Carbon nanotubes (CNTs) were dispersed in gas atomized Cu47.5Zr47.5Al5 (CZA) and Cu50Zr50 (CZ) amorphous powders, in an effort to elucidate the mechanisms of adhesion of CNTs onto amorphous metallic powders. CNTs were homogenously dispersed in water using a zwitterionic (ZW) surfactant. Then CZA and CZ powders were submersed in the ZW-CNT suspensions with varying amounts of dwell time in an ultrasonic bath. The ZW-CNT-metal powder suspensions were dried, and CNT-metal composite powders were obtained after decomposition of the surfactant by calcination. Zeta potential measurements on ZW-CNT-metal powder suspensions and scanning electron microscopy investigation into the CNT-metal composite powders both indicated an ideal dwell time, for a specific alloy composition, of metallic powders in ZW-CNT suspension to achieve optimal adhesion of CNTs onto amorphous metallic powder surfaces. The results are rationalized on the basis of hydrolysis of metal ions into suspension creating a net positive charge on the metallic powder surfaces, and the interaction between the charged powder surfaces and the charged hydrophilic head groups of ZW, which has the other end attached to CNTs.
Carbon nanotubes (CNTs) have inner diameters of ~1 nm and high length/diameter ratio[ 1], and typically multi-walled CNTs have up to several tens of graphitic shells with adjacent shell separation of ~0.34 nm. CNTs possess high stiffness and axial strength as a result of the strong carbon-carbon sp2 bonding[ 2]. The high elastic modulus, high strength, large aspect ratio, and excellent chemical stability that originates from their seamless cylindrical graphitic structure render CNTs an ideal reinforcement to improve mechanical performance of monolithic materials[ 3]. However, the difficulty with uniformly dispersing CNTs constitutes a barrier to their application as composite reinforcements.
Monolithic bulk metallic glass (BMG) generally exhibits a brittle nature[ 4] , [ 5]. However, it has been shown that reinforcement of secondary phase dispersions can enhance ductility as well as strength[ 6]. The enhanced ductility in BMG-CNT composites is attributed to the inhibition of shear band flow deformation by the composite structure, which concomitantly increases strength[ 6]. Typically, plastic deformation of BMGs occurs in a highly localized manner through the formation of shear bands[ 7]. In an unconfined geometry, failure of the BMG occurs typically along a single shear band that cuts across the sample at an angle of about 45° with respect to the compression axis; the shear band is in the direction of maximum resolved shear stress[ 8]. An approach to enhancing plasticity in BMG composites is to prevent a single shear band from traversing through the sample and to generate multiple shear bands[ 9]. It is important to note that the interaction of shear bands triggers their multiplication and increases the flowability of the BMG, and thus a higher global compressive strain to failure is achieved. It is hypothesized that CNTs in a BMG matrix will contribute to this type of behavior[ 6]. Many investigations involve additions of CNTs with high contents into the bulk metallic glass matrix, up to 10 vol. % in some reports[ 10]. However, the addition of high content CNTs can cause the formation of a detrimental hard quasicrystalline ZrC phase for Zr-based BMG[ 11]. It is proposed that lowering the CNT content and simultaneously ensuring homogenous dispersion into starting powders will allow for a reduction in deleterious phases and accordingly enhance the overall mechanical behavior.
CNTs usually agglomerate in bundles in order to minimize the surface energy. Due to Van der Waals forces, it is difficult to uniformly disperse highly entangled CNT bundles, resulting in composites with only modest, if any, improvement in mechanical behavior. Aggregates are generally a hurdle to most CNT applications, because they hinder the formation of a continuous three-dimensional network that efficiently carries mechanical loads of the composite[ 12]. Therefore, it is important to separate CNTs and uniformly disperse them in metallic powders. Typically there are two different approaches to dispersing CNTs into metallic powders: mechanical (or physical) methods and chemical methods. Mechanical dispersion methods, such as ball milling, effectively separate CNTs from each other, but can also break them, causing a decrease in the aspect ratio and consequently lower contribution to improving mechanical behavior[ 13] as well as create defects on the CNTs walls. Chemical methods change the surface energy of CNTs using surfactants or functionalization, thereby reducing CNTs' tendency to agglomerate and improving their wetting of or adhesion onto various surfaces. However, aggressive chemical functionalization, such as using acids at high temperatures, can damage the outer layer of CNTs and create defects. In contrast, a zwitterionic surfactant, which utilizes dipole-dipole electrostatic interaction for CNT dispersion, has been shown to have little or no damage to the CNTs surface[ 14] , [ 15].
In this study, stable aqueous suspensions of CNTs were prepared, utilizing a zwitterionic surfactant, 3-(N,N-dimethylstearylammonio)-propanesulfonate (ZW). Ultra-violet-visible (UV-vis) spectroscopy was used to quantitatively characterize colloidal stability of the CNT suspension as a function of ZW concentration. Cu47.5Zr47.5Al5 (CZA) and Cu50Zr50 (CZ) (at.%) powders were immersed in the ZW-CNT suspensions having dwell time from 1 to 6 h in solution in order to understand the adhesion-debonding behavior of CNTs onto-from metal powder surfaces. CNT-metal composite powders were obtained after drying of ZW-CNT-metal powder suspensions and thermal decomposition of ZW. Zeta potential measurements were performed on ZW-CNT-metal powder suspensions and scanning electron microscopy (SEM) investigations were carried out into the CNT-metal composite powders, in an effort to understand the mechanisms of dispersion of CNTs and their adhesion onto amorphous metallic powder surfaces in aqueous suspensions with surfactant. In view of the above, the present study was undertaken to elucidate the underlying interactions for adhesion of CNTs onto amorphous metallic powder particles.
Master ingots of CZ and CZA alloys were prepared by arc-melting a mixture of pure Cu (99.99%), Zr (99.9%) and Al (99.99%) in a Zr-gettered Ar atmosphere. Each ingot was re-melted at least 5 times to achieve chemical homogeneity. The weight loss of each sample during alloying was less than 0.1%. Subsequently, gas atomization was performed on the master ingots using high purity He as the cooling gas. The ingots were heated to ~1273 K under vacuum in a quartz tube using an induction-heating coil, injected through a nozzle of 1.5 mm in diameter, and atomized by high-pressure Ar gas with a dynamic pressure of ~12 MPa.
ZW was purchased from Sigma-Aldrich (St. Louis, MO, USA) and multi-walled CNTs from NanoArmor (Houston, TX, USA), of 20-40 nm in diameter, 10-20 μm in length, 95% in purity. Suspensions with varying concentration of ZW (0.04-0.10 wt%) in de-ionized water was heated to 353 K to dissolve the ZW in solution for 10 min with a fixed CNT concentration (0.1wt%) and ultrasonicated for 15 min. The CZ or CZA powder of 1g was submersed in 100 mL of the 0.06 wt% ZW-0.1wt% CNT suspensions with varying amounts of dwell time in solution from 1 to 6 h. The ZW-CNT-metal powder suspensions were dried at 423 K in vacuum, and the dried powders were subsequently annealed in a vacuum furnace at 693 K for 10 min to decompose a majority of the surfactant to obtain CNT-metal composite powders. The appropriate decomposition temperature of ZW surfactant was determined to be about 410°C by thermal gravimetric analysis (TGA) (Perkin Elmer TGA7). Differential scanning calorimetry (DSC) (Perkin Elmer DSC7) was used to determine the appropriate crystallization temperature of CZ and CZA powders. The atmosphere in the sample chamber of both methods was purged by flowing Ar gas for 30 min and tests were performed at the heating rates of 40°C/min.
Phase identification of atomized CZ and CZA powders was performed using X-ray diffraction (XRD) (Scintag XRD) with a monochromatic Cu Kα radiation over 2 θ range of 20°-80°. UV-vis spectroscopy of ZW-CNT suspensions was performed on a Perkin Elmer Lambda 750 to observe the overall dispersion quality of CNTs in suspensions by measuring light absorbance as a function of wavelength from 280 to 800 nm. Zeta potential measurements were performed on ZW-CNT, ZW-metal powder and ZW-CNT-metal powder suspensions, using a Brookhaven Instruments ZetaPlus. Suspensions were diluted by a factor of 10 before measurements as recommended by the manufacturer to determine changes in electrokinetic potential as a function of time at a fixed pH of ~7. The microstructures of CNT-metal composite powders were examined by SEM (FEI XL30).
Fig. 1 shows the XRD spectra of gas atomized CZ and CZA powders with different particle sizes. Crystalline CuZr peaks are identified in the XRD spectra when the particle sizes are >53 μm and >75 μm for CZ and CZA powders, respectively. The presence of crystalline phases in powders with larger particle sizes is attributed to the fact that the critical cooling rate necessary to attain a fully amorphous phase cannot be satisfied when the powder particle size exceeds a certain value[ 16]. To maintain the amorphous structure of the CZ and CZA powders, particles sizes were limited <53μm and <75μm for CZ and CZA, respectively.
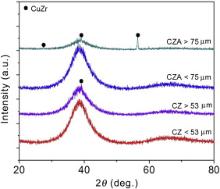 | Fig. 1. XRD spectra of gas atomized CZ and CZA powders, where crystalline peaks are labeled and identified as CuZr. |
Fig. 2 shows UV-vis light absorbance spectra of ZW-CNT suspensions with varying ZW concentration of 0.04-0.1 wt% and fixed CNT concentration of 0.1 wt%. It can be seen that for all ZW concentrations, there is a major absorption peak at ~283 nm in the spectrum, which is consistent with published results and ascribed to separated CNTs[ 17]. Moreover, potential variations in absorption peak may be resulted due to differences in a surfactant or polymer's head and tail groups on dispersion efficacy of CNTs[ 18]. Bundled CNTs have been reported to not have light absorption in the wavelength range of 200-900 nm[ 19], and absorption in this regime is due to individual CNTs. Therefore, for all ZW concentrations, CNT bundles are separated into individual CNTs and the colloidal CNT suspensions are stable. 0.06 wt% ZW was selected to study the adhesion with amorphous metallic powders in order to minimize the ZW content while maintaining a high level of efficacy for CNT dispersion quality.
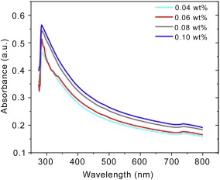 | Fig. 2. Ultra Violet/visible light absorbance spectra of ZW-CNT suspensions with varying ZW concentration and fixed CNT concentration of 0.1 wt%. |
Fig. 3(a) shows the zeta potential (ζ) of different ZW suspensions as a function of time up to 6 h. In addition, the ζ of CNT aqueous suspension without surfactant was found to be -30 mV[ 17]. Suspensions with a ζ not larger than -15 mV are expected to be stable[ 20], and the stability increases with decreasing ζ[ 21]. The ζ of the ZW-CNT suspension was determined to be about -40 mV. Therefore, ZW improves the stability of the CNT suspension throughout the entire dwell time range. By contrast, the ζ of the ZW-CNT-CZ suspension initially decreased with time until a minimum of ~-36 mV was reached at 3-4 h, and then increased with time and attained a value of 0 mV at 6 h. Comparing the ζ curve of ZW-CNT-CZ to that of ZW-CNT, it follows a similar trend by shifting ζ to a more negative value. It is worthy to note that the minimum was reached at 3 h in the former while 5 h in the latter. Similarly, Fig. 3(b) shows that the ζ of the ZW-CNT-CZA suspension initially decreased and then increased with time. However, a minimum of ~ -32 mV was attained at 1 h, and the value increased to 0 mV at 3 h.
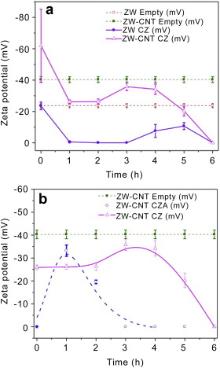 | Fig. 3. (a) Zeta potential of different ZW suspensions as a function of time; (b) Zeta potential of different ZW-CNT suspensions as a function of time. |
Fig. 4 displays SEM micrographs of CZA-CNT and CZ-CNT composite powders with varying dwell time of metallic powders in ZW-CNT suspension. It can be seen that for CZA-CNT composite powders with 1 h dwell time of CZA powders in ZW-CNT suspension, there is a high density of CNTs attached to the CZA powder surfaces, while after 4 h dwell time in suspension, CNTs are barely seen on the powder surfaces. In contrast, for CZ-CNT composite powders with 1 h dwell time of CZ powders in ZW-CNT suspension, very few CNTs are present on the CZ powder surfaces, while after 4 h dwell time in suspension, large amounts of CNTs are evident on the powder surfaces. The SEM images indicate that the optimum dwell time in suspension for adhesion of CNTs onto CZA and CZ powder surfaces is 1 h and 4 h, respectively, and if the dwell time exceeds the optimum time, debonding of CNTs occurs from the powder surfaces. These results are consistent with the ζ measurements showing the minimum of ζ at 1 h and 4 h for ZW-CNT-CZA and ZW-CNT-CZ suspensions, respectively. It is proposed that different hydrolysis and/or oxidation behavior between CZ and CZA leads to the different optimum dwell time in suspension, which is discussed in detail in the discussion section.
When fine powder particles or CNTs are dispersed in a liquid, a charged interface develops in most instances between the surfaces of the particles or CNTs and the bulk liquid[ 22]. The charged interface consists of two parts; the inner region is called the Stern layer where ions are strongly bound, and the outer region is a diffuse layer where ions are loosely associated[ 22]. Inside the diffuse layer exists a theoretical boundary where the ions and particles form a stable entity[ 23]. The ions outside this boundary, which define the shear plane, determine ζ. ζ is a measure of colloidal stability and is defined as the electrical potential at the interface powder-aqueous interface. ζ is typically measured as a function of pH[ 24], while an alternative method of implementing ζ measurements is to measure the electrokinetic potential as a function of time, at a constant pH (of ~7 in this study). Moreover, electrostatic interactions between CNTs-CNTs and metal-CNTs create a diffuse electrical double layer, which are ultimately responsible for dispersion stability and adhesion, respectively.
Colloidal dispersions tend to coagulate due to Van der Waals attractive forces. To promote stability, some mechanisms are required to overcome the Van der Waals attraction. In general, there are three mechanisms for dispersing fine particles or CNTs in water: (i) repulsion among the particles or CNTs, (ii) steric hindrance, and (iii) the reduction of hydrophobic linkages among dispersed particles or CNTs. Rosen noted that surfactants with a short hydrophobic section are more effective at dispersing suspensions, but are less efficient[ 25]. The use of surfactants has been reported to facilitate dispersion of CNTs in suspensions through the first two mechanisms. In the case of ionic surfactants, their adsorption onto CNTs imparts an effective charge onto CNTs[ 21], and the dipole-dipole electrostatic interaction between adsorbed surfactant molecules prevents CNTs from agglomeration by counteracting Van der Waals forces[ 14] , [ 26].
ZW surfactants are interesting because of their many unique properties, which are minimally influenced by added electrolyte, and changes with temperature or pH[ 27]. In general, ZW surfactants have both hydrophobic and hydrophilic ends. The hydrophilic end, which is the head of the ZW surfactant, contains both positively and negatively charged groups, and electrostatic interactions easily occur between neighboring positive and negative charges[ 15]. It is important to ensure complete surfactant dissolution and ionization in water. However, at room temperature there is virtually no solubility of ZW in water. At temperatures below the Krafft point, the solubility is limited because the surfactant has a long alkyl chain[ 28]. Therefore, the suspensions must be heated to 353 K to enable dissolution of ZW into de-ionized water.
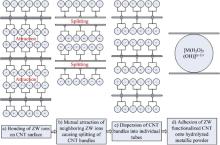
The mechanism for dispersing CNTs using the ZW surfactant is to overcome CNTs' inherent Van der Waals forces through the electrostatic interaction among ZW's charged head groups. Jiang et al. found that for an anionic surfactant sodium dodecyl sulfate (SDS), the adsorption onto a CNT bundle occurs through the interaction of the alkane groups, the hydrophobic tail segment, of SDS with the CNT bundle surface[ 17]. Similarly, for ZW, adsorption of ZW with CNT preferentially occurs through the interaction of the alkane group, the difference of which is that the hydrophobic tail segment of ZW forms an ordered hemi-cylindrical structure around the CNT at the solid-aqueous interface[ 29], as schematically depicted in Fig. 5(a). Furthermore, Fugetsu et al. ascertained that in aqueous solutions water molecules are absorbed on the ZW alkyl tail group forming hydrogen bonds between them. These hydrogen bonds aggregate on the CNT surface leading to the formation of a self-assembled monolayer along the length of the CNT[ 14]. Fig. 5(b) shows the mutual attraction of neighboring hydrophilic ZW head groups splitting CNT bundles. It is thought that after CNT splitting, a self-assembled monolayer (SAM) of ZW is formed around each individual CNT surface[ 29]. Therefore, the CNTs can be completely separated, and un-bundled CNTs are dispersed in the ZW surfactant solution[ 14], as seen in Fig. 5(c).
Hydration of metal ions occurs in a series of reactions. First the metal ion is solvated by about six water molecules (aka hydration), in what is known as the primary hydration sphere, that surround the metal ions[ 23]. Most of the heat released in this reaction is due to the enthalpy of hydration. Second, the hydrated ion can now undergo step-wise hydrolysis in which it delivers one or more H+ to the bulk solvent producing an acidic solution represented by the equilibrium Eq. (1), though the same scheme applied for any element, differing only by degree[ 30]. The first step in Eq. (1) shows the interaction of the cation for oxygen to an attached water molecule resulting in the splitting-off of one H+, which mimics the behavior of a Lewis acid donating an H+ proton. Each step in the hydrolysis reduces the charge as hydroxyl-cations are formed and subsequent protons are released; eventually a neutral species forms[ 20]. The positive charge on the metal ions draws an electron cloud from the O-H bonds of the water molecules, and accordingly increases the O-H bonds' polarity, facilitating breakage of the bonds.
[ M(H2O)6] z++H2O⇔[ M (H2O)5 (OH)](z-1)++H3O+ (1)
The charge and radius of a cation influence its relative tendency for hydration. The hydration enthalpy, entropy and Gibb's free energy can be determined, by Eqs. (2), (3) and (4)[ 31]:
ΔH=-0.86 A-4.6 z/ d (±~8.4kJmol-1) (2)
ΔS=1.69 A+34.9 z/ d (~±25.1 JK-1mol-1) (3)
Δ G=Δ H- TΔ S (4)
where A is a factor that represents the stability of the MOH species of the cation, z is the cation charge, d is the interatomic distance of cations, and T is the absolute temperature. From these equations, the hydration Gibb's free energy, which predicts the relative tendency of hydration of a particular ion, typically decreases with the charge and increases with the ionic radius. Zr ions have virtually no hydration in water up to 573 K[ 32], and Table 1 shows the values of hydration enthalpy, entropy and Gibb's free energy for Al3+ and Cu2+ at room temperature. The Gibb's free energy of hydration for Al3+ is significantly lower than that for Cu2+, indicating that Al3+ has the strongest tendency of hydration among the three cations[ 31].
| Table 1. Tabulation of Δ H, Δ S and Δ G for hydration of Al and Cu at T = 25°C |
Hydration results in a positive charge of a metal powder particle, which imparts electrostatic attraction with the ZW head groups and allows ZW heads to attach to the powder particle surface. Considering that the tails (hydrophobic ends) of ZW are attached to a CNT, the CNT is connected, via the several ZW molecules adsorbed on it, to the powder particle, and after the removal of ZW by decomposition, adhesion of the CNT onto the powder particle surface occurs. Adhesion of CNTs onto powder particle surface after ZW decomposition occurs through the presence of oxygen on the Cu-CNT interface due to oxidation from exposure to water and atmosphere. Park et al. reported a significant enhancement in binding energies between Cu and carbon atoms due to the presence of oxygen[ 33], thus oxygen promotes electron exchange between Cu and carbon atoms, enabling ionic bonding between the powder particle and CNT surfaces. This process is schematically shown in Fig. 5(d). Adhesion of CNTs on powder surfaces is critical to inhibiting re-agglomeration of CNTs, and to enabling ultimate role of CNTs in improving mechanical properties of the composites by preventing a single shear band from traversing through the composites and by generating multiple shear bands.
ZW surfactants undergo a self-assembly process onto CNT surfaces forming monolayers[ 27], which creates an electrostatic double layer. As mentioned earlier, ZW surfactants have both positive and negative head groups with an implied neutral net charge, although Kamenka et al. found that ZW monolayers form micelles and that these micelles have been shown to be have a small net negative charge[ 34]. Adhesion of CNTs functionalized with ZW (net negative charge) onto metallic powder surfaces is considered to occur through interaction of hydrolyzed metallic powder particles (net positive charge) with the electrical double layer. Debonding of CNTs from the powder surfaces (net neutral charge) may also occur if the electrical double layer is depleted due to an increased number of ions by hydrolysis. Electrostatic forces arise when similarly charged electric double layers are overlapped. According to the Derjaguin approximation, interaction potential in aqueous colloidal systems has two main components, namely, Van der Waals forces and electrostatic double layer interactions[ 35]. The interaction between the metal powder and CNT is thought to be analogous to interaction between two non-identical planes due to the particle size-CNT diameter ratio[ 22]. The adhesion of CNTs to metallic powder particles occurs, in the presence of a surfactant alkyl chain through electrostatic double layer interaction that naturally forms whenever particles or CNTs with surfaces carrying ionizable groups are suspended in a polar solvent like water[ 36]. The electrostatic double layer results from the build-up of opposite charge to the surface charge, ions tend to spread out or homogenize their distribution in order to increase the width and entropy which attracts ions to the surfaces[ 37]. Hydrolysis released protons into the suspension that already contains a finite concentration of anions and cations. Increased ion concentration at the contact leads to degraded attraction of the next layers in the electron cloud on the surface, hence debonding of CNT from powder surface occurs after the hydrolysis reaction of the metallic powder in suspension has completed. Ultimately the cooperative reaction of adsorption of ZW onto the CNT increasing the electrostatic double layer allows for stable dispersion of the CNT in suspension, while hydrolysis allows for attraction of the functionalized CNT onto the amorphous metallic powder surface, and debonding of the CNT occurs at the completion of hydrolysis releasing an abundance of ions into the suspension. Therefore, in order to synthesize composite amorphous metallic powder with CNTs an understanding of the underlying interactions of cooperative mechanism have been clarified.
It was shown that the ζ of ZW-CNT suspension did not vary as a function of time. In contrast, ζ of ZW-CNT-metal powder suspensions changed as a function of time, indicating changes in adsorption of ZW head groups onto metal powder particles with the dwell time of CZ or CZA powders in ZW-CNT suspension. The decrease in ζ of ZW-CNT-metal powder suspensions, or the increase in the absolute magnitude of ζ, is thought to indicate more adhesion affinity of ZW head groups onto the amorphous metallic powder surfaces.
CNTs were dispersed in water using a ZW surfactant, and then CZA and CZ powders were submersed in the ZW-CNT suspension with varying amounts of dwell time in an ultrasonic bath. CNT-metal composite powders were obtained after drying of the ZW-CNT-metal powder suspensions and thermal decomposition of the surfactant. Zeta potential measurements were performed on ZW-metal powder, ZW-CNT, and ZW-CNT-metal powder suspensions, and the microstructures of CNT-metal composite powders were examined by SEM. The mechanisms are discussed for dispersion of CNTs in aqueous suspension and adhesion of CNTs onto metal powder particles. The following conclusions can be drawn.
(1)The mechanism for dispersion of CNTs using ZW surfactant is the formation of self-assembled monolayers at the CNT-aqueous ZW interface creating a mutual attraction between neighboring ZW head group enabling the CNT to overcome its inherent Van der Waals attraction.
(2)Hydrolysis of metal ions into aqueous suspension creates a net polarity on amorphous metallic powder particle surfaces, which allows for interactions with CNTs in surfactant suspension and accordingly adhesion of CNTs onto the powder particles.
(3)Zeta potential measurements of ZW-CNT-metal powder suspensions and SEM study of CNT-metal composite powders indicated an optimum dwell time of metal powders in ZW-CNT suspension for optimal adhesion of CNTs onto metal powder particle surfaces. The difference in tendency of hydration between Al3+ and Cu2+ causes the difference in optimum dwell time between CZA and CZ powders. Hydrolysis of Al3+ occurs significantly more readily than that of Cu2+.
(4)Electrostatic double layer in conjunction with minimizing the ion concentration due to hydrolysis is necessary for adhesion of CNT onto amorphous metallic powder to occur without subsequent debonding of CNTs from the amorphous metallic powder surfaces.
Acknowledgments
This work was supported by the Materials Design Institute (program manager Dr. Dan Thoma), funded by the LANL/UC Davis Education Research Collaboration, Los Alamos National Laboratory (LANS Subcontract No. 75782-001-09), and the UC Lab Fees Research Program- Contingency Funds. The authors also thank Profs. Adam Moule and Tom Young for usage of their UV/vis spectrometer and zeta meter, respectively.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|