In this paper, we have reported the synthesis of FeS2 of higher band gap energy (2.75 eV) by using capping reagent and its successive application in organic–inorganic based hybrid solar cells. Hydrothermal route was adopted for preparing iron pyrite (FeS2) nanoparticles with capping reagent PEG-400. The quality of synthesized FeS2 material was confirmed by X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy, Fourier transform infrared, thermogravimetric analyzer, and Raman study. The optical band gap energy and electro-chemical band gap energy of the synthesized FeS2 were investigated by UV–vis spectrophotometry and cyclic voltammetry. Finally band gap engineered FeS2 has been successfully used in conjunction with conjugated polymer MEHPPV for harvesting solar energy. The energy conversion efficiency was obtained as 0.064% with a fill-factor of 0.52.
The FeS2 in pyrite phase is an important material because of its environmental compatibility and high stability towards photo corrosion[ 1]. It has attracted significant scientific interest and has numerous technological applications[ 2], [ 3]. Owing to their large potential capacities in application of devices, iron–sulfur based materials have been extensively studied by Kirkeminde et al.[ 4]. The photo-sensing behavior mainly depends on the absorbance and the direct band gap of the materials. The indirect band gap of FeS2 was measured as 0.95 eV by Ennaoui et al., which is suboptimal for single junction photovoltaic application[ 5]. To improve the band gap of pyrite FeS2, enormous efforts have been made. By controlling the environmental conditions of reaction, it is possible to synthesize high quality FeS2 nanoparticle with desired property. Improvement in absorbance of FeS2 can be done by tuning the nanomorphology. FeS2 nanoparticles with different morphology have been fabricated by using a variety of synthetic methods[ 6]. Sun and Ceder studied and tried the technique to tune the band gap of FeS2 by controlling the particle size[ 7]. As it had been reported so far, there are various phases of FeS2, such as mackinawite (tetragonal), troilite (hexagonal), pyrrhotite (monoclinic), smythite (hexagonal), pyrite (cubic), marcasite (orthorhombic) and greigite (cubic)[ 7]. The solids consisting only of iron and sulfur are known to occur naturally at lower temperature (below 200 °C). The structural growth strongly depends upon the reaction temperature and the vapor pressure of the solvent. Wadia et al.[ 8] reported that iron pyrite (FeS2) is significantly attractive in both cost and availability for the application of thin film technology. Like FeS2 there are very few nano-semiconducting materials, which can meet the large scale need, outside quantum confined systems[ 8]. As the pyrite phase has more extensive stability in natural environment in spite of its lower band gap, the use of this phase is enormous. Unfortunately, the iron pyrite based solar energy harvesting devices have been plagued by performance problems. The science behind its underperformance is still not well understood. After hard research work and a prolonged study it is identified that the various phases of FeS2 produce surface defects near the surface of thin film and grain boundaries that limit the open circuit voltage of the photovoltaic devices. Ennaoui et al. reported pyrite single crystal based photo electrochemical cells, which show low open circuit voltage of 187 mV and fill-factor (FF) of 0.5[ 9]. The phase purity was attributed to low open circuit voltage, which was reported by Thomas et al.[ 10]. Ganta et al.[ 11] successively synthesized superstrate type FeS2 and CdS ink-based solar cell with efficiency of 0.03% and open circuit voltage ( Voc) of 565 mV. Several recent publications exhibited that successful and robust synthesis of pyrite nanocrystals and many efforts have been directed toward exploring their optoelectronic applications[ 12], [ 13], [ 14], [ 15], [ 16]. Most recently (in the year 2012) Kirkeminde et al. reported inorganic solar cell based on ITO/PEDOT:PSS/(TFB) poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(4,4-(N-(4-s-butylphenyl)) diphenylamine)]/FeS2:CdS/Al structure, which showed more promising results with a PCE of 1.1% and Voc of 0.79 V[ 17], but the absorption and charge transportation highly depends on the CdS quantum dot, rather FeS2. Bi et al.[ 14] synthesized FeS2 NCs with improved stability in air and observed a photo response in an ITO/FeS2 NC/Al device, but the result is not upto the mark. Moreover, not surprisingly, no rectification behavior was observed, as the pyrite formed ohmic contacts at the ITO (indium tin oxides) and aluminum interfaces. Despite these recent works and renewed interest in pyrite FeS2 over the past several years, no high performance device has been made yet based on this material. Further investigation on the fundamental properties of pyrite FeS2 and deployment in alternative device architectures are required to explore the potential of pyrite FeS2 in photovoltaics.
Here we have studied a different technique to improve the band gap energy of FeS2 by changing the architectural growth. Capping reagent has been introduced to influence the vapor pressure of the solution, which can effectively control the morphology via controlling the growth rate. The optical absorption of the synthesized material was studied by UV–vis absorption spectroscopy. Depending upon the morphological growth, energy absorption of as-synthesized iron pyrite was noticed as very low compared to other inorganic semiconductors. The optical band gap energy estimated from UV–vis absorption data was significantly improved. This fact was in agreement with the absorbing behavior of iron pyrite, for its modified architecture and epitaxial growth. The electrochemical band gap energy was calculated from oxidation (corresponding to valence band) and reduction (corresponding to conduction band) states with the help of cyclic voltammetry (CV). Thermal stability of the FeS2 nanoparticle was studied with thermogravimetric analyzer (TGA). It is obvious that by controlling the growth of morphology with chemical surfactant, the band gap would successively tune up for application in high temperature semiconducting electronic devices. Thus our synthesized semiconducting material with higher band gap and thermal stability can be applied in photovoltaics at high temperature. It is noteworthy that it has still remained as a challenging unexplored arena for the researchers. In the fore step we have fabricated ITO/PEDOT:PSS/MEH-PPV:FeS2/Al based hybrid solar cell and characterized the device to estimate different cell parameters. The polymer MEHPPV has a wide research application for its higher absorption with band gap energy of 2.2 eV, as a semiconducting donor polymer in fabrication of efficient thin film hybrid solar cell. This was the early challenge to harvest solar energy by mostly abundant iron-pyrite conjugated with MEHPPV. The subject still remains unexplored. The synthesized FeS2 of higher band gap energy with corresponding energy levels demonstrates the successive charge transportation phenomena from MEHPPV donor to FeS2 acceptor and also explains the improvement of open circuit voltage of the device. This is the uniqueness of this work.
Hydrated ferric chloride (FeCl3·6H2O), hydrated sodium sulfide (Na2S·9H2O), ammonium hydroxide (NH4OH), ethanol, chloroform and polyethylene glycol (PEG-400) of AR grade were procured from Merck. Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEHPPV), poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS) and sulfur powder were purchased from Sigma Aldrich. All these chemicals were used without further purification.
In this work FeS2 nanoparticles were synthesized by adopting hydrothermal synthesis to curtail the manufacturing cost[ 18]. 20 ml 0.1 mol/L ferric chloride aqueous solution was prepared to mix with 20 ml 0.1 mol/L Na2S aqueous solution. The mixture was stirred for 30 min with the help of magnetic stirrer. By maintaining the pH value of the solution within 6–7, NH4OH aqueous solution was added drop-wise to the mixture. A black–brown precipitate was observed, and 10 ml PEG-400 was added. 0.2 mg sulfur powder was mixed simultaneously with the solution and was sonicated for 24 h. The desired mixture was then transferred into a linear Teflon autoclave to be heated at 120 °C for 2 days. Then the nanoparticles were collected by washing with ethanol and distilled water repeatedly by centrifuge technique.
Before device fabrication, the solutions of MEHPPV and synthesized FeS2 nano-particle were prepared separately in chloroform with concentration of 10 mg/mL and 2 mg/mL, respectively. Solutions were mixed at appropriate weight ratio under vigorous stirring by magnetic stirrer for 2 h to get the desired mixture. As a buffer transparent layer, PEDOT:PSS was spun on the patterned and pre-cleaned ITO substrate (cleaned with acetone, ethanol and de-ionized water repeatedly under ultra-sonic bath) at 1200 r/min for 2 min and then dried at 100 °C. The active layers of blended solution of MEH-PPV:FeS2 composite was prepared via spin coating at 2000 r/min for 2 min in open atmosphere with the help of SCU-2007 spin coating unit. Solvent evaporation has been done by heating the samples at 100 °C for 10 min under vacuum oven. Thereafter, aluminum electrode as back contact was deposited on to the active film with shadow mask by thermal evaporation technique with the help of 12A4D HHV vacuum coating unit.
The characterization of blackish FeS2 nanopowder was done by recording powder X-ray diffraction (XRD) spectra with the help of Bruker D8-X-ray Diffractometer, Raman spectroscopy, field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) of JEOL make. To investigate the functional groups, which are responsible for homogenous dispersion in chloroform medium, Fourier transform infrared (FTIR) spectra were recorded with the help of FTIR-8400S Spectrophotometer of Shimadzu. The thermal stability of the sample was measured with DTG-60 thermogravimetric and differential thermal analyzer of Shimadzu. The sample was heated at a rate of 10 °C min-1 in a nitrogen atmosphere starting from 50 to 800 °C. The electrochemical measurements were carried out using a Gamry reference 750 potentiostat in a three-electrode configuration. CV was performed using a conventional three-electrode system. The working electrode was a photocatalyst powder-modified glassy carbon electrode (GCE) (3 mm diameter), the reference electrode was an Ag/AgCl (in 3 mol/L NaCl) electrode, and the counter electrode was a platinum wire. The electrochemical band gap energy of the material was measured from CV data and the optical band gap energy was evaluated from UV–VIS absorption data, recorded with the help of 2401PC Shimadzu Spectrophotometer. To study the energy quenching of the composite, the photoluminescence spectra were recorded with the help of Cary Eclipse Fluorescence Spectrophotometer from Varian. The current density vs voltage characteristic of the device was measured with Keithley 2400 sourcemeter interfaced with PC.
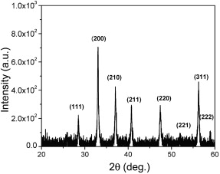
Fig. 1 represents the XRD spectra of the synthesized material. The XRD peaks from each responsible Bragg's ( hkl) planes for diffraction were recorded as (111), (200), (210), (211), (220), (221), (311) and (222) at 2 θ = 28.5, 33, 37, 40.7, 47.4, 51.9, 56.3, and 58.9 deg, respectively, which is related to FeS2 pyrite (cubic structure) with phase purity[ 13]. This phase is approved by JCPDS card no. 42-1340. Few extra peaks may be due to the attenuation and the presence of noise. Analysis of the XRD pattern using Scherrer's broadening equation, estimates the particle size[ 19]. From XRD spectrum by measuring the broadening of intensity at half maxima in radian and considering wavelength ( λ) = 0.154 nm (Cu Kα), the particle size of the material was evaluated with the auxiliary equation λ/ Bcos θ as 19 nm, where B represents the broadening constant and θ is the Bragg's angle.
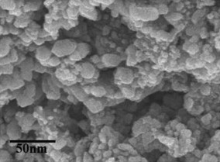
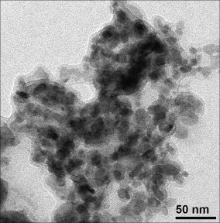
Fig. 2 and Fig. 3 represent FE-SEM and TEM images of the as-synthesized FeS2. These FE-SEM and TEM images exhibited that the size of the particles are in nano range (10–100 nm). The coagulations of the particles were observed from these electron microscopic imaging. The morphology of the particles is indistinguishable, which may be due to the coagulations of the nanoparticles. This is crudely related with the chain length of the capping reagent and the solution vapor pressure.
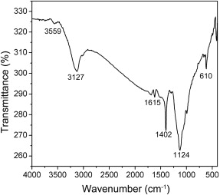
Fig. 4 represents FTIR spectroscopy of FeS2 nanoparticles. This spectrum exhibited O–H stretching as peak at 3559 cm-1 and a broad peak centered around 3127 cm-1. The absorption near 1402 cm-1 corresponds to the C–H bending vibration which came from the capping reagent (PEG-400). In addition, the band at 1124 cm-1 represented the asymmetric S–O stretching of the sulfate species, while the peak at 610 cm-1 was the consequence of the disulfide stretching (S–S)[ 20].
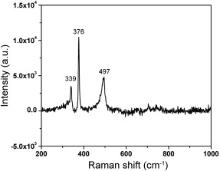
To study the chemical bonds and symmetry of molecules the vibrational information has been gathered from Raman spectroscopy. The active modes of Raman spectra consist of a symmetric mode ( Ag), a doubly degenerate ( Eg), and three triply degenerate modes. Fig. 5 represents the Raman spectra of FeS2 nanoparticle, recorded in the wave number range from 200 to 1000 cm-1. In this spectra sharp peaks were observed at 339 and 376 cm-1, which are the characteristic active modes for FeS2 corresponding to the S2 libration ( Eg) and in phase stretching vibration of S–S dimer ( Ag), respectively. In the Eg mode, the S atoms are displaced perpendicularly to the dimer axes. The peak at 497 cm-1 corresponds to the coupled libration and stretching ( Tg) modes or their combination[ 21], [ 22]. The above peaks exhibit the pyrite cubic structure[ 23].
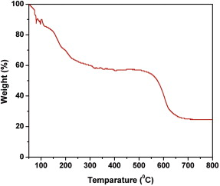
Fig. 6 represents the thermal degradation of mass with decomposition, which was recorded with increasing temperature starting from 50 to 800 °C by TGA. This TGA curve shows a drastic weight loss at around 50–250 °C. It may be caused by dehydration of the hydroxyls. Thereafter the degradation was not observed till 600 °C, which indicates the thermal stability of the sample. Therefore, it is obvious from this graph that FeS2 is thermally stable up to 600 °C. After that the sample started to be decomposed sharply. Finally the sample was decomposed to 25% of its initial mass at temperature 800 °C. It may be due to the dissociation of FeS2 into iron oxide. The important thing is to be noted that the high thermal constancy of FeS2, dignified the material for the application in high temperature environment (i.e., greater than room temperature).
The UV–vis absorption spectra of FeS2 nanoparticle, dispersed in DMF solution is given in the inset of Fig. 7. These data were analyzed with Tauc's equation and considering the intercept of the sharp linear plot ( αhν)2 vs incident photon energy ( hν) at incident energy axis. The direct optical band gap energy of the nanomaterial at room temperature was measured as 2.75 eV ( Fig. 7). With these UV–vis spectral data considering Davis and Mott's equation, the curve α (absorption coefficient) vs hν (incident photon energy) was plotted to estimate direct optical band gap energy of FeS2 nanomaterial ( Fig. 8). From this plot, the direct band gap energy of the nanomaterial was calculated as 2.74 eV, which is not much different from the value estimated by Tauc's equation. The direct band gap of FeS2, reported so far is in the range from 0.7 to 2.6 eV[ 24]. In this study, the effective direct band gap at room temperature of the nanomaterial, as measured from Tauc's and Davis–Mott's equation is appreciably high. It might be due to the epitaxial growth of the nanocrystal during hydrothermal synthesis[ 25], [ 26], [ 27], or due to the Burstein–Moss effect[ 28], [ 29]. According to Burstein–Moss effect, depending upon the morphological growth of nanoparticles the absorption edge is pushed to higher energies, as a result of all states close to the conduction band being populated.
2 nanoparticles.'>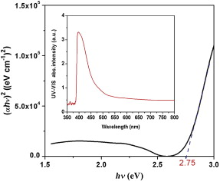 | Fig. 7. Tauc's plot and band gap of FeS2 nanoparticles. |
2 nanoparticles.'>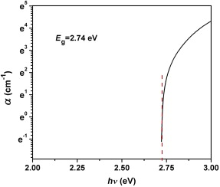 | Fig. 8. Devis-Mott's plot and band gap of FeS2 nanoparticles. |
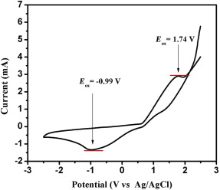
Fig. 9 shows the cyclic voltammogram of dispersed FeS2 nanoparticles. The voltammogram shows an oxidation peak, i.e., the ionization potential at approximately +1.74 V and a reduction peak, i.e., the electron affinity at about -0.99 V. In order to estimate the electrochemical band gap energy, the conduction and valence band energy ( ECB and EVB) were calculated with the help of following equations
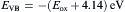
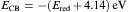
as ECB = -3.15 eV and EVB = -5.88 eV, where Eox and Ered are the onset potentials of the oxidation and reduction relative to an Ag reference electrode, respectively. The value 4.14 eV represents the difference between the vacuum level potential of the normal hydrogen electrode and the potential of the Ag/AgNO3 electrode. Calculating the difference Δ E = ECB - EVB[ 30], the energy band gap of FeS2 was estimated as 2.73 eV.
Fig. 10 represents the photoluminescence (PL) spectra of MEHPPV and MEHPPV:FeS2 composite in chloroform medium, in the wavelength range from 450 to 750 nm. The figure shows that for the composite, PL excitation peak has been shifted from 558 to 565 nm. The deviation of excitation peak of composite from MEHPPV in chloroform medium indicates the energy quenching with red shift. The PL intensity is significantly reduced to 0.3 times of the value of MEHPPV during formation of composite with FeS2. The static energy quenching of MEHPPV with incorporation of FeS2 nanoparticle was observed distinctly in Fig. 9. Thus it is obvious that in MEHPPV:FeS2 composite, FeS2 nanoparticle acts as a semiconducting acceptor. The PL quenching is the evidence for exciton dissociation. When the photogenerated excitons are dissociated, the probability for recombination should be significantly reduced. This is the ultrafast electron transfer phenomena from donor to acceptor and it is expected to increase the exciton dissociation efficiency in photovoltaic devices[ 31] and [ 32].
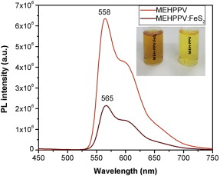 | Fig. 10. Photoluminesence spectra of MEHPPV and MEHPPV:FeS2 composite. |
Fig. 11 represents the current density–voltage ( J– V) characteristic of the device with structure ITO/PEDOT:PSS/MEHPPV:FeS2/Al. The effective diameter of each cell is 2 mm. The J– V characteristic of the device was recorded under the illumination of white light with incident power density of 80 mW cm-2. From these characteristic curves the short circuit current density ( Jsc) and open circuit voltage ( Voc) were measured as 0.13 mA cm-2 and 0.72 V, respectively. The maximum power, maximum voltage, energy conversion efficiency, and fill-factor were calculated as 0.049 mW cm-2, 0.5 V, 0.064% and 0.52 for the device, sequentially ( Table 1). The series and shunt resistances ( Rs and Rsh) of the device, as measured from J– V curve are 53 Ω and 2.13 kΩ, respectively. From CV analysis of FeS2 by knowing the positions of energy levels, the exciton dissociations and charge transportation path way from donor polymer (MEHPPV), with HOMO energy -5.1 eV and lowest unoccupied molecular orbital (LUMO) energy at -2.7 eV, to acceptor (FeS2) can be explained. The energy band diagram ( Fig. 12) approved the concerned motivation and experimental effort consecutively. Thus newly found FeS2 with higher band gap energy can be a good candidate for solar energy harvesting. To improve the efficiency of the device the thicknesses of different layers should be optimized along with material synthesis conditions.
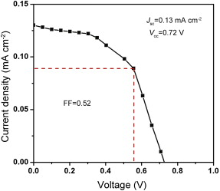 | Fig. 11. Density–voltage ( J– V) characteristic curve of ITO/PEDOT:PSS/MEHPPV:FeS2/Al device. |
| Table 1. Characteristics data of the device |
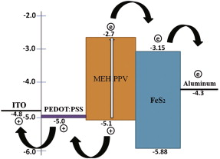 | Fig. 12. Energy band diagram for the array ITO/PEDOT:PSS/MEHPPV:FeS2/Al with charge transportation path way. |
In this experimental study, the energy band gap of iron pyrite (FeS2) nanoparticle was successively improved to be 2.74 eV by improving the synthesis technique with capping reagent. The morphological growth of nanoparticle with capping reagent PEG-400 improved the surface to volume ratio and thermal stability of semiconducting FeS2. By incorporating synthesized FeS2 nanoparticle with MEHPPV we have succeeded to harvest solar energy. The energy conversion efficiency can be improved further by fabricating the device under inert atmosphere within glove box. Thus the non-toxic FeS2 semiconducting nanoparticle with higher band gap energy can be applied for harvesting solar energy.
This work was supported by University Grants Commission (UGC), Govt. of India under project 39-508/2010 (SR). The authors acknowledge Madhusudan Nandy of Department of Chemistry, Jadavpur University and Priyanka Das of Department of Chemistry, West Bengal State University, Barasat for their valuable advice and enormous technical assistance.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|