Cesium tungsten bronze (Cs
In the past decades, tungsten oxides (WO3) have attracted great interests due to its many unique characteristics such as electrochromic [1], photoelectrochemical [2], photocatalytic activity [3] and gas sensing effects [4]. In recent years, the tungsten bronze (M xWO3), which is formed by doping monovalent ions such as H+, Li+, Na+, K+, Cs+ and other monovalent ions into WO3, have been paid more attention owing to their excellent optical and electrical properties [5], [6], [7], [8], [9], [10], [11] and [12]. Particularly, it was reported that the dispersions of cesium tungsten bronze (Cs xWO3) particles showed a remarkable near-infrared (NIR) light shielding while retaining a high transmittance of visible light, and Cs xWO3 will have great potential applications in the field of architectural and automotive window glasses [13].
At present, transparent heat insulation particles as a kind of green energy-saving materials, have great potential application prospects in the field of heat insulation of automotive and architectural windows glass. Some transparent conductive oxides such as antimony tin oxide (ATO), indium tin oxide (ITO) and zinc-aluminum oxide (ZAO) etc. have been reported to have better NIR shielding and heat insulation properties, and these transparent conductive oxides usually can shield the NIR light with wavelength λ > 1500 nm [14], [15] and [16]. By contrast, cesium tungsten oxides (Cs xWO3) have more desirable visible light transmittance and NIR shielding properties. It was reported that Cs xWO3 particles could shield the NIR light with wavelength λ > 1100 nm and this excellent heat shielding properties of Cs xWO3 is very suitable for the architectural and automotive window glass heat insulation [17]. However, the formation of Cs xWO3 with excellent transparency and NIR shielding often needs reduction atmosphere, and annealing in the reduction atmosphere (H2/N2) is also usually required [13].
Very recently, synthesis of Cs xWO3 powders with excellent transparency and NIR shielding by solvothermal reaction at 180–200 °C by using WCl6 and CsOH as raw materials have been studied by our research group and Guo et al. [18], [19] and [20]. However, the raw materials WCl6 are expensive and harmful to the environment owing to its easy hydrolysis and volatilization of HCl. Thus, it is necessary to prepare Cs xWO3 powders by using more moderate raw materials so as to realize the large-scale synthesis and practical applications of Cs xWO3 powders.
Therefore, in this work, Cs xWO3 powders have been prepared through hydrothermal process by using Na2WO4·2H2O and Cs2CO3 as raw materials. In order to further improve the properties of Cs xWO3, the effects of N2 annealing on the microstructure, NIR shielding and heat insulation properties of Cs xWO3 have been investigated systematically.
The Cs xWO3 powders were prepared according to our previous studies [21] and [22]. Firstly, a precursor solution with appropriate citric acid concentration and n(Cs): n(W) = 0.3:1 was prepared by using sodium tungstate and cesium carbonate as raw materials. Then the precursor solution was transferred into a Teflon-lined autoclave and heated at 190 °C for 72 h, and finally pure Cs xWO3 powders could be obtained after being washed with water and ethanol three times, respectively and dried at 80 °C overnight. In this work, Cs xWO3 powders were prepared in the precursor solution with the concentration of citric acid being 0.55 mol/L and 1.1 mol/L, respectively.
Then the as-prepared Cs xWO3 powders with 1.1 mol/L citric acid were annealed in the N2 atmosphere in a tubular furnace at 500–800 °C for 30–120 min with the flow of N2 at 400 mL/min.
The phase composition of the samples was determined by X-ray diffraction analysis (XRD, D/max-3B, Japan). The morphology of the samples was observed by scanning electron microscopy (SEM, JEOL JSM-6460LV, Japan).
The diffuse reflectance spectra (DRS) of Cs xWO3 powders were characterized by using a Lambda 35 ultraviolet visible (UV–Vis) spectrophotometer at 300–1100 nm range with BaCl2 as background. In order to evaluate the NIR shielding properties, the Cs xWO3 powders were dispersed in the polyvinyl alcohol (PVA) solution with the concentration of 0.1 g/mL. Then the dispersion solution was coated on the glass plate for two layers. The transmittance spectra of the glass plate coated with Cs xWO3 film were measured by PerKin Elmer Lambda 950 UV–Vis-NIR spectrophotometer at 300–2500 nm range.
The heat insulation of Cs xWO3 film was evaluated by using an insulated box (10 cm × 5 cm × 10.5 cm) made from foam board. There was a rectangular window (5 cm × 10 cm) on the top of the box. The bottom of the box was covered with a black iron plate. Red water thermometer was contacted closely with the black iron plate. Put the glass coated with Cs xWO3 film on the window of the insulated box and record the interior temperature when the box was irradiated by a 250 W infrared lamp. Then the curves of temperature increment dependence on irradiation time could be obtained. The heat insulation of Cs xWO3 film glass was evaluated by comparison with that of the blank glass.
Fig. 1 shows the XRD patterns of Cs xWO3 powders prepared by hydrothermal reactions in the precursor solution with different concentrations of citric acid. It is found that the diffraction patterns of Cs xWO3 powders prepared from the precursor solution with different concentrations of citric acid agree well with that of hexagonal cesium tungsten bronze Cs0.32WO3 (JCPDS 83-1334). It is obvious that the diffraction peaks become stronger with increasing citric acid content, which indicates that higher concentration of citric acid is favorable for obtaining higher crystallinity of Cs0.32WO3.
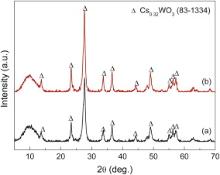 | Fig. 1. XRD patterns of Cs xWO3 powders prepared by hydrothermal reaction from precursor solutions with different concentrations of citric acid: (a) 0.55 mol/L, (b) 1.10 mol/L. |
As is known that the valence state of tungsten (W) in the tungsten oxide (WO3) is hexavalent (+6), while the valence state of tungsten in the cesium tungsten bronze (Cs xWO3) should be lower than the hexavalent (+6). Thus reduction atmosphere in the reaction system is necessary for the synthesis of Cs xWO3. In this study, citric acid, an organic carboxylic acid containing one hydroxyl and three carboxyl groups, was chosen as reducing agent to provide the reduction atmosphere.
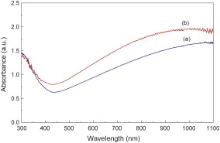
Fig. 2 shows the UV–Vis absorption spectra of Cs xWO3 powders prepared by hydrothermal reaction with different concentrations of citric acid. The as-prepared Cs xWO3 powders have a high absorption in the NIR region, and the Cs xWO3 powders prepared from the precursor solution with 1.1 mol/L citric acid exhibit higher absorption in the NIR region than Cs xWO3 prepared with 0.55 mol/L citric acid. It was reported that Cs xWO3 particles have higher NIR light absorption property, which is the main factor for achieving better NIR shielding ability [20].
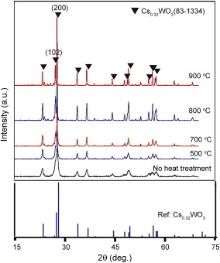
Fig. 3 shows the XRD patterns of Cs xWO3 powder after N2 annealing at different temperatures for 2 h. The diffraction peaks of (102) crystal plane become more obvious and stronger with the increase of N2 annealing temperature, which indicates that the Cs0.32WO3 crystals can grow more completely with the increase of N2 annealing temperature. However, after N2 annealing at 800 and 900 °C, some tiny diffraction peaks also appear. This result indicates that too high annealing temperature may lead to the formation of other impurity crystal phase.
Fig. 4 shows the SEM images of Cs xWO3 powders after N2 annealing at different temperatures. The Cs xWO3 particle size becomes bigger with the increase of N2 annealing temperature. Particularly, the 800 °C-annealed samples exhibit micrometer-sized crystalline grains.
 | Fig. 4. SEM images of Cs xWO3 powders after N2 annealing at different temperatures: (a) no annealing, (b) 500 °C for 2 h, (c) 700 °C for 2 h, (d) 800 °C for 30 min. |
Fig. 5 shows the transmittance spectra of Cs xWO3 synthesized from the precursor solution with 1.10 mol/L citric acid and followed by N2 annealing at different temperatures for 2 h. In comparison with the Cs xWO3 products without annealing, the 500 °C-annealed Cs xWO3 products exhibit the best visible light transmittance and the highest NIR shielding ability, whereas the Cs xWO3 products annealed at 600 and 700 °C exhibit higher NIR shielding ability but lower transmittance in the visible light. In addition, the NIR shielding properties decrease again when the N2 annealing temperature is higher than 800 °C. The Cs xWO3 products after N2 annealing at 800 °C exhibit lower NIR shielding ability in spite of its excellent transparency in the visible light. Thus it can be concluded that the Cs xWO3 particles after N2 annealing at 500 °C exhibit the best visible light transmittance and the highest NIR shielding properties.
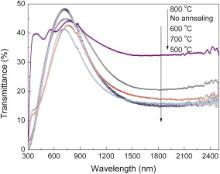 | Fig. 5. Transmittance spectra of Cs0.3WO3 films, in which the Cs xWO3 powders were annealed in the N2 atmosphere at different temperatures for 2 h. |
In addition, it is found that a new inflexion appears in the curves at wavelength 330–380 nm after N2 annealing at 600–800 °C, which indicates that the microstructure of Cs xWO3 may have been changed and re-oxidation of Cs xWO3 may be possible by N2 annealing at higher temperature. Moreover, too high annealing temperature may lead to the quick growth of the Cs xWO3 crystals and thus the NIR absorption ability decreases again. It is reasonable that the particle size and surface morphology of the Cs xWO3 particles have effects on the visible light transparency and NIR shielding properties.
The elemental valence of the Cs xWO3 before and after N2 annealing was investigated by X-ray photoelectron spectroscopy (XPS). Fig. 6(a) shows the W4 f XPS profiles of Cs xWO3 before and after N2 annealing at different temperatures. It is obvious that the two peaks in the W4 f show a shift to lower binding energies after N2 annealing, which indicates that the chemical state of W has been changed due to the partial reduction of W6+ to W5+. In order to further explain the chemical state of W in Cs xWO3, the W4 f XPS spectra of Cs xWO3 after N2 annealing at 700 °C is shown in Fig. 6(b). The W4 f core-level spectrum can be fitted to two spin-orbit doublets, which are associated with the two different oxidation states of W atoms. As was reported by Guo et al. [23], the main peaks, with W4 f5/2 at 37.9 eV and W4 f7/2 at 35.8 eV, are attributed to the W atoms in the 6+ oxidation state, and the second doublet with a lower binding energy at 34.6 and 36.7 eV is characteristic of W atoms in the 5+ oxidation state. It is suggested that the non-stoichiometric cesium tungsten bronzes Cs xWO3 are reduced compounds, which can also be expressed as the formula Cs xW x5+W1– x6+O3, while the ideal oxidized stoichiometric cesium tungsten oxides should be expressed as a formula of Cs xWO3+ x/2. According to the energy band and the free electron theory, the visible light transparency and NIR shielding properties of Cs xWO3 should be related with the plasmon resonance of free electrons, the interband transition and small polarons. Whereas, the concentration of free carrier and oxygen vacancies have great effects on the plasmon resonance frequency of free electrons and the band gap energy, which will directly affect the starting wavelength of the NIR shielding and visible light transparency properties. So, the visible light transparency and NIR shielding properties of Cs xWO3 could be further improved by adjusting and controlling the concentration of free carriers in its microstructure.
 | Fig. 6. W4 f XPS profiles of the as-synthesized Cs xWO3 after N2 annealing at: (a) different temperatures, (b) 700 °C for 2 h. |
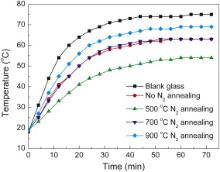
Fig. 7 shows the heat insulation curves of Cs xWO3 films, which were prepared from the dispersions of Cs xWO3 powders after N2 annealing at different temperatures. The heating rates of glass coated with Cs xWO3 products all are obviously lower than that of the blank glass, which indicates that the Cs xWO3 films have excellent heat insulation performance. In particular, the 500 °C-annealed Cs xWO3 products in the N2 atmosphere exhibit the best heat insulation, and the highest heat insulation temperature difference between the blank glass and the Cs xWO3 film glass could reach 21 °C. Obviously, the excellent heat insulation of the 500 °C-annealed Cs xWO3 products is closely associated with its excellent NIR shielding properties.
Cesium tungsten bronze (Cs xWO3) particles with hexagonal Cs0.32WO3 structure were synthesized through hydrothermal synthesis by using cheaper sodium tungstate and cesium carbonate as raw materials. The higher citric acid concentration in the precursor solution is favorable for obtaining Cs0.32WO3 crystals with higher crystallinity and higher NIR absorption ability.
Subsequent N2 annealing has great influences on the particle size, crystallinity and NIR shielding ability of Cs xWO3 powders. The NIR shielding and heat insulation of Cs xWO3 products could be further improved by annealing the Cs xWO3 powders in the N2 atmosphere. In particular, the 500 °C-annealed Cs xWO3 particles in the N2 atmosphere exhibit the best NIR shielding and heat insulation, and the highest heat insulation temperature difference between the blank glass and the Cs xWO3 film could reach 21 °C.
This work was financially supported by the National Natural Science Foundation of China (No. 51278074), and the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (the [42nd, 20111139]).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|