Hydroxyapatite (HA) nano-powder was synthesized via wet chemical technique in a used precipitation reaction, in which Ca(OH)2 and H3PO4 were used as precursors. Deionised water was used as a diluting media for the reaction and ammonia was used to adjust the pH. The synthetic HA nano-powder has some medical applications such as a coating material in orthopaedic implants and in dental. HA powder has been studied at different temperatures from 100 to 800 °C to achieve the stoichiometric Ca/P ratio 1.667. The optimum temperature was found to be 600 °C. Above this temperature, the HA powder decomposed to CaO. The crystallite size of HA powder was found to be in the range of 8.47–24.47 nm. The crystallographic properties were evaluated by X-ray diffraction, Fourier transform infrared spectroscopy, energy dispersive X-ray spectroscopy and scanning electron microscopy. The results show that, high purity of nano-hydroxyapatite powders could be obtained at low temperatures, and the crystallinity, crystallite size and Ca/P ratio of the resulting nanoparticles were found to be dependent on the calcination temperature. When Ca/P ratio exceeded 1.75, formation of CaO phase was observed.
Hydroxyapatite (HA) [Ca10(Po4)6(OH)2] is a naturally existing mineral in the inorganic component of human bone and tooth enamel. The crystal size of HA in natural human bone is in nano range. The constituent elements of HA are primarily calcium and phosphorus, with a stoichiometric Ca/P ratio of 1.667 [1]. HA is composed primarily of calcium and phosphorous with hydroxide ions that are eliminated at elevated temperatures. HA and other related calcium phosphate minerals have been utilized extensively as implant materials for many years due to its excellent biocompatibility and bone bonding ability and also due to its structural and compositional similarity to that of the mineral phase of hard tissue in human bones [1], [2] and [3]. HA coatings have good potential as they can exploit the biocompatible and bone bonding properties of the ceramic, while utilizing the mechanical properties of substrates such as Ti–6Al–4V and other biocompatible alloys. While the metallic materials have the required mechanical properties, they benefit from the HA which provides an osteoconductive surface for new bone growth, anchoring the implant and transferring load to the skeleton, helping to combat bone atrophy [1] and [4]. Their Ca/P ratio of 1.52 ± 2.0 makes them an excellent choice for most dental and orthopaedic applications in the form of bioceramic coatings. The quality of a coating is closely dependent on the overall attributes and characteristics of the synthesized powders. Such attributes include phase composition, purity, crystallinity, particle size, particle-size distribution, specific surface area, density and particle morphology. These important factors determine the resulting success of the HA coating deposited onto orthopaedic implants through plasma thermal spraying due to poor mechanical properties of HA. The recent trend in bioceramic research is focused on improving their mechanical and biological properties using nanotechnology [5]. Common methods used to produce synthetic nano-crystalline HA include precipitation [6], hydrothermal [7], hydrolysis [8], mechanochemical [9] and sol-gel [10] method.
In the present work, nano-sized HA powder was synthesized via wet chemical precipitation method using calcium hydroxide, orthophosphoric acid and ammonia as precursors, and its crystallographic properties were characterized.
In the present work, calcium oxide (CaO) (S D Fine Chem Limited, Mumbai, India), orthophosphoric acid (H3PO4) (Fisher Scientific, Mumbai, INDIA), and ammonium hydroxide (NH4OH) (Fisher Scientific, Mumbai, India) were used as starting materials. Firstly, an analytical weighing scale was used to accurately weigh CaO powder. 1.42 mol (79.55 g) CaO powder was added to 500 ml of deionised water in a 1000 ml beaker and vigorously stirred at 1000 r/min at 20 °C for 24 h to react and form a suspension of Ca(OH)2 in an excess of deionised water. The beaker was covered in order to avoid possible contamination via contact with atmospheric conditions. The temperature of the reaction (20 °C) was maintained by a thermostat-controlled water bath.
An analytical weighing scale was used to accurately weigh the required quantity of orthophosphoric acid. 97.32 g of 85% H3PO4 was added to Ca(OH)2 solution at a rate of 1.5 ml/min. During the course of the acid addition, the pH of the solution was monitored via a handheld pH meter with an accuracy of ±0.2. The reactants were stirred for further 24 h to aid the maturation stage, under continuous stirring conditions at 1000 r/min, held at the respective reaction temperature of 20 °C. 0.28 mol (9.94 g) NH4OH, was added to the HA slurry after 24 h ripening period to stabilise the pH of the super saturation solution to above 9. To maintain a Ca/P ratio of 1.67 the solution was kept above 9 [1] and [5]. A pH of 10 has been considered to produce the correct stoichiometric condition by which only pure single phase HA is formed [11].
Assay samples were taken for analysing the composition of mixture in the barrel. A small crucible was filled with a sample of the mixture in the mixing barrel and dried in a drying oven for 1 h at 100 °C. The dried samples were then placed in a furnace and sintered at 1200 °C for 1 h. When the assays were cooled, they were removed from the furnace and ground using a motor and pestle. Scanning electron microscopy (SEM, HITACHI model S-3700N at DTU) was used to observe the morphology and the particle size of calcined HA powder. Elemental phase composition of the HA powder was analysed using energy dispersive X-ray spectroscopy (EDX) (HITACHI model S-3700N at DTU). The X-ray diffraction (XRD) pattern of the final HA nanoparticles was obtained with Cu Kα radiation ( λ = 0.15406 nm) on a diffractometer (RIGAKU MINIFLEX at AMU). The XRD patterns were recorded in a 2 θ range of 20°–60° with a step size of 0.02° and step duration of 0.5 s. The mean crystallite size ( D) of the particles was calculated from XRD line broadening measurement using the Scherrer equation [12]:
where λ is the wavelength of Cu Kα, β the full width at half maximum of the HA line and θthe diffraction angle.
The following reactions were involved in the formation of HA during the precipitation reaction:
CaO+H2O→Ca2(OH) (1)
10Ca2(OH)+6H34PO→Ca1066(4PO)2(OH)+18H2O (2)
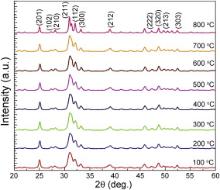
Fig. 1 shows the XRD pattern of HA from 100 to 800 °C. The crystallite size was calculated by Scherrer equation with the most intense plane that is 211 of eight calcined HA samples for each temperature. The results are given in . It can be observed that with increasing the calcination temperature the crystallite size also increases. Similar phenomenon was observed previously [5] and [13]. Hydroxyapatite has two intrinsic properties that enable it to form an amorphous phase. First, it is made up of phosphates which are glass formers, and second, the composition of hydroxyapatite is very close to the eutectic composition, thus inducing a liquidus effect [14]. It has also been reported from XRD spectra that HA calcined at higher temperatures exhibited good crystallinity as the peaks become narrow and sharp moving from 100 to 800 °C. Also, it shows only a little or no activity towards bioresorption which is important for the formation of chemical bonding with surrounding hard tissues [5], [15] and [16]. As it is evident from the XRD spectra, the amorphous HA powders were obtained at lower temperatures (100–200 °C). Similar phenomena have been observed by others [5] and [13]. This indicates that HA powder prepared in this study is a mixture of crystalline as well as amorphous phases with the major content being the amorphous phase of HA. It is expected to be metabolically more active than the fully developed crystalline hydroxyapatite structure which otherwise is insoluble in the physiological environment [5] and [17].
| Table 1. Crystallite size ( D) at different calcination temperatures |
The spectra in Fig. 1 are typically in agreement with those published in literature; all XRD spectra obtained have characteristic peaks consistent with the International Centre for Diffraction [JCPDS 2001] files for calcium phosphate. The predominant HA phase was confirmed with JCPDS files number 09-432.
This suggests that no foreign elements, such as sodium (Na2+), ammonium (NH4+), potassium (K+), chloride (Cl-) and nitrate (NO3-) ions, were involved in the synthesis reaction, as there is strong evidence to suggest that these ions are easily incorporated into the crystal lattice leading to the formation of known stoichiometric HA. These elements are usually introduced into the precipitating systems with the reactants. The absence of these elements can be attributed to the nature of the raw materials used as precursors. In fact, it has been shown that chloride ions enter into the crystal lattice substituting hydroxyl groups while potassium ions are found to substitute calcium ions into the HA crystal lattice forming locally non-stoichiometric (i.e. impure) HA islands in the bulk crystal. Sodium ions also show the evidence of substitutions. In order to avoid contamination of the products, use of calcium nitrate and phosphoric acid instead of calcium chloride salts and potassium dihydrogen phosphate, respectively is advantageous because the presence of potassium ions is avoided and the nitrate ions are too large to substitute hydroxyl groups in the crystal lattice of HA. These substitutions were avoided when Ca(OH)2 and H3PO4 reactants were used to prepare our samples. For all samples of the HA studied no CaO was observed. This indicates that either small or no carbonation of HA has occurred during the synthesis of HA although no foreign elements were found.
The Ca/P stoichiometry of calcined HA at different temperatures (100–800 °C) was analysed by EDX. Fig. 2 shows that HA powder with a Ca/P ratio near 1.67 that is at 600 °C and below, indicates no CaO content and that with a Ca/P ratio near 1.75 and above, and showed the formation of minor amounts of CaO for calcined samples at 800 °C. Other researchers have reported similar formation of CaO in sol-gel processing of HA [5], [18] and [19].
SEM observation was performed at Delhi Technical University (DTU). The HA sample is coated with gold and placed in a HITACHI MODEL-S-3700N. The images were recorded at different temperatures ranging from 100 to 800 °C ( Fig. 3). It is known that spherical powders, in general, have better rheological properties than irregular powders and, thus, produce better coatings for hip implants [1] and [11]. In order to produce dense, high-quality HA powder for purpose like hip implant coating, it is very important to predict or control granule spherical morphology [20]. It has also been demonstrated that an increase in synthesis temperature leads to an increase in HA precipitates size.
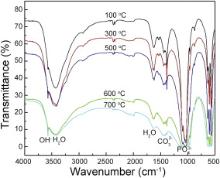
Fourier transform infrared spectroscopy (FT-IR) was performed at Centre of Excellence in Materials Science (Nanomaterials) A.M.U. Aligarh. FT-IR machine (PARKIN ELMER) with resolution of 4000–400 cm-1 and reference material of KBR were used. FT-IR patterns presented in Fig. 4 confirm the formation of HA calcinated at different temperatures of 100–700 °C. The spectra possessed an (OH)-1 group in the region of 3577 cm-1 and (PO4)3- group comes out from the region of 1075 cm-1. From this analysis the formation of HA is confirmed. The peaks are quite sharp at intermediate temperatures (500–600 °C) and as the temperature increases peaks are going to be weak as shown in Fig. 4, which is in agreement with published data [5], [21] and [22]. A weak band of CO32- was detected in the region around 1420 cm-1. H2O band was also observed at around 1650 cm-1 and 3350 cm-1 [5] and [23]. The characteristic bands from inorganic carbonate ion suggest that carbon gets dissolved in the organics from atmosphere and does not pyrolyse completely and may instead dissolve into the HA crystal. Since carbonates are constituents of bone structures [5] and [24] the presence of CO32- may improve the bioactivity of HA rather than being a cause of concern [5].
Wet chemical precipitation method is used in the present work due to its high reproducibility, simplicity as well as economical benefits it offers on industrial scale. One of the main advantages of this method is that the water is its only byproduct. For the reported method, calcium hydroxide and orthophosphoric acid are used as precursors. This process shows that high purity of nano-hydroxyapatite powders could be obtained at low temperatures. The crystallinity, crystallite size and Ca/P ratio of the resulting nanoparticles were found to be dependent on the calcination temperature. When Ca/P ratio exceeded 1.75, the formation of CaO phase was observed.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|