Fig. 8(a)) reveal that the oxides are mainly composed of α-Al2O3 with minor amounts of NiAl2O4, NiO and Nb2O5. Al-deficient layer (around 7 μm) formed under the oxide scale evidently after 50 h oxidation at 1000 °C. The oxide scales formed on the sputtered coating at 900 and 1000 °C are compact, adherent and much thinner than that formed on the substrate (Figs. 5(b and d) and 6(b and d)), which are mainly composed of α-Al2O3 with a small amounts of NiAl2O4 (Fig. 8(b)). Some Mo-rich phase precipitates in the coating after oxidation of 50 h. After 50 h isothermal oxidation at 1100 °C, the oxide scale formed on the surface of the M951 cast alloy stratified and spalled seriously. Combined with the XRD results (Fig. 8(a)) and EDX analysis, it is found that the external layer of the scale consists of NiAl2O4 and NiO and the internal layer is α-Al2O3. Internal oxides formed in some areas of the substrate surface. The central part of the internal oxides is Nb-rich oxide, which is surrounded by α-Al2O3 (Fig. 7(b)). The thickness of the Al-deficient layer at 1100 °C is about 16 μm, which is thicker than that at 1000 °C. The oxide scale formed on the sputtered coating at 1100 °C is also compact and adherent, consisting mainly of α-Al2O3 with NiAl2O4, but a little bit thicker than that at 900 °C and 1000 °C.
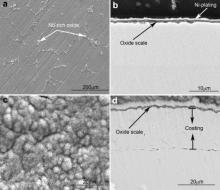 | Fig. 5. Surface and cross-sectional morphologies of cast M951 alloy (a) and (b) and its sputtered nanocrystalline coating (c) and (d) after oxidation at 900 °C for 50 h. |
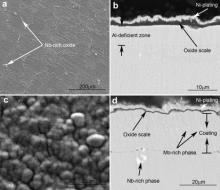 | Fig. 6. Surface and cross-sectional morphologies of cast M951 alloy (a) and (b) and its sputtered nanocrystalline coating (c) and (d) after oxidation at 1000 °C for 50 h. |
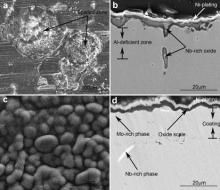 | Fig. 7. Surface and cross-sectional morphologies of cast M951 alloy (a) and (b) and its sputtered nanocrystalline coating (c) and (d) after oxidation at 1100 °C for 50 h. |
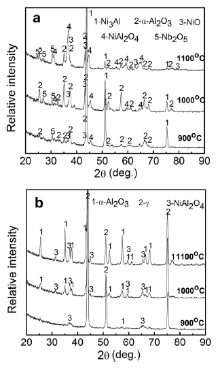 | Fig. 8. Glancing incidence XRD patterns of M951 alloy (a) and its sputtered nanocrystalline coating (b) after oxidation at 900-1100 °C for 50 h. |
3.2.2. Cyclic oxidation
The cyclic oxidation kinetics curves of M951 cast alloy and its sputtered coating at 1000 °C are shown in Fig. 9. It can be seen from the figure that both the alloy and coating have rapid mass gain at the early stage of the oxidation. After this fast oxidation period, the mass gain of the coating increased slowly while mass loss occurred for the cast alloy, which indicates that spallation of the oxide scale formed on the cast alloy occurred during the cyclic oxidation process.
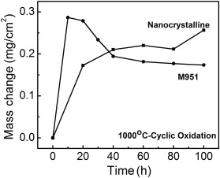 | Fig. 9. Cyclic oxidation kinetics curves of cast M951 alloy and its sputtered nanocrystalline coating at 1000 °C. |
Fig. 10 presents the surface and cross-sectional morphologies of the M951 cast alloy and its sputtered coating after cyclic oxidation at 1000 °C. Severe spallation of the oxide scale occurred for the cast alloy, which is identical with the kinetics results (Fig. 9). Unlike the oxide scale formed on the alloy at 1000 °C after isothermal oxidation, the scale formed after cyclic oxidation was stratified, and the structure was similar to that formed after isothermal oxidation at 1100 °C. Internal oxidation also took place, as shown in Fig. 10(b), which is similar to the results obtained after isothermal oxidation at 1100 °C for 50 h as well. An Al-deficient layer around 1.2 μm thick was observed. However, the oxide scale formed on the sputtered coating is thin, compact and adherent (Fig. 10(c) and (d)), and consists only of α-Al2O3 (Fig. 11), which is similar to that formed at 1000 °C after isothermal oxidation (Fig. 6(d)). Neither cracking nor spalling of the oxide scales was observed.
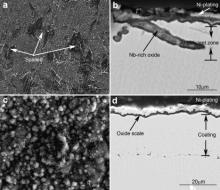 | Fig. 10. Surface and cross-sectional morphologies of cast M951 alloy (a) and (b) and its sputtered nanocrystalline coating (c) and (d) after cyclic oxidation at 1000 °C. |
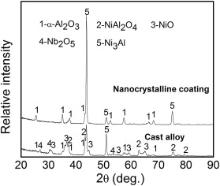 | Fig. 11. Glancing incidence XRD patterns of M951 alloy and its sputtered nanocrystalline coating at 1000 °C after cyclic oxidation. |
TEM was employed to investigate the growth of the oxides in the initial stage. Fig. 12 is the XRD patterns of the M951 cast alloy and its sputtered coating after initial oxidation at 1000 °C. It can be seen that NiO, α-Al2O3, NiCr2O4 and (Al0.9Cr0.1)2O3 formed on the M951 cast alloy, while only α-Al2O3 was detected on the sputtered nanocrystalline coating. The scanning transmission electron microscopy (STEM) image and the corresponding elemental X-ray mappings are shown in Fig. 13, Fig. 14, Fig. 15 and Fig. 16. The oxide scale formed on the cast M951 alloy after 1 h oxidation is mainly composed of α-Al2O3 with minor (Al0.9Cr0.1)2O3 according to the glancing incidence XRD and the elemental X-ray mapping results. Although NiO and NiCr2O4 were not observed by TEM, they were detected by XRD, indicating that the distribution in the oxide scale was not continuous. The oxide scale formed on the cast alloy after 10 h oxidation consists of double layers. Combined with the glancing incidence XRD and the elemental X-ray mapping results, it could be obtained that the outer layer is NiO and the inner layer is the mixed oxides of Ni, Al and Cr, Al2O3, NiCr2O4 and (Al0.9Cr0.1)2O3, while the oxide adjacent to the substrate is merely Al2O3 (Fig. 15). Nb and Mo-rich oxides were also observed in the mixed oxides layer. The oxide scale formed on the sputtered coating after 1 h oxidation is merely α-Al2O3 (Figs. 12(b) and 14), which indicates that nanocrystallization can promote the rapid formation of the α-Al2O3 at initial oxidation. After 10 h oxidation, almost pure α-Al2O3 layer formed on the surface of the sputtered coating, and some blocks of Ni, Cr and Al mixed oxides were observed on the top of the α-Al2O3 scale. However, Ni and Cr oxides were not detected by XRD, indicating the amount of them formed on the sputtered coating were quite few. After oxidation, the grains of the coating coarsened, but the grain sizes were still in order of micrometer (Fig. 17). Twining of the grains (Fig. 17) in the sputtered coating was observed after 10 h oxidation at 1000 °C, indicating the plastic deformation of the coating occurred during oxidation and cooling process. Nb and Mo-rich phase precipitated at the grain boundary of the coating, as shown in Fig. 14.
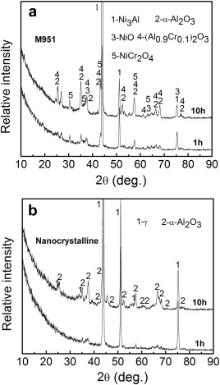 | Fig. 12. Glancing incidence XRD patterns of the M951 cast alloy (a) and its sputtered coating (b) after initial oxidation at 1000 °C. |
 | Fig. 13. STEM image and the corresponding elemental X-ray mappings of M951 cast alloy after 1 h oxidation at 1000 °C. |
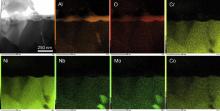 | Fig. 14. STEM image and the corresponding elemental X-ray mappings of M951 sputtered coating after 1 h oxidation at 1000 °C. |
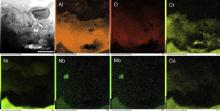 | Fig. 15. STEM image and the corresponding elemental X-ray mappings of M951 cast alloy after 10 h oxidation at 1000 °C. |
 | Fig. 16. STEM image and the corresponding elemental X-ray mappings of M951 sputtered coating after 10 h oxidation at 1000 °C. |
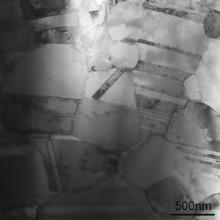 | Fig. 17. Twining of the grains in the sputtered coating after 10 h oxidation at 1000 °C. |
Nanocrystalline coatings in two kinds of statuses were employed in the hot corrosion test in molten Na2SO4 + 25 wt% K2SO4 salt at 850 °C. One was in the as-sputtered condition and the other was pre-oxidized at 1000 °C for 100 h in air. Hot corrosion kinetics curve is shown in Fig. 18. It can be seen that both of the as-sputtered and the pre-oxidized coatings improved the corrosion resistance of the substrate alloy significantly. The mass changes of the pre-oxidized coating during the whole corrosion process are very small, indicating that accelerated oxidation didn't occur for 100 h. For the as-sputtered samples, obvious mass loss occurred after corrosion for 55 h, meanwhile it was observed that accelerated corrosion occurred at the edges and hole of the samples, which indicated the end of the incubation and the start of the propagation.
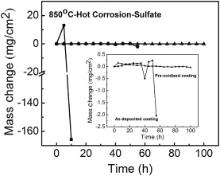 | Fig. 18. Hot corrosion kinetics curves of cast M951 alloy and its sputtered coating in Na2SO4 + 25 wt% K2SO4 salt at 850 °C. |
The surface and cross-sectional morphologies of M951 alloy and its sputtered coating after hot corrosion in molten Na2SO4 + 25 wt% K2SO4 salt at 850 °C are shown in Fig. 19. Severe corrosion took place for the M951 cast alloy after 10 h. Loose oxide scale around 500-μm-thick formed on the surface of the substrate (Fig. 19(b)), which cracked and spalled seriously. Spherical Ni2S3 formed on the surface of the scale. The bright parts of the scale were Ni rich phase, while the dark parts were the mixtures of the oxides and sulfides of the alloy elements, such as Cr, Al, Ni. At the corrosion frontier a large amount of CrS formed. The oxide scales formed on the surfaces of the as-sputtered coating (Fig. 19(c)) after 55 h corrosion and the pre-oxidized coating after 100 h corrosion were thin, compact and adherent to the substrate, which were composed mainly of Cr2O3 and α-Al2O3 (Fig. 20). Mo-rich phase precipitated (Fig. 19(d)) in the pre-oxidized coating but not in the as-sputtered coating, indicating the precipitation took place in the pre-oxidation process at 1000 °C.
 | Fig. 19. Surface and cross-sectional morphologies of the samples after corrosion in Na2SO4 + 25 wt% K2SO4 salt at 850 °C: (a) and (b) the cast M951 alloy corroded for 10 h, (c) and (d) the as-sputtered coating corroded for 55 h, (e) and (f) the pre-oxidized coating corroded for 100 h. |
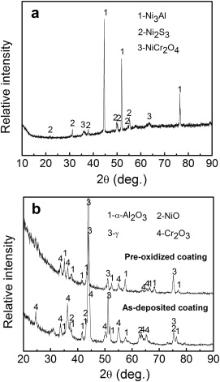 | Fig. 20. XRD patterns of the samples after corrosion in Na2SO4 + 25 wt% K2SO4 salt at 850 °C: (a) the cast M951 alloy corroded for 10 h, (b) the as-sputtered coating corroded for 55 h and the pre-oxidized coating corroded for 100 h. |
M951 cast alloy belongs to the “alumina former” group, because its ratio of Cr:Al (mass%) is less than 4. Different from the “chromia former” group, external alumina scale is able to form on the M951 cast alloy, which has been proved by the above results. However, the alumina scale formed on the surface of the cast alloy tended to spall during thermal cycles. The continuous spallation of the alumina scale led to accelerated consumption of aluminum in the alloy. So after prolonged oxidation, continuous and protective Al2O3 scale could not form any longer and the oxidation resistance of the alloy decreased.
The chemical composition of the M951 sputtered coating is the same as that of its cast alloy, but the grain size of the coating is in nanometer magnitude, which facilitated outward diffusion of the alloy elements from the coating and inward diffusion of oxygen. So the selective oxidation of Al element was promoted, and Al2O3 scale formed rapidly during the early stage of oxidation, which prevented the formation of the oxides of the other coating elements.
The adhesion of the alumina scale to the sputtered nanocrystalline coating was superior. The stresses generated in the oxide scale can be released by plastic deformation of oxide scale and the substrate alloy and/or cracking and spalling of the scale. The factors enhancing the plastic deformation of the scale and the alloy will decrease the trend of scale cracking and spalling. Reduction of the grain size of the oxide scale can enhance the diffusion creep rate of the oxide scale, and grain refinement of the metal substrate is also beneficial to the stresses relieving in the oxide scale by the substrate deformation[ 32]. In the present study, the grain size of the oxide scale formed on the cast alloy and the sputtered coating did not show much difference. However, the grain size of as-sputtered nanocrystallized film is about two to three orders of magnitude smaller than that of the cast alloy, so the plastic deformation for the former is much easier than that for the latter. Although after oxidation, the grains of the coating coarsened, the grain sizes of the coating were still in micrometer magnitude, which was much smaller than that of the cast alloy. So the stresses of oxide scales formed on the nanocrystallized alloy may be relieved by the plastic deformation of metallic substrates adjacent to the scales[ 33], and this may play a dominant role in relieving thermal stresses during cooling. Twining of the coarsened grains in the sputtered coating provided a convincing evidence that the plastic deformation of the coating indeed happened during oxidation and cooling process.
The melting point of Na2SO4 + 25 wt% K2SO4 is lower than 850 °C. Therefore, at the test temperature, the mixed sulfate is molten. The corrosion of the cast M951 can be explained by the fluxing mechanism of hot corrosion[ 34]. The refractory metal elements in M951 alloy such as molybdenum and tungsten have high affinity with oxygen. In the early stage of corrosion, Al2O3 as well as the oxides of refractory metal elements such as MoO3 and WO3 formed at the same time. The oxides of refractory metal elements reacted with the O2- in the molten sodium sulfate:
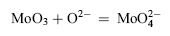 | (1) |
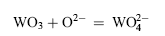 | (2) |
So O2- near the interface of melt-alloy was consumed, which caused the adjacent melt to become acidic. Al2O3 on the surface of the alloy dissolved into the melt:
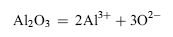 | (3) |
Al3+, 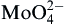
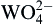
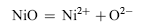 | (4) |
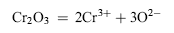 | (5) |
The grain size of the nanocrystalline coating is in nanometer magnitude, which facilitates outward diffusion of aluminum from the coating and inward diffusion of oxygen. So the selective oxidation of Al element was promoted, and Al2O3 scale formed rapidly during the early stage of oxidation, which prevented the formation of MoO3 and WO3 or decreased in the initial stage of corrosion. However, after a longer time and many cycles of cooling and heating, microcracks might appear somewhere in the Al2O3 scale formed on the coating surface. Sulfur might permeate through microcracks into the coating and induce the formation of Cr sulfides, which consequently caused the concentration of oxygen ions to increase in the melts. The dissolution of Al2O3 in the melts increased. However, nanocrystalline also facilitated outward diffusion of chromium and formation of Cr2O3 scale, which prevented the formation of NiO as well as MoO3 and WO3. Therefore, accelerated corrosion could not occur quickly, and the incubation period of corrosion was prolonged.
After pre-oxidation, a continuous, dense and adherent Al2O3 scale formed on the surface of the sputtered nanocrystalline coating, which prevented the formation of MoO3 and WO3 when the pre-oxidized sample was corroded in the molten Na2SO4 + 25 wt% K2SO4 salts. So the melts could not become acidic. And when the microcracks appeared somewhere in the Al2O3 scale after longer time, as stated above, the formation of Cr sulfides and Cr2O3 prevented the formation of NiS and NiO as well as MoO3 and WO3, and the incubation period of corrosion was prolonged. The Al2O3 scale formed on the sputtered nanocrystalline coating after pre-oxidation was thicker and denser than that formed in the molten salts at the initial stage of corrosion, so the incubation period of corrosion of the pre-oxidized sample was longer than that of the as-sputtered sample. Even after 100 h corrosion, the accelerated corrosion didn't occur for the pre-oxidized samples.
(1)The as-sputtered M951 coating is composed of γ phase of very fine grain size (30-200 nm) with the preferential growth texture of (111) parallel to the interface.
(2)Nanocrystallization promoted rapid formation of Al2O3 scale during the early stage of oxidation and enhanced the adhesion of the oxide scale, which improved the oxidation resistance of the substrate alloy.
(3)The sputtered nanocrystalline coating apparently improved the hot corrosion resistance of the cast alloy in mixed sulfate by the formation of a continuous Al2O3 and Cr2O3 mixed oxide layer on the surface of the coating, and the pre-oxidation treatment of the coating led to an even better effect.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51071163), the National Key Basic Research and Development Program (“973 Program”, Nos. 2010CB631206 and 2012CB625100), and the National High Technology Research and Development Program of China (No. 2012AA03A512).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|