Nanodiamond (ND) powder was successfully activated by wet chemical method and by exposure of UV/O3 in a chamber followed by mixing in triethylenetetramine (TETA) solution. The reinforcement role of activated ND in the mechanical properties of epoxy matrix was studied. Both treatments, i.e. acid and UV/O3 provide ND surface with chemical functionalities for adhesion with epoxy resin. Fourier transform infrared spectroscopy was utilized to confirm the attachment of surface groups to the ND particles. The low content of acid and UV/O3 activated ND was dispersed ultrasonically in the epoxy matrix separately to make nanocomposites. The mechanical properties of the nanocomposites were investigated under three point bending. The strong interactions among activated ND particles and the epoxy resin provide efficient load transfer interfaces, which enhances the mechanical properties of the composites. It was found that the flexural strength, modulus, and toughness of 0.1 wt% ND loaded nanocomposites have been enhanced up to 85%, 57%, and 39%, respectively for UV/O3 treated ND powder. It is also found that the optimum ND concentration to achieve maximum reinforcement is 0.1 wt% while higher concentrations lead to decrease in mechanical properties. The significant improvement of the mechanical properties of the ND/epoxy nanocomposites is attributed to the good dispersion of the functionalized ND in epoxy matrix.
Having the diamond structure on a nanometre scale, nanodiamonds (NDs) exhibit exceptional hardness, fracture strength, environmental inertness, and optoelectronic properties attractive for a variety of important applications, including field-emission displays, nanotribology, miniaturized mechanical and optoelectronic systems, and biomedical nanodevices[ 1], [ 2], [ 3]. The readily available detonation NDs (DNDs) and the recent breakthrough in disintegrating them into stable aqueous colloidal solution of ND particles having diameters of 4-5 nm provide new fundamental and applied opportunities. The ND surface is multifunctional and can simultaneously contain hydride, hydroxyl, carboxyl, ketone, ester, lacton, and other groups. The aggregation degree of individual diamond nanoparticles may be sufficiently high with respect to the conditions of synthesis and further refinement. The potentials of employing ND as reinforcements in polymer matrix have been severely limited, however, because of the difficulties associated with dispersion and aggregation of ND during processing and the poor interfacial interactions between ND and the polymer matrix. The interfacial interactions can be improved through functionalization of ND before utilizing as filler in epoxy matrix. In this case, significant efforts have been directed towards the establishment of chemical functionalities on the ND surface[ 4], [ 5], [ 6], [ 7], [ 8], [ 9], [ 10], [ 11].
In the present paper, we report the purification of the DNDs through a mild surface modification method using the ultra-violet/ozone (UV/O3) treatment as well as acid treatment. The reinforcement effect of these active fillers on mechanical properties of the epoxy matrix has also been studied. The advantage of using UV/O3 treated ND as reinforcement in epoxy matrix has been observed.
ND powder was purchased from Heyuan Zhonglian Nanotechnology Co., having phase purity higher than 98%. The colour of the as-received ND powder is grey and the average size of the ND particle is around 5 nm according to the supplier. In this study, NDs are classified into three types: (i) as-produced NDs; (ii) UV ozone (UV/O3) treated NDs; and (iii) acid treated NDs. Prior to UV/O3 and acid treatment, the as-received ND powder was placed in a crucible, and oxidized in a furnace at 440 °C for 5 h to remove the non-diamond impurities present in the powder[ 12]. These impurities include traces of metals and non-diamond carbon content. Amorphous and graphitic carbons are generally considered as non-diamond carbon content and traces of metals are embedded into the amorphous carbon shells[ 11]. Then, NDs was subjected to UV/O3 cleaner (Jelight 144AX-220) emitting radiation of 28 μW/cm2 by low-pressure mercury vapour grid lamp. The distance between UV lamp and sample tray was fixed at 10 mm. UV/O3 treatment consisted of two stages, i.e. UV/ozone exposure (1 h) and ozone exhaust (10 min) at an ambient temperature and pressure[ 13]. The UV/O3 treated ND powder was further functionalized through excess triethylenetetramine (TETA, Aldrich). The ND mixed with TETA solution was sonicated using 15UDH sonicator (frequency 24 kHz, Hielscher Ultrasonics GmbH, Teltow, Germany) at 60 °C for 1 h. The excess TETA was removed with a pipet and the powder was rinsed several times with acetone and filtrated using centrifuge machine (Select Spin Spectra-6C, USA). When the last rinse was completed, a few drops from the solution were mixed with deionized water and the pH of this solution was measured with pH paper to ensure complete washing out of the excess TETA. Acid treated NDs were prepared by dispersing NDs in a mixture of nitric and sulphuric acids for 24 h. The existence of carboxylic groups at NDs was detected using Thermo Electron Corporation IR 200 FTIR (Fourier transmission infrared) spectrometer. The Rutherford backscattering spectroscopy (RBS) for elemental analysis was performed using 5UDH Pelletron Tandem accelerator at Experimental Physics Lab, NCP, Islamabad. The dispersion stability test for ND was performed by dispersing the sample for 90 min in 50 ml tall glass bottle using an ultrasound bath (frequency 24 kHz, Hielscher Ultrasonics GmbH, Teltow, Germany) and then the sample was allowed to settle for 72 h.
The nanocomposites were prepared by UV ozone followed by mixing TETA solution (named as series II) and acid treated (named as series I) functionalized ND powder. The matrix used was Diglycidyl Ether of Bisphenol-A (DGEBA) epoxy and triethylenetetramine (TETA) used as hardener for the curing of DGEBA. The ratio of epoxy to hardener was about 10 pph (parts per hundred). First of all epoxy was degassed for 1.5 h by using ultrasonicator (1510R-DTH, Branson Ultrasonics Co., USA), then ND powder was mixed with degassed epoxy. The whole mixture was sonicated at 45 °C for 1 h. Afterwards the hardener was mixed through mechanical mixing. The mixture was then casted into the moulds and left over night for the evaporation of ethanol, used as solvent for pre-dispersion of ND particles before adding them to epoxy. Standard curing process was adopted for the curing of the composite samples. Image analysis of nanocomposites prepared from activated NDs was performed by scanning electron microscopy (SEM, JED 2300). The moulded nanocomposite plates were cut into 70 mm long × 12.7 mm wide × 3 mm thick samples according to the specification, ASTM standard D790 for three point bending. Reported values are averages of the results obtained with at least five samples.
The FTIR spectra of as-produced, acid treated and UV/O3 treated ND samples are presented in Fig. 1. Carboxylic functional groups were not detected from as-produced NDs. The bands around 1100 cm-1 and 3433 cm-1 corresponding to C-O-C and O-H stretching from surface -COOH group respectively are present in all three samples. The one of the main differences in the spectra occurs in the region between 1625 and 1790 cm-1. The features between these are mostly related to water and OH bending. There is a strong peak of C=O stretching around 1782 cm-1 in acid and UV/O3 treated samples (Fig. 1(b) and (c)), suggesting the presence of carbonyl species. This peak is absent in as-received sample (Fig. 1(a))[ 14], [ 15], [ 16], [ 17]. The spectra of the ozone treated sample indicate that the presence of these groups became more obvious with UV/O3 exposure, providing evidence that UV/O3 is beneficial to improving functionalization of ND particles.
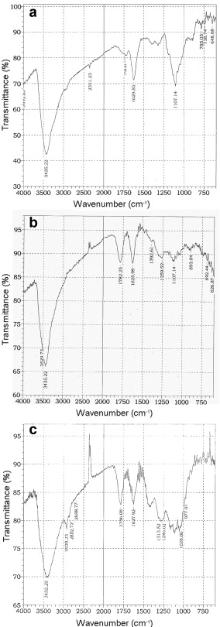 | Fig. 1. FTIR spectra of as-received (a), acid treated series I (b) and UV/O3 exposed series II (c) ND powder. |
Fig.2(a)-(c) showed the elemental analysis by RBS, an important IBA (ion beam analysis) technique of as-received, acid treated and UV/O3 treated ND powder. The RBS spectra were recorded using 4.501 MeV beam of He2+ ions focused on a diameter of 2 mm by a high-precision system of quadrupole magnetic lenses. Backscattered ions were collected on a silicon surface barrier detector at an angle of 170° as measured with respect to the incident direction of the beam. The spectra shown here were recorded with He2+ integrated charge of 20lC. The setup chamber was maintained at a background vacuum of approximately 1.33 × 10-4 Pa (10-6 Torr). The detected elements are C with broad signal, and O with very small signal in as-received ND (Fig. 2(a)). The content of oxygen kept on increasing with treatment method. It is higher for UV/O3 treated sample (Fig. 2(c)) as compared to acid treated sample (Fig. 2(b)) as can be seen from the backscattered counts from oxygen signal.
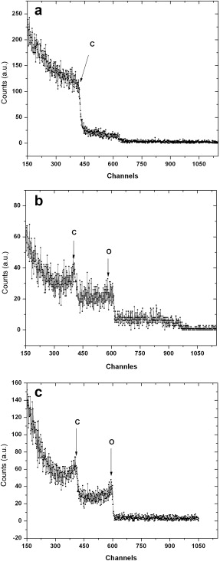 | Fig. 2. RBS spectra for as-received (a), acid treated series I (b) and UV/O3 exposed series II (c) ND powder. |
Macroscopically, several marked differences exist between as-received ND and treated ND. The most distinguishing characteristic that differentiates as-received ND from treated ND is the differences in optical properties. Fig. 3 is a photograph of solutions of both as-received ND and treated ND (using both methods) dispersed in water. ND specimens with a concentration of 0.1% (w/w) were prepared by ultrasonic dispersion. The dispersion stability of UV ozone is found to be better than the acid treated ND as observed from some precipitation of ND at the bottom of the cuvette in acid treated sample (Fig. 3(b)). Whereas the UV/O3 treated ND remain dispersed even after 48 h. The properties and stability of dispersions were shown to be significantly affected by the pretreatment of particles. UV/O3 exposure may increase the polar surface energy or polarity of the nanoparticle surface. In views of this, it can be said that the enhanced interaction of the nanofiller with polymer arising from the increase of polar surface energy is likely to promote good adhesion. Therefore, dry oxidation treatment can be an alternative functionalization method for improving the dispersion and afterwards for the distribution of nanomaterials in a polymer matrix to achieve good mechanical strength.
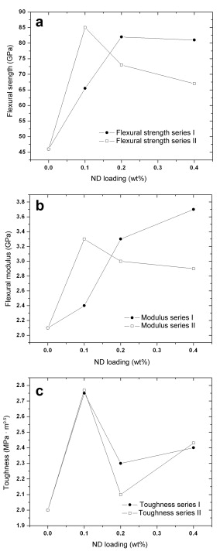
The mechanical properties under flexural load as a function of ND concentration in epoxy matrix were evaluated. Mechanical properties such as ultimate tensile strength (UTS), flexural strength, modulus, and toughness for nanocomposites are listed in Table 1 and corresponding curves are shown in Fig. 4. It is clear from the figures that there is first increasing trend up to 0.1 wt% followed by decline in properties. The loading of 0.1 wt% ND has provided the highest strengthening (Fig. 4(a)) both for series I and II. The maximum enhancement in flexural strength, modulus, and toughness is 85%, 57%, and 39%, respectively for series I and 42%, 12%, and 37%, respectively for series II.
| Table 1. Summary of mechanical properties for Epoxy/ND composites |
It has been shown that toughness (Fig. 4(c)) is greatly enhanced with ND particles (~39% for series I and ~37% for series II). These ND particles shear as an additional energy absorbing mechanism to conventional crack pinning and deflection due to the large aspect ratio. The increase in toughness can be attributed to the introduction of hard ND into the matrix. ND has removed the inherent brittleness thus resisting the crack initiation with the applied external force. By increasing ND concentration to 0.4 wt%, higher stress by aggregated particles above a critical ND level causes decline in mechanical properties but the mechanical properties of all nanocomposites are higher than that of neat epoxy. Thus, there should be an optimum ND level, the magnitude of which depends on the effectiveness of dispersion methods used. The ND content above the optimum level of 0.1 wt% still provides properties superior to neat epoxy resin (Table 1). The optimum ND level found for the strength, modulus and toughness can be attributed to a dispersion state with homogenous distribution of the finest particles (as observed from SEM image in Fig. 5), and the smaller values at higher ND levels are due to the reduced degree of dispersion.
 | Fig. 5. Electron microscopy images of Epoxy/ND nanocomposites with ND loading of 0.1 wt% (a) and 0.4 wt% (b). |
The mechanical properties, of series I, are higher than those of series II. It is due to the improved surface functionality of UV ozone (plus mixing in TETA solution) treated ND, which also helped better dispersion of nanofiller in the matrix as shown in Fig. 3(c). The better dispersion, due to functional groups on the surface of ND and the epoxy ring, gave rise to higher properties than that of acid treated ND in epoxy matrix. The toughness decreased by further increasing ND loadings above 0.1% for both series, because higher amount of ND loading causes mixing difficulties and increases aggregation of the particles. Whereas for higher ND concentration other mechanical properties including flexural strength and modulus decreased for series II as compared to series I. The possible reason is that the number of amine related groups added to the epoxy may not have been adjusted according to the amount of curing agent (TETA). To maximize the reinforcing effect of ND on the epoxy matrix, all amino groups present on the surface of the filler must react with all epoxide groups. The stoichiometric ratio for Epon 828 to TETA is 10 pph (parts per hundred)[ 18]. But when aminated ND is incorporated into aminated epoxy system, amine groups on the surface of ND shifts the curing stoichiometry[ 19]. It can lower the mechanical response at higher concentration.
In earlier studies, the optimum levels for enhanced mechanical properties have been found to be 0.3 wt% for ND/epoxy nanocomposites[ 20] and resulting in the increase in tensile strength and tensile modulus, 52.7% and 54.2% higher than that of pure epoxy, respectively. It is shown there that the improved stress transfer from the matrix to the reinforcing phase is due to the carboxylic acid functional groups developed onto the ND surface. Improvement of ~60% in Young's modulus for 3.5% ND loading and of ~700% for high ND loading (up to 25 vol.%) were also reported[ 17] , [ 21]. The addition of 0.1 wt% of ND increased the yield strength and toughness to about 12% and 5%, respectively[ 22]. In the present study, the critical ND concentration is reasonably low (0.1 wt%) and obtained properties are higher compared to earlier observations.
The improvement in mechanical properties in such a low content of ND (0.1 wt%) is due to the improved functional groups developed onto the ND surface. Such groups are helpful in providing an in situ chemical integration of the ND into the epoxy matrix. The formation of covalent bonds between the ND and epoxy resin facilitates load transfer between the ND and epoxy matrix, which contributes to the improvement of the mechanical properties of the nanocomposites. The improved dispersion at 0.1 wt% compared to 0.4 wt% can be observed from the SEM images in Fig. 5(a and b). The declined trend in the mechanical properties in higher concentration is due to the aggregation of the ND in the form of clusters. The aggregates of ND within the epoxy matrix are the defect areas, which affect the interaction of the ND particles with the epoxy matrix[ 23]. In fact, epoxy matrix does not wet the ND particles surface area within the cluster, which reduces the effective linkage density between the ND particles and epoxy matrix. As a result matrix cannot transfer the load efficiently to the ND particles. Further, the poor dispersion of ND within epoxy network affects the inter chain bonding, which may also be responsible for the lower mechanical properties above 0.1 wt%[ 5] , [ 8].
In fact, as the size of aggregate area increases the matrix cannot completely penetrate between the particles and wet them, which can be seen from the SEM image of nanocomposites prepared with 0.4 wt% ND Fig. 4(b). Therefore, a weak interface develops between the matrix and the ND particles in these regions. The reason is that modified ND particles, without degrading properties of matrix, cannot fully play their reinforcement part in nanocomposites prepared with high content of ND (i.e. above 0.1 wt%) due to weak interface formation as a result of agglomeration of particles.
The NDs treated by dry and wet oxidizing were utilized in nanocomposite as reinforcement fillers. The prepared ND/epoxy nanocomposites were studied for the enhancement of mechanical properties. The functional groups developed on the surface of the ND particles helped to increase interactions among ND and epoxy resin. The UV ozone treated ND showed better dispersion stability in water. The mechanical properties of treated ND showed significant improvements in strength, modulus and toughness. Optimum ND concentration for enhancement in mechanical properties was determined to be 0.1 wt% for aminated ND particles. The UTS, Young's modulus, yield strength and toughness enhanced to ~34%, 57%, 85% and 39%, respectively. Higher concentrations of ND >0.1 wt% resulted in decreased mechanical properties, due to the poor dispersion of ND particles at higher concentrations in the epoxy matrix.
The financial support of the Centre of Excellence for Science & Advanced Technology, and Higher Education Commission of Pakistan is gratefully acknowledged. The authors acknowledge the cooperation of Experimental Physics Labs (EPL) members for technical support.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|