A simple synthetic method has been described to prepare anisotropic gold nanoparticles (AuNPs) possessing unique optical and structural properties at room temperature, and subsequently the nanoparticles have been stabilized by temperature-sensitive poly(
A great deal of attention has been centered on the modification of gold nanoparticles (AuNPs); tuning their optical, electrical, and chemical properties has potential applications in the fields of biomedicine, sensors, electronics, and catalysis [1], [2], [3], [4], [5], [6], [7] and [8]. Some of the most attractive features of modified AuNPs are their structure-dependent absorption properties (e.g., size and shape) that can easily be examined with a conventional spectrophotometer [6], [8], [9], [10], [11], [12] and [13]. The strong absorption properties of metallic nanoparticles, including gold, readily allow for converting light energy to heat upon light irradiation via the photothermal heating process [14], [15] and [16]. As uniform spherical AuNPs are often prepared by citrate/borohydride reduction methods to exhibit strong but narrow absorption bands in the visible range [1], [10], [17], [18], [19] and [20], structurally modified AuNPs (including rods [21] and [22], plates [23], prisms [24], and core-shells [25], [26] and [27]) can possess tunable, intense, and broad absorption bands across the visible to near-IR areas. Thus, these nanoparticles can serve as photothermally-induced heating materials via irradiation—even with a common broadband light source (e.g., fluorescent light or sunlight)—for various optically-driven applications [3], [4], [6], [25], [28] and [29].
The modification of AuNPs into various nanostructures often requires multiple steps in the presence of surfactants, but the resulting nanoparticles sometimes exhibit limited stability for further use [30], [31] and [32]. Based on our previous study, kinetically-driven partially aggregated anisotropic AuNPs possessing an intense and broad absorption band can be prepared at room temperature via one-pot synthesis. Unfortunately, these unstable nanoparticles undergo a fast, irreversible reshaping process to form thermodynamically-stable polydisperse AuNPs within a few days at room temperature. While this transformation process can be retarded at low temperatures to maintain the anisotropic structure, the stability at room or higher temperatures for extended periods of time is required for their practical applications. As such, we attempted to further improve a strategy to overcome the limited stability of the anisotropic AuNPs by introducing water-soluble linear poly( N-isopropylacrylamide), or poly(NIPAM). Poly(NIPAM) exhibits abrupt structural changes above and/or below the lower critical solution temperature (LCST) [33], [34] and [35], which allows for the development of interesting temperature-responsive materials for various biological applications [16], [36], [37], [38], [39] and [40]. Although poly(NIPAM) does not possess specific functional groups to strongly interact with AuNPs, the anisotropic AuNPs can maintain their original structures and absorption properties in the presence of linear poly(NIPAM). In addition, the concentration of the linear polymer can systematically affect the size and roughness of AuNPs during their formation, and the resulting nanoparticles can exhibit an extended stability at room temperature. These stable nanoparticles with strong and broad absorption properties, as well as a greater surface area, can serve as efficient photothermal heating devices under a broad wavelength of light for a wide range of possible applications, including cancer therapy [41] and [42], tissue welding [2], surface enhanced Raman scattering [43] and [44], medical imaging [3] and [5], and catalytic/biological systems [4], [45], [46], [47] and [48].
l(+)-ascorbic acid (AA, 99.0%), potassium carbonate (K2CO3, 99.0%), ammonium persulfate (APS, 98.0%), N, N′-methylene-bis-acrylamide (BIS, 99%), and gold (III) chloride hydrate(HAuCl4·H2O, >99.9% trace metals basis) were all from Aldrich and were used as received. N-Isopropylacrylamide (NIPAM, Aldrich, 99%) was recrystallized in hexane (Fisher, 99.8%) and dried under vacuum before use. The potassium bromide (KBr, Fisher, >99%) was dried at 50 °C overnight and kept in the desiccator prior to use. The water used in all reactions was purified to a resistance of 18 MΩ (Nanopure water system; Barnstead/Thermolyne) and filtered through a 0.2 μm membrane to remove any impurities. Glassware was cleaned with an aqua regia solution, treated in a base bath (saturated KOH in isopropyl alcohol), and then rinsed with pure water.
Linear poly( N-isopropylacrylamide), or poly(NIPAM), was prepared according to the conventional radical polymerization method [46] and [49]. Recrystallized NIPAM (0.10 g, 0.88 mmol) and APS (0.0010 g, 0.0044 mmol) were mixed with 100 mL water in a 250 mL round-bottom flask containing a stir bar. Pure argon gas was purged through the reaction mixture for 1 h to completely remove oxygen prior to polymerization. The reaction flask was heated to 70 °C and stirred for 6 h (milky white). The final solution was then cooled down to room temperature (colorless) and used without further purifications. The average molecular weight of the linear poly(NIPAM) that stabilized the AuNPs was measured to be ~55 kg/mol. The polydispersity index (PDI) value was ∼3.5 which implies the high distribution of molecular weight.
Cross-linked poly(NIPAM) nanoparticles, ∼550 nm in diameter, were also prepared under similar conditions [34], [36], [46] and [49]. A reaction flask containing NIPAM (1.0 g, 8.8 mmol), BIS (0.10 g, 0.65 mmol), APS (0.12 g, 0.53 mmol), and 200 mL water was equipped with a stir bar and reflux condenser with an inlet for argon gas. The flask was purged with argon for 1 h and kept at 70 °C for 6 h. The resulting mixture was cooled and used without further purification.
1 mL of 1 wt% gold salt (HAuCl4·H2O, 0.028 mmol) solution was mixed with a 49 mL solution containing potassium carbonate (0.013 g, 0.091 mmol) in a 125 mL Erlenmeyer flask. The solution was vigorously stirred for another 40 min and kept in the refrigerator overnight prior to use. Then 100 mmol/L (or 28.0 mmol/L) l-ascorbic acid (AsA, 2.40 mL) was rapidly added to a cold K–Au solution (40 mL) in a 50 mL glass vial. After 5 min of vigorous stirring, the solution was transferred into a series of 25 mL glass vials with magnetic stir bars (5.3 mL of solution was in each glass vial), and followed by the addition of varying amounts of linear poly(NIPAM) (0%, 10%, 20%, 30%, 50%, 100%, 160%, and 300%, molar ratio). These final solutions were stirred for another 15 min, and then allowed to rest for 1 h so the poly(NIPAM) could stabilize the AuNPs.
Similarly, selected amounts of linear poly(NIPAM) (0%–300%) were mixed with 5 mL of cold K–Au solution in a 25 mL glass vial containing a magnetic stir bar. The solution was vigorously stirred for 15 min, and 0.30 mL of 100 mmol/L AsA was rapidly introduced into the vial. A color change was observed within 30 s (from colorless to purple, light blue, or dark blue), indicating the formation of various AuNPs. The addition of more linear poly(NIPAM) to the solution resulted in notably longer times for the color change to occur. The resulting solutions were additionally stirred for 15 min prior to analysis.
To confirm the interaction between the cross-linked poly(NIPAM) nanoparticles and anisotropic AuNPs, ∼50% of the polymer nanoparticles were mixed with freshly prepared anisotropic AuNPs. The resulting mixture was aged at room temperature overnight and centrifuged (Sorvall Legend X1 Centrifuge Series, Thermo Scientific) at 3000 r/min for 15 min (×2) prior to analysis. The precipitate was re-suspended in water and subjected to analysis.
Scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS)/zeta potential, Fourier transform infrared (FT-IR) spectroscopy, Raman, and UV–Vis spectroscopy were used to characterize the structure, size distribution, and absorption property of various AuNPs stabilized by poly(NIPAM), as well as the presence of adsorbed polymer layer around AuNPs. Additionally, gel-permeation chromatography (GPC) was employed to characterize the molecular weight of linear poly(NIPAM).
An FEI-Quanta 450 instrument operating at 20 kV was used to analyze the general size distribution, and a Zeiss 10 TEM operating at an accelerating voltage of 60 kV was used to evaluate the detailed morphology of AuNPs. All samples were deposited from the solution onto silicon wafers (for SEM) and 300 mesh carbon-coated copper grids (for TEM) and then completely dried at room temperature overnight.
The DLS analysis and zeta potential measurement were performed with an analyzer (ZetaPALS, Brookhaven Instruments Corp., New York) equipped with a 35 mW solid state laser (90° and 15° angular measurements); the diameters and surface charges of the AuNPs stabilized by poly(NIPAM) were measured at 20 °C. All samples for the DLS analysis were diluted in pure water. The data were collected from an average of five measurements over 100 s. For the zeta potential measurement, all samples were analyzed without dilution at 20 °C.
To examine the presence of a linear poly(NIPAM) layer around AuNPs, an FT-IR (Perkin–Elmer Spectrum One) and Raman spectroscopy (Enwave Optronics, ProRaman-L-785B) were employed. For FT-IR analysis, a solution containing AuNPs stabilized by linear poly(NIPAM) was concentrated by centrifugation at 3000 r/min for 15 min (×2). This solution and 20 mL of linear poly(NIPAM) solution were dried in an oven overnight. Each sample was then mixed with KBr powder to prepare a pellet for transmission IR measurement. The spectra were collected for 512 scans at a resolution of 4 cm-1. For Raman analysis, 10 mL of AuNPs with and without linear poly(NIPAM) in solution were purified at 3000 r/min for 15 min (×2), re-suspended in 1.0 mL of pure water, and briefly sonicated prior to analysis. The diode laser was operated in continuous wave mode at 785 nm with a 30 mW-output power. The data were collected with 300 s integration.
To characterize the absorption properties of various AuNPs stabilized by poly(NIPAM), an Agilent UV–Vis spectrometer was used over the wavelength range of 300–1100 nm. All samples were prepared in water and transferred to a quartz UV–Vis cell. For the absorption band patterns of AuNPs stabilized by the linear poly(NIPAM) upon heating and cooling, 0.1 mL of a 10 times concentrated sample solution was initially placed into two centrifuge tubes with 0.9 mL of water. An aliquot (0.1 mL) of the sample solution was transferred to a Hellma quartz ultra-micro UV–Vis cell (0.1 mL capacity) for the reversible changes in the absorption bands above and below the LCST. A quartz cuvette was then placed in a warm water bath to increase the temperature of the solution well above LCST ≥45 °C and was monitored by a dual temperature thermometer (Fisher Scientific). The same solution was cooled below room temperature (≤25 °C) to collect the absorption bands. This heating and cooling process was repeated several times.
Molecular weight (MW) of polymer was measured relative to poly(acrylic acid) standards kit (10 standards, 900–1,000,000 Da) using a Waters gel-permeation chromatography (GPC) system equipped with Breeze 2 software, ultrahydrogel linear (7.8 mm × 300 mm) column, a Waters 2489 UV detector, and a 2414 refractive index detector. Polymer samples (10 μL) were manually injected, and pure water was used as an eluent at an isocratic flow rate of 0.5 mL/min for 20 min at 30 °C and for all measurements.
The reduction of HAuCl4 in a basic solution containing K2CO3 by an excess amount of l-ascorbic acid [1], [32] and [50] at room temperature often results in the formation of relatively large kinetically-driven anisotropic gold nanoparticles (AuNPs) with some degree of aggregation [1], [4], [32] and [51]. Fig. 1 shows the representative SEM images and UV spectra of AuNPs prepared in the absence of linear poly( N-isopropylacrylamide) by using 28 and 100 mmol/L AsA. While the use of a low concentration of AsA results in large spherical nanoparticles ( Fig. 1(a)), the high concentration of AsA leads to the formation of rough polydisperse AuNPs ( Fig. 1(c)). These nanoparticles undergo a rapid reshaping process to form thermodynamically-stable discrete AuNPs (i.e., smaller and/or more spherical than those from the freshly prepared sample with a low concentration of AsA) at room temperature ( Fig. 1(b) and (d)). The corresponding absorption bands ( Fig. 1(e)) of fresh AuNPs reduced by 28 and 100 mmol/L AsA initially exhibit a strong and broad absorption band from visible to near-IR areas ( λmax ~636 and ~740 nm, respectively). Upon the reshaping of the nanoparticles into stable polydisperse AuNPs, a significant shift of the absorption bands to the lower and narrower wavelengths was often observed [4] and [10]. The reshaping of the nanoparticles with a notable blue shift of the absorption bands could be slowed down by storing them at low temperatures (<~4 °C), but this process was eventually completed within a few weeks regardless of the concentration of AsA. In addition, the nanoparticles at low temperatures are significantly limited in their practical applications. Thus, maintaining the structure and absorption property of the anisotropic AuNPs is a challenging task.
Upon the addition of the water-soluble linear poly(NIPAM) to the pre-formed anisotropic AuNPs prepared by 100 mmol/L AsA, the stability of the AuNPs in the presence of the polymer was monitored at room temperature ( Fig. 2). The SEM images of the fresh and one week aged anisotropic AuNPs at room temperature show the same results regardless of the polymer concentrations, strongly indicating that the preservation of their original structures is due to the increased stability provided by the linear polymer. The overall UV spectra and solution colors are observed in the digital photos and also remain almost identical; however, the AuNPs with less than 100% of the linear polymer often exhibit slightly broad and weak absorption bands, as well as a faded solution color after being aged for a week. The faded solution color is presumably due to the local aggregation and adsorption of a small amount of anisotropic AuNPs in solution and on the surface of plastic container, respectively. The structures of these AuNPs still remain the same even after aging the solutions (a week aged sample shown in Fig. 2(c)) for a few more weeks.
The absorption intensities at 740 nm and zeta potentials of bare anisotropic AuNPs, and anisotropic AuNPs with 10% and 300% linear poly(NIPAM), have also been monitored respectively, as a function of time ( Fig. 3). The anisotropic AuNPs with linear poly(NIPAM) show a slight decrease in absorbance at 740 nm over a period of a week, but the bare anisotropic AuNPs exhibit a significant decrease of absorption intensities in only 2 days, which is presumably caused by their rapid structural changes to polydisperse nanoparticles. The resulting nanoparticles exhibit a largely blue-shifted absorption band (~550 nm), but their position and intensity cannot be precisely measured (even with the same batch of sample) due to the unstable nature of the initial anisotropic AuNPs and the varying temperature of the environment during the reshaping process. This transformation process was also examined by slow decrease (from -30 mV to -40 mV) of zeta potentials and increase of polydispersity index (PDI) as a function of time (this observation could be also caused by the formation of locally aggregated AuNPs). Although zeta potentials of anisotropic AuNPs with poly(NIPAM) slowly increase (from -30 mV to -20 mV), these nanoparticles do not show significant changes of absorption patterns and morphologies in a week, indicating the extended stability of overall structures. The gradual increase of particle diameters and PDIs as a function of time is also observed which supports the formation of partial aggregation of AuNPs in solution. While the post-insertion of the linear poly(NIPAM) into the pre-formed anisotropic AuNPs could slowly develop partial aggregation, the reshaping process of the AuNPs can be significantly hindered apparently, supporting the presence of the stabilizing capability of the polymer for the AuNPs.
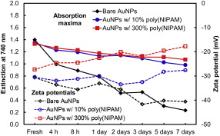 | Fig. 3. Absorption intensities at 740 nm and zeta potentials of bare anisotropic AuNPs, and anisotropic AuNPs with 10% and 300% linear poly(NIPAM), respectively, as a function of time. |
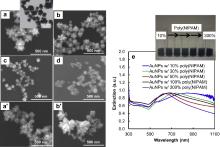
To verify the presence of interaction between the AuNPs and linear poly(NIPAM), gold ions were reduced with 100 mmol/L AsA under various amounts of the polymer (10%–300%). The representative SEM/TEM images and absorption spectra of fresh AuNPs and those one week aged solution were prepared with different polymer-gold ion ratios ( Fig. 4). While the AuNPs prepared with low concentrations of linear poly(NIPAM) (10%–30%, based on the initial polymer to Au3+ ratio) exhibit a similar roughness to the bare anisotropic AuNPs, notably smaller AuNPs with decreased surface roughness are formed with a high concentration of the polymer (≥50% polymer). Specifically, the minimum required amount of the linear polymer needed to maintain the anisotropic structures is estimated to be 10%. The average diameter of these nanoparticles (~120 nm by DLS) is slightly larger than that of the bare anisotropic AuNPs (~100 nm), presumably due to the presence of the polymer and additional partial aggregations during the formation of the AuNPs. The use of less than 10% of polymer in solution often results in the reversible aggregation of the AuNPs. The employment of greater than 50% of polymer in solution, however, limits the formation of the highly anisotropic structure of the AuNPs. These systematic changes in size and shape as a function of the polymer concentration clearly suggest the presence of interactive forces between the AuNPs and linear poly(NIPAM). The anisotropic structures of these AuNPs are maintained for a few weeks at room temperature ( Fig. 4(a′) and (b′)). Although microscopic analyses do not show the distinct aggregation/agglomeration of the anisotropic AuNPs prepared with the linear polymer, slightly higher PDI values from the DLS analysis indicate the possible local aggregation of the nanoparticles in the solution. This observation could also be explained by the slightly decreased surface charge of AuNPs in the presence of linear poly(NIPAM) (e.g., ≥-28 mV for 300% polymer) compared to that of bare AuNPs (≤-31 mV).
The distinctive color of fresh gold solution as a function of the polymer concentration signifies the formation of slightly different structures (e.g., shape and size) of the AuNPs (digital photo, Fig. 4). The UV spectra of a series of fresh poly(NIPAM)–AuNPs display a combination of two main absorption bands: a small peak at 530 nm and an intense distinctive broad absorption band in the range of 650–1100 nm which could be supported by the formation of anisotropic AuNPs with partial aggregation. The absorption band of the anisotropic AuNPs prepared with 10% linear polymer exhibits a much broader absorption range than that of the nanoparticles without the polymer; this may be due to the presence of the additional aggregation/agglomeration of the AuNPs in the solution. The absorption maxima are then gradually positioned at lower wavelengths with the increase in the concentration of the polymer, implying the decrease in size and surface roughness of the nanoparticles [4]. After aging the samples for a week at room temperature, the absorption bands and overall structures remain nearly the same, which could indicate the enhanced stability of the anisotropic AuNPs by the linear poly(NIPAM). The intensity of the absorption spectra somewhat decreases, and the colors of the aged solution slightly fade from dark blue to light blue, which may be due to the partial irreversible aggregation of the nanoparticles in solution [50], although any notable aggregation of the AuNPs was not observed from the microscope images nor from overall zeta potential, and DLS measurements except the slightly increase of PDI values. Thus, the linear poly(NIPAM) introduced during the formation of AuNPs clearly provided the extended stability of the anisotropic gold structures via weak interactive forces (i.e., van der Waals, dipole and dipole interaction between adsorbed AsA on AuNPs and linear poly(NIPAM), and/or hydrogen bonding). In a separate study, the formation of anisotropic AuNPs using the NIPAM monomer was not successful and often resulted in the fast agglomeration and aggregation in the solution at room temperature. As a whole, this water-soluble linear poly(NIPAM) may serve two functions during and after the formation of anisotropic AuNPs. Firstly, a very weak interaction with AuNPs at low concentrations may not significantly affect the final structure of AuNPs during the formation process. Secondly, the polymer could increase the stability of the resulting nanoparticles so they can maintain their strong and broad absorption bands at room temperature.
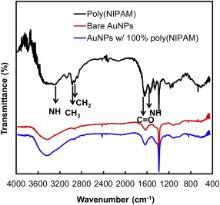
IR and Raman analyses were carried out to confirm the presence of the adsorbed poly(NIPAM) around the AuNPs as a stabilizer. Fig. 5 shows the representative IR spectra of AsA-reduced AuNPs in the presence and absence of poly(NIPAM) and pure poly(NIPAM). The spectrum of the pure poly(NIPAM) sample possesses broad major peaks of N–H stretch (~3280 cm-1), CH3 and CH2 stretch (~2984 cm-1 and ~2970 cm-1), C O stretch (~1660 cm-1), N–H bend (~1545 cm-1), and CH3 asymmetric stretch (~1470 cm-1) [35], [52], [53] and [54]. Although the linear poly(NIPAM) exhibits these major peaks, IR spectra do not clearly show the difference between the AsA-reduced AuNPs with and without the linear poly(NIPAM) under our conditions. This observation could be presumably due to the relatively broad peaks and presence of adsorbed AsA around the surface of AuNPs [1], which apparently overlapped with most characteristic peaks of the linear poly(NIPAM). In addition, residual water moisture in poly(NIPAM)-based materials often caused peak broadening and loss of spectral resolution [39], [55] and [56]. Similarly, Raman analysis did not allow for verifying the presence of linear poly(NIPAM) around the surface of AuNPs (data not shown).
O stretch (~1660 cm-1), N–H bend (~1545 cm-1), and CH3 asymmetric stretch (~1470 cm-1) [35], [52], [53] and [54]. Although the linear poly(NIPAM) exhibits these major peaks, IR spectra do not clearly show the difference between the AsA-reduced AuNPs with and without the linear poly(NIPAM) under our conditions. This observation could be presumably due to the relatively broad peaks and presence of adsorbed AsA around the surface of AuNPs [1], which apparently overlapped with most characteristic peaks of the linear poly(NIPAM). In addition, residual water moisture in poly(NIPAM)-based materials often caused peak broadening and loss of spectral resolution [39], [55] and [56]. Similarly, Raman analysis did not allow for verifying the presence of linear poly(NIPAM) around the surface of AuNPs (data not shown).
The absorption properties of anisotropic AuNPs stabilized by two different concentrations (100% and 300%) of the linear poly(NIPAM) were also examined well above(≥45 °C) and below (≤25 °C) the lower critical solution temperature (LCST). While the polymer to gold ratio of 1:1 (~100% polymer) did not show the changes to the absorption bands at these high and low temperatures (data not shown), the polymer to gold ratio of 3:1 (~300% polymer) exhibits a slightly broader and red-shifted absorption band above the LCST ( Fig. 6). This slight change in the absorption band is probably due to the increased refractive index of the surrounding environment of AuNPs caused by the collapsed linear polymer layers [16], [36] and [57]. Upon cooling below the LCST, the absorption band recovered its original shape due to the swelling of the linear polymer layers on the surface of the AuNPs. Although IR and Raman analyses did not provide conclusive evidence of the presence of the polymer on the AuNPs as a stabilizer, these reversible absorption patterns upon heating and cooling caused by the swelling and collapsing of the linear polymer layers strongly suggested the adsorbed linear poly(NIPAM) layers around AuNPs. In addition, the structures of the resulting AuNPs after the heating and cooling process remained almost the same as before the process.
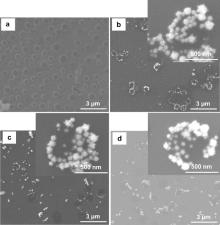
To further confirm the attractive forces between poly(NIPAM) and AuNPs, 50% cross-linked poly(NIPAM) nanoparticles (~550 nm in diameter) were mixed with freshly prepared anisotropic AuNPs. SEM images clearly show the preferential accumulation of the anisotropic AuNPs on the surface of the polymer nanoparticles ( Fig. 7). The overall structure (shape, size, and roughness) of these AuNPs is still preserved even after a month at room temperature ( Fig. 7(d)), strongly implying the presence of the stabilizing forces from the poly(NIPAM). This investigation can undoubtedly support the idea that the poly(NIPAM) can significantly slow the reshaping process and therefore increase the stability of the anisotropic AuNPs. As such, poly(NIPAM) and its derivatives can serve as a good stabilizing agent to prepare various anisotropic AuNPs.
The weak interactive forces of poly(NIPAM) in regards to AuNPs (via possible van der Waals, dipole and dipole interaction between adsorbed AsA on AuNPs and linear poly(NIPAM), and/or hydrogen bonding) play an important role in stabilizing the kinetically-driven anisotropic AuNPs. The preservation of the original structures and absorption bands of these nanoparticles at room temperature strongly indicated the enhanced stability in the presence of the polymer layer. Although vibrational spectroscopy (i.e., IR and Raman) studies did not provide the evidence of the adsorbed poly(NIPAM) on AuNPs, the systematic control of the size and surface roughness as a function of polymer concentration, reversible absorption patterns above and below LCST, and the interaction between cross-linked poly(NIPAM) nanoparticles and AuNPs clearly demonstrated the presence of the poly(NIPAM) around AuNPs. The increased stability of these anisotropic AuNPs, possessing a large surface area and a broad absorption band across the visible to near–IR range, may allow for their practical use as optically-driven/enhanced devices.
We gratefully acknowledge the financial support from the Cottrell College Science Award of Research Corporation and Illinois State University. This research is also supported by Korea Ministry of Environment as The Eco-Innovation Project (Global Top project, No. GT-SWS-11-01-0040-0). We thank Dr. M.E. Cook for assistance with the SEM and TEM measurements as well.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. |
|
| 42. |
|
| 43. |
|
| 44. |
|
| 45. |
|
| 46. |
|
| 47. |
|
| 48. |
|
| 49. |
|
| 50. |
|
| 51. |
|
| 52. |
|
| 53. |
|
| 54. |
|
| 55. |
|
| 56. |
|
| 57. |
|